1516:
321:
298:
195:
220:
579:
1689:
enzyme, substrate levels build up in the CNS. The substrate, L-methylmalonyl-CoA hydrolyzes to form methylmalonate (methylmalonic acid), a neurotoxic dicarboxylic acid that, due to the poor dicarboxylic acid transport capacities of the blood-brain barrier, is effectively trapped within the CNS, leading to neurological debilitation. To combat these effects perioperative anti-catabolic regimes and no diet discontinuation are recommended.
572:
327:
226:
1437:, are metabolized via methylmalonate semialdehyde (MMlSA) or propionyl-CoA (Pr-CoA) to a common compound - methylmalonyl-CoA (MMl-CoA). MCM catalyzes the reversible isomerisation of l‐methylmalonyl‐CoA to succinyl‐CoA, requiring cobalamin (vitamin B12) in the form of adenosylcobalamin (AdoCbl) as a cofactor. As an important step in propionate catabolism, this reaction is required for the degradation of odd-chain
40:
5315:
1752:
1491:
1501:
1755:
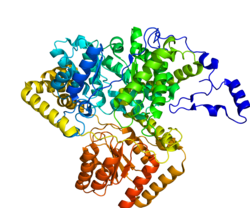
MCM active site. Corrin ring and α-axial ligand (DMB): (yellow), β-axial ligand: (green), substrate/product: (cyan), residues interacting with β-axial ligand: glu370, asn366, gly91, ala139 (blue), residues interacting with substrate: gln197, his244, arg207, tyr89 (red), and his610: (orange). Rendered
1688:
of methylmalonic acidemia. However, detrimental neurological effects can continue to plague patients even after a successful operation. It is thought that this is due to the widespread presence of methylmalonyl-CoA mutase throughout the central nervous system. Due to the loss of functionality of the
1716:(III) bond, the C and Co atoms each acquire one of the electrons that formed the cleaved electron pair bond. The Co ion, therefore, fluctuates between its Co(III) and Co(II) oxidation states . Hence, the role of coenzyme B-12 in the catalytic process is that of a reversible generator of a
66:
2376:
Manoli I, Sysol JR, Li L, Houillier P, Garone C, Wang C, Zerfas PM, Cusmano-Ozog K, Young S, Trivedi NS, Cheng J, Sloan JL, Chandler RJ, Abu-Asab M, Tsokos M, Elkahloun AG, Rosen S, Enns GM, Berry GT, Hoffmann V, DiMauro S, Schnermann J, Venditti CP (August 2013).
1974:
Ballhausen D, Mittaz L, Boulat O, Bonafé L, Braissant O (December 2009). "Evidence for catabolic pathway of propionate metabolism in CNS: expression pattern of methylmalonyl-CoA mutase and propionyl-CoA carboxylase alpha-subunit in developing and adult rat brain".
1731:
subfamily of adenosylcobalamin-dependent enzymes. Furthermore, it is classified as class I, as it is a ‘DMB-off’/’His-on’ enzyme. This refers to the nature of the AdoCbl cofactor in the active site of methylmalonyl CoA. AdoCbl is composed of a central
2944:
Lubrano R, Elli M, Rossi M, Travasso E, Raggi C, Barsotti P, Carducci C, Berloco P (August 2007). "Renal transplant in methylmalonic acidemia: could it be the best option? Report on a case at 10 years and review of the literature".
2216:
Dündar H, Özgül RK, Güzel-Ozantürk A, Dursun A, Sivri S, Aliefendioğlu D, Coşkun T, Tokatli A (August 2012). "Microarray based mutational analysis of patients with methylmalonic acidemia: identification of 10 novel mutations".
1705:
1748:-610 to bond with Co, instead of DMB (the reason for the ‘DMB-off’/’His-on’ notation). Binding of histidine-610 residue increases the rate of homolytic β-axial ligand – Co bond breakage by a factor of 10.
2129:
Martínez MA, Rincón A, Desviat LR, Merinero B, Ugarte M, Pérez B (April 2005). "Genetic analysis of three genes causing isolated methylmalonic acidemia: identification of 21 novel allelic variants".
1932:
Keyfi F, Sankian M, Moghaddassian M, Rolfs A, Varasteh AR (January 2016). "Molecular, biochemical, and structural analysis of a novel mutation in patients with methylmalonyl-CoA mutase deficiency".
1760:
Other important residues of methylmalonyl-CoA mutase include
Histidine-244, which acts as a general acid near the substrate and shields the radical species from side reactions involving oxygen,
4500:
1740:, an upper axial ligand (β-axial ligand), and a lower axial ligand (α-axial ligand). In methylmalonyl-CoA mutase, the β-axial ligand 5’-deoxy-5’-adenosine reversibly dissociated to give the
2982:
Frenkel EP, Kitchens RL (December 1975). "Intracellular localization of hepatic propionyl-CoA carboxylase and methylmalonyl-CoA mutase in humans and normal and vitamin B12 deficient rats".
1337:
of the protein is cleaved, forming the fully processed monomer. The monomers then associate into homodimers, and bind AdoCbl (one for each monomer active site) to form the final, active
3591:
3259:
Jansen R, Kalousek F, Fenton WA, Rosenberg LE, Ledley FD (February 1989). "Cloning of full-length methylmalonyl-CoA mutase from a cDNA library using the polymerase chain reaction".
4193:
4217:
4173:
3688:
3514:"Expression and kinetic characterization of methylmalonyl-CoA mutase from patients with the mut- phenotype: evidence for naturally occurring interallelic complementation"
334:
233:
4839:
3290:"Immunochemical studies of fibroblasts from patients with methylmalonyl-CoA mutase apoenzyme deficiency: detection of a mutation interfering with mitochondrial import"
2257:
Takahashi-Íñiguez T, García-Arellano H, Trujillo-Roldán MA, Flores ME (January 2011). "Protection and reactivation of human methylmalonyl-CoA mutase by MMAA protein".
1243:
1789:, and allows for the transfer of the AdoCbl cofactor to the enzyme active site. Furthermore, if the bound AdoCbl accrues oxidative damage during normal functioning,
4493:
3778:
4773:
1262:
4222:
3584:
1720:. The C-Co(III) bond is weak, with a dissociation energy = 109 kJ/mol, and appears to be further weakened through steric interactions with the enzyme. The
3341:
Zoghbi HY, O'Brien WE, Ledley FD (November 1988). "Linkage relationships of the human methylmalonyl CoA mutase to the HLA and D6S4 loci on chromosome 6".
2687:, Meier TW, Evans PR, Leadlay PF (October 1998). "Stabilization of radical intermediates by an active-site tyrosine residue in methylmalonyl-CoA mutase".
4486:
4212:
4168:
4018:
3683:
3405:
Fenton WA, Hack AM, Willard HF, Gertler A, Rosenberg LE (April 1982). "Purification and properties of methylmalonyl coenzyme A mutase from human liver".
817:
156:
3101:"Genetic characterization of a MUT locus mutation discriminating heterogeneity in mut0 and mut- methylmalonic aciduria by interallelic complementation"
798:
4274:
3783:
4744:
3062:
Crane AM, Martin LS, Valle D, Ledley FD (May 1992). "Phenotype of disease in three patients with identical mutations in methylmalonyl CoA mutase".
4014:
2329:"Pre-clinical efficacy and dosing of an AAV8 vector expressing human methylmalonyl-CoA mutase in a murine model of methylmalonic acidemia (MMA)"
3577:
3144:"Heterozygous mutations at the mut locus in fibroblasts with mut0 methylmalonic acidemia identified by polymerase-chain-reaction cDNA cloning"
3735:
1911:
63:
3021:"Cloning and expression of a mutant methylmalonyl coenzyme A mutase with altered cobalamin affinity that causes mut- methylmalonic aciduria"
1893:
5335:
3875:
3698:
2649:
Buckel W, Friedrich P, Golding BT (October 2012). "Hydrogen bonds guide the short-lived 5'-deoxyadenosyl radical to the place of action".
320:
4388:
3985:
3708:
2072:"Molecular basis for dysfunction of some mutant forms of methylmalonyl-CoA mutase: deductions from the structure of methionine synthase"
1595:
2610:"Proton transfer from histidine 244 may facilitate the 1,2 rearrangement reaction in coenzyme B(12)-dependent methylmalonyl-CoA mutase"
4626:
4545:
4188:
3678:
1028:
1021:
297:
5034:
4876:
4183:
4100:
4009:
2292:
Buck NE, Wood LR, Hamilton NJ, Bennett MJ, Peters HL (November 2012). "Treatment of a methylmalonyl-CoA mutase stopcodon mutation".
3713:
2891:
2743:"Structures of the human GTPase MMAA and vitamin B12-dependent methylmalonyl-CoA mutase and insight into their complex formation"
1255:
3855:
1880:
1859:
2741:
Froese DS, Kochan G, Muniz JR, Wu X, Gileadi C, Ugochukwu E, Krysztofinska E, Gravel RA, Oppermann U, Yue WW (December 2010).
4783:
4613:
4601:
4550:
4247:
3728:
3723:
1206:
1182:
5190:
1876:
2169:"Functional characterization and categorization of missense mutations that cause methylmalonyl-CoA mutase (MUT) deficiency"
219:
194:
4622:
4398:
4393:
4110:
3773:
2569:"Conformational changes on substrate binding to methylmalonyl CoA mutase and new insights into the free radical mechanism"
1515:
1855:
4609:
136:
4336:
4287:
3754:
1374:
The mature enzyme is a homodimer with the N-terminal CoA binding domain and the C- terminal cobalamin-binding domain.
333:
232:
5305:
4967:
4940:
4042:
3758:
3659:
3654:
3569:
1411:
5175:
3177:
Nham SU, Wilkemeyer MF, Ledley FD (December 1990). "Structure of the human methylmalonyl-CoA mutase (MUT) locus".
2532:
Mancia F, Smith GA, Evans PR (June 1999). "Crystal structure of substrate complexes of methylmalonyl-CoA mutase".
5291:
5278:
5265:
5252:
5239:
5226:
5213:
4977:
4926:
4916:
4902:
4710:
4383:
4378:
4331:
4292:
3937:
2878:
Ledley FD, Rosenblatt DS (1997). "Mutations in mut methylmalonic acidemia: clinical and enzymatic correlations".
326:
225:
5185:
1200:
5139:
5082:
4893:
4705:
4540:
3918:
3563:
1696:
model has proven an adequate and accurate way of studying the effects of MMA, and potential treatment methods.
1631:
1583:
1462:
1281:
1093:
862:
144:
3436:"Cloning and expression of mutations demonstrating intragenic complementation in mut0 methylmalonic aciduria"
2794:"Energetics of interaction between the G-protein chaperone, MeaB, and B12-dependent methylmalonyl-CoA mutase"
1187:
5340:
5087:
4935:
4911:
4730:
4646:
4557:
4072:
4047:
3975:
3957:
3718:
2379:"Targeting proximal tubule mitochondrial dysfunction attenuates the renal disease of methylmalonic acidemia"
843:
1772:-89 which stabilizes reactive radical intermediates and accounts for the stereo-selectivity of the enzyme.
1768:
of the β-axial ligand forces interaction between the β-axial ligand radical species and the substrate, and
1304:-dependent enzyme catalyzes the isomerization of methylmalonyl-CoA to succinyl-CoA in humans. Mutations in
4689:
4638:
4605:
4459:
4252:
4105:
2696:
2490:"Importance of the histidine ligand to coenzyme B12 in the reaction catalyzed by methylmalonyl-CoA mutase"
1794:
1606:
inherited inborn error of metabolism, characterized by recurrent episodes of vomiting, lethargy, profound
1599:
1407:
1309:
1744:. The α-axial ligand 5,6-dimethylbenzimidazole (DMB) is involved in organizing the active site to enable
1267:
5108:
5027:
4986:
4869:
4583:
4454:
3818:
3554:
1741:
1721:
1175:
5180:
1623:
4754:
4684:
4572:
4125:
3899:
3632:
3622:
3608:
3301:
3219:
2390:
2083:
1110:
208:
3559:
123:
5144:
4991:
4834:
4269:
4265:
4178:
4063:
3693:
2701:
2684:
1603:
1474:
1203:
1105:
1333:
in length. Upon entry to the mitochondria, the 32 amino acid mitochondrial leader sequence at the
1127:
983:
5345:
5077:
4950:
4945:
4725:
4311:
3860:
3738:– this diagram does not include the pathway for β-leucine synthesis via leucine 2,3-aminomutase)
3604:
3479:"Clustering of mutations in methylmalonyl CoA mutase associated with mut- methylmalonic acidemia"
3087:
3007:
2970:
2903:
2000:
1670:
1666:
168:
1004:
979:
953:
932:
3208:"Molecular cloning of L-methylmalonyl-CoA mutase: gene transfer and analysis of mut cell lines"
17:
4513:
3703:
3535:
3500:
3465:
3422:
3393:
3358:
3329:
3276:
3247:
3194:
3165:
3130:
3079:
3050:
2999:
2962:
2932:
2895:
2856:
2815:
2774:
2714:
2666:
2631:
2590:
2549:
2511:
2470:
2418:
2358:
2309:
2274:
2234:
2198:
2146:
2111:
2049:
1992:
1949:
1681:
1547:
1194:
116:
56:
1661:(demonstrates very low activity in presence of excess AdoCbl). Over half of the mutations of
5123:
5118:
5092:
5020:
4862:
4808:
4803:
4676:
4530:
4428:
3525:
3490:
3455:
3447:
3414:
3383:
3350:
3319:
3309:
3268:
3237:
3227:
3186:
3155:
3120:
3112:
3071:
3040:
3032:
2991:
2954:
2924:
2887:
2846:
2805:
2764:
2754:
2706:
2658:
2621:
2580:
2541:
2501:
2460:
2452:
2408:
2398:
2348:
2340:
2301:
2266:
2226:
2188:
2180:
2138:
2101:
2091:
2039:
2031:
1984:
1941:
1677:
413:
344:
288:
243:
1163:
578:
5170:
5154:
5067:
4996:
4004:
3880:
3763:
1814:
388:
164:
1139:
4478:
3305:
3223:
2915:
Ludwig ML, Matthews RG (1997). "Structure-based perspectives on B12-dependent enzymes".
2394:
2087:
1284:
1098:
571:
5319:
5208:
5149:
3495:
3478:
3160:
3143:
2995:
2769:
2742:
2465:
2440:
2413:
2378:
2353:
2328:
2193:
2168:
2044:
2019:
1988:
1751:
1619:
1611:
1238:
3460:
3435:
3434:
Qureshi AA, Crane AM, Matiaszuk NV, Rezvani I, Ledley FD, Rosenblatt DS (April 1994).
3388:
3371:
3324:
3289:
3242:
3207:
3125:
3100:
3045:
3020:
2851:
2834:
2585:
2568:
1218:
732:
727:
722:
717:
712:
707:
691:
686:
670:
665:
660:
655:
650:
645:
640:
635:
625:
620:
5329:
5113:
5072:
4365:
4145:
3793:
3418:
3354:
3272:
3190:
2928:
2439:
Takahashi-Iñiguez T, García-Hernandez E, Arreguín-Espinosa R, Flores ME (June 2012).
2106:
2071:
1685:
1551:
1213:
607:
2974:
2907:
2070:
Drennan CL, Matthews RG, Rosenblatt DS, Ledley FD, Fenton WA, Ludwig ML (May 1996).
2004:
1594:
A deficiency of this enzyme is responsible for an inherited disorder of metabolism,
1410:(CNS). MCM resides in the mitochondria, where a number of substances, including the
39:
5062:
4749:
4735:
4657:
4450:
4322:
4150:
3768:
3091:
3011:
1717:
1627:
1615:
1607:
1579:
1490:
1482:
406:
185:
148:
172:
5286:
5221:
5057:
4130:
4096:
3372:"Isolation and characterization of methylmalonyl-CoA mutase from human placenta"
2344:
2230:
2142:
2035:
1916:
National Center for
Biotechnology Information, U.S. National Library of Medicine
1898:
National Center for
Biotechnology Information, U.S. National Library of Medicine
1737:
1575:
1458:
1438:
1434:
1330:
1298:
1222:
5314:
3294:
Proceedings of the
National Academy of Sciences of the United States of America
3212:
Proceedings of the
National Academy of Sciences of the United States of America
2383:
Proceedings of the
National Academy of Sciences of the United States of America
2305:
2270:
2076:
Proceedings of the
National Academy of Sciences of the United States of America
1945:
489:
4854:
4593:
4509:
4437:
4413:
4282:
4260:
4242:
4232:
4203:
4052:
3745:
3636:
3600:
2958:
1567:
1555:
1450:
1446:
1422:
1414:
1359:
1338:
1334:
305:
202:
152:
2626:
2609:
5260:
5234:
4885:
4535:
4441:
4302:
4121:
4117:
4092:
3980:
3966:
3952:
3928:
3232:
2759:
2403:
2096:
1786:
1781:
protein, fills the important role of aiding cofactor loading and exchange.
1761:
1745:
1728:
1563:
1454:
1426:
762:
549:
427:
372:
359:
271:
258:
160:
3530:
3513:
3314:
2966:
2819:
2810:
2793:
2778:
2670:
2662:
2635:
2553:
2515:
2506:
2489:
2474:
2422:
2362:
2313:
2278:
2238:
2202:
2150:
2053:
1996:
1953:
1724:
reaction is unusual in biology, as is the presence of a metal-carbon bond.
3539:
3504:
3469:
3426:
3397:
3362:
3333:
3280:
3251:
3198:
3169:
3134:
3083:
3054:
2936:
2899:
2860:
2718:
2594:
2456:
2115:
1500:
1068:
1063:
4567:
4405:
4369:
4356:
4088:
4033:
3909:
3871:
3828:
3797:
1769:
1461:, funneling metabolites from the breakdown of these amino acids into the
1052:
907:
888:
3003:
2835:"Inhibition of the human methylmalonyl-CoA mutase by various CoA-esters"
2167:
Forny P, Froese DS, Suormala T, Yue WW, Baumgartner MR (December 2014).
1793:
protein fosters exchange of the damaged cofactor for a new AdoCbl via a
1712:
The MCM reaction mechanism begins with homolytic cleavage of AdoB12's C-
1151:
630:
4409:
3995:
3890:
3867:
3842:
3833:
3669:
3075:
2184:
1571:
1430:
1391:
1170:
874:
829:
3451:
3116:
3099:
Raff ML, Crane AM, Jansen R, Ledley FD, Rosenblatt DS (January 1991).
3036:
2710:
2545:
93:
89:
85:
5273:
5043:
4889:
4516:
4159:
3846:
3645:
3612:
1832:
1827:
1765:
1733:
1713:
1693:
1559:
1442:
1418:
1403:
1399:
1383:
1326:
1250:
1146:
1134:
1122:
1036:
784:
127:, MCM, methylmalonyl-CoA mutase, Methylmalonyl Coenzyme-A mutase, MUT
2892:
10.1002/(SICI)1098-1004(1997)9:1<1::AID-HUMU1>3.0.CO;2-E
1382:
Methylmalonyl-CoA mutase is expressed in high concentrations in the
3206:
Ledley FD, Lumetta M, Nguyen PN, Kolhouse JF, Allen RH (May 1988).
2833:
Taoka S, Padmakumar R, Lai MT, Liu HW, Banerjee R (December 1994).
5247:
4818:
4813:
4023:
1750:
1703:
1395:
1387:
1363:
1323:
1319:
1657:(demonstrates no activity even in presence of excess AdoCbl), or
747:
743:
4960:
4955:
4798:
4793:
4788:
4778:
4768:
4561:
1822:
1806:
1777:
1158:
140:
5016:
4858:
4482:
3573:
1602:(also referred to as methylmalonic aciduria or MMA). MMA is an
587:
1704:
1673:
comprise a significant remaining fraction (approximately 14%)
1316:
2020:"Genetic and genomic systems to study methylmalonic acidemia"
1618:
in infancy, and may cause early death. Complications include
3019:
Crane AM, Jansen R, Andrews ER, Ledley FD (February 1992).
1468:
Methylmalonyl-CoA mutase catalyzes the following reaction:
5012:
2441:"Role of vitamin B12 on methylmalonyl-CoA mutase activity"
1649:
protein) can lead to methylmalonyl acidemia. Mutations to
1645:(encodes a chaperone protein of methylmalonyl-CoA mutase,
1554:, a substance formed from the catabolism and digestion of
3288:
Fenton WA, Hack AM, Kraus JP, Rosenberg LE (March 1987).
396:
3555:
1578:, or odd-chain fatty acids. The product of the enzyme,
1764:-370, whose hydrogen bond with the 2’-OH group of the
5303:
561:
4218:
Branched-chain alpha-keto acid dehydrogenase complex
4174:
Branched-chain alpha-keto acid dehydrogenase complex
3689:
Branched-chain alpha-keto acid dehydrogenase complex
5199:
5163:
5132:
5101:
5050:
4976:
4925:
4901:
4827:
4718:
4702:
4674:
4655:
4636:
4591:
4582:
4523:
4436:
4426:
4364:
4354:
4320:
4301:
4231:
4202:
4158:
4143:
4081:
4061:
4032:
3994:
3965:
3950:
3927:
3908:
3889:
3841:
3826:
3817:
3792:
3744:
3668:
3644:
3631:
2294:
2259:
1261:
1249:
1237:
1232:
1212:
1193:
1181:
1169:
1157:
1145:
1133:
1121:
1116:
1104:
1092:
1087:
1082:
997:
972:
946:
925:
1872:
1870:
1868:
1851:
1849:
1847:
3512:Janata J, Kogekar N, Fenton WA (September 1997).
343:
242:
4840:Electron-transferring-flavoprotein dehydrogenase
2792:Padovani D, Labunska T, Banerjee R (June 2006).
2608:Maiti N, Widjaja L, Banerjee R (November 1999).
1293:, is a protein that in humans is encoded by the
4745:Complex III/Coenzyme Q - cytochrome c reductase
3779:Aminocarboxymuconate-semialdehyde decarboxylase
2736:
2734:
2732:
2730:
2728:
2527:
2525:
2434:
2432:
2252:
2250:
2248:
2162:
2160:
2488:Vlasie M, Chowdhury S, Banerjee R (May 2002).
1969:
1967:
1965:
1963:
1877:GRCm38: Ensembl release 89: ENSMUSG00000023921
1402:, and liver, and in low concentrations in the
5028:
4870:
4494:
3585:
3370:Kolhouse JF, Utley C, Allen RH (April 1980).
2065:
2063:
1406:. The enzyme can be found all throughout the
8:
1727:Methylmalonyl-CoA mutase is a member of the
4223:3-hydroxy-2-methylbutyryl-CoA dehydrogenase
1856:GRCh38: Ensembl release 89: ENSG00000146085
1676:Common treatment methods for MMA include a
1546:The substrate of methylmalonyl-CoA mutase,
5035:
5021:
5013:
4877:
4863:
4855:
4715:
4588:
4501:
4487:
4479:
4433:
4361:
4213:Branched-chain amino acid aminotransferase
4169:Branched-chain amino acid aminotransferase
4155:
3962:
3838:
3823:
3684:Branched-chain amino acid aminotransferase
3641:
3592:
3578:
3570:
2327:Chandler RJ, Venditti CP (November 2012).
1229:
758:
603:
384:
283:
180:
74:
4194:Methylmalonate semialdehyde dehydrogenase
3562:at the U.S. National Library of Medicine
3529:
3494:
3459:
3387:
3323:
3313:
3241:
3231:
3159:
3124:
3044:
2850:
2809:
2768:
2758:
2700:
2625:
2584:
2505:
2464:
2412:
2402:
2352:
2192:
2105:
2095:
2043:
3784:Aminomuconate-semialdehyde dehydrogenase
2445:Journal of Zhejiang University Science B
1785:protein favors association with the MCM
1386:, in intermediate concentrations in the
733:short-chain fatty acid catabolic process
5310:
4015:1-Pyrroline-5-carboxylate dehydrogenase
3407:Archives of Biochemistry and Biophysics
2018:Chandler RJ, Venditti CP (2016-10-01).
1843:
1641:(encodes methylmalonyl-CoA mutase), or
1329:in 1955. In its latent form, it is 750
27:Mammalian protein found in Homo sapiens
4275:Betaine-homocysteine methyltransferase
1513:
1478:
1362:location of 6p12.3 and consists of 13
1079:
728:positive regulation of GTPase activity
29:
3736:Template:Leucine metabolism in humans
3440:The Journal of Clinical Investigation
3142:Jansen R, Ledley FD (November 1990).
3105:The Journal of Clinical Investigation
3025:The Journal of Clinical Investigation
348:
309:
304:
247:
206:
201:
7:
3876:Guanidinoacetate N-methyltransferase
1927:
1925:
1536:
1528:
1495:
4389:4-Hydroxyphenylpyruvate dioxygenase
3986:Formiminotransferase cyclodeaminase
3709:Isovaleryl coenzyme A dehydrogenase
3376:The Journal of Biological Chemistry
2839:The Journal of Biological Chemistry
2798:The Journal of Biological Chemistry
2747:The Journal of Biological Chemistry
2614:The Journal of Biological Chemistry
2494:The Journal of Biological Chemistry
1596:methylmalonyl-CoA mutase deficiency
641:intramolecular transferase activity
4731:Complex II/Succinate dehydrogenase
4627:Pyruvate dehydrogenase phosphatase
4189:3-hydroxyisobutyrate dehydrogenase
3679:3-Hydroxybutyryl-CoA dehydrogenase
3483:American Journal of Human Genetics
3148:American Journal of Human Genetics
2996:10.1111/j.1365-2141.1975.tb00885.x
1989:10.1016/j.neuroscience.2009.08.028
1308:gene may lead to various types of
994:
969:
943:
922:
898:
879:
853:
834:
808:
789:
566:
484:
422:
401:
25:
4184:3-hydroxyisobutyryl-CoA hydrolase
4101:Gamma-glutamylcysteine synthetase
4010:Pyrroline-5-carboxylate reductase
3477:Crane AM, Ledley FD (July 1994).
2333:Molecular Genetics and Metabolism
2219:Molecular Genetics and Metabolism
2131:Molecular Genetics and Metabolism
2024:Molecular Genetics and Metabolism
671:protein homodimerization activity
646:methylmalonyl-CoA mutase activity
5313:
2929:10.1146/annurev.biochem.66.1.269
2567:Mancia F, Evans PR (June 1998).
1598:, which is one of the causes of
1514:
1499:
1489:
577:
570:
332:
325:
319:
296:
231:
224:
218:
193:
38:
4755:Complex IV/Cytochrome c oxidase
3856:Serine hydroxymethyltransferase
18:Methylmalonyl coenzyme A mutase
4602:Pyruvate dehydrogenase complex
4270:Homocysteine methyltransferase
4248:Methionine adenosyltransferase
3729:Methylglutaconyl-CoA hydratase
3724:Methylcrotonyl-CoA carboxylase
2984:British Journal of Haematology
713:homocysteine metabolic process
588:More reference expression data
550:More reference expression data
1:
4623:Pyruvate dehydrogenase kinase
4399:Fumarylacetoacetate hydrolase
4394:Homogentisate 1,2-dioxygenase
4111:Gamma-glutamyl transpeptidase
3774:3-hydroxyanthranilate oxidase
3714:α-Ketoisocaproate dioxygenase
3389:10.1016/S0021-9258(19)85795-2
2917:Annual Review of Biochemistry
2852:10.1016/S0021-9258(18)31741-1
2586:10.1016/s0969-2126(98)00073-2
1653:can be categorized as either
1637:Either mutations to the gene
1550:, is primarily derived from
317:
216:
4726:Complex I/NADH dehydrogenase
3419:10.1016/0003-9861(82)90088-1
3355:10.1016/0888-7543(88)90135-8
3273:10.1016/0888-7543(89)90300-5
3191:10.1016/0888-7543(90)90259-W
1315:MCM was first identified in
524:Epithelium of choroid plexus
438:Epithelium of choroid plexus
5336:Genes on human chromosome 6
4337:Methylmalonyl CoA epimerase
4288:Cystathionine beta synthase
3755:Indoleamine 2,3-dioxygenase
2345:10.1016/j.ymgme.2012.09.019
2231:10.1016/j.ymgme.2012.05.014
2143:10.1016/j.ymgme.2004.11.011
2036:10.1016/j.ymgme.2005.07.020
1582:, is a key molecule of the
1291:methylmalonyl-CoA isomerase
708:cobalamin metabolic process
621:modified amino acid binding
5362:
4968:Phosphoenolpyruvate mutase
4941:Bisphosphoglycerate mutase
4546:Oxoglutarate dehydrogenase
4261:regeneration of methionine
4243:generation of homocysteine
4043:Ornithine aminotransferase
3759:Tryptophan 2,3-dioxygenase
3660:Glutaryl-CoA dehydrogenase
3655:Saccharopine dehydrogenase
2306:10.1016/j.bbrc.2012.09.133
2271:10.1016/j.bbrc.2010.11.141
1946:10.1016/j.gene.2015.10.002
1524:
1522:
1509:
1507:
1412:branched-chain amino acids
1280:Methylmalonyl-CoA mutase (
723:post-embryonic development
5191:Michaelis–Menten kinetics
4917:Precorrin-8X methylmutase
4711:oxidative phosphorylation
4384:Tyrosine aminotransferase
4379:Phenylalanine hydroxylase
4332:Propionyl-CoA carboxylase
4293:Cystathionine gamma-lyase
3938:L-threonine dehydrogenase
3619:
2959:10.1007/s00467-007-0460-z
1912:"Mouse PubMed Reference:"
1894:"Human PubMed Reference:"
1632:progressive renal failure
1537:methylmalonyl-CoA mutase
1498:
1488:
1479:methylmalonyl-CoA mutase
1228:
1067:
1062:
1058:
1051:
1035:
1016:
1001:
976:
965:
950:
929:
918:
905:
901:
886:
882:
873:
860:
856:
841:
837:
828:
815:
811:
796:
792:
783:
768:
761:
757:
741:
666:identical protein binding
606:
602:
585:
569:
560:
547:
496:
487:
434:
425:
395:
387:
383:
366:
353:
316:
295:
286:
282:
265:
252:
215:
192:
183:
179:
134:
131:
121:
114:
109:
82:
77:
60:
55:
50:
46:
37:
32:
5083:Diffusion-limited enzyme
5002:Methylmalonyl-CoA mutase
4706:electron transport chain
4666:Methylmalonyl-CoA mutase
4541:Isocitrate dehydrogenase
4342:Methylmalonyl-CoA mutase
3919:D-cysteine desulfhydrase
3564:Medical Subject Headings
3560:Methylmalonyl-CoA+Mutase
3518:Human Molecular Genetics
2627:10.1074/jbc.274.46.32733
1775:The processing protein,
1708:MCM's reaction mechanism
1584:tricarboxylic acid cycle
1463:tricarboxylic acid cycle
1083:methylmalonyl-CoA mutase
1029:Chr 17: 41.25 – 41.27 Mb
4936:Phosphoglycerate mutase
4912:Lysolecithin acylmutase
4647:Glutamate dehydrogenase
4558:Succinate dehydrogenase
4551:Succinyl CoA synthetase
4073:Glutamate dehydrogenase
4048:Ornithine decarboxylase
3976:Histidine ammonia-lyase
3719:Leucine 2,3-aminomutase
3233:10.1073/pnas.85.10.3518
2760:10.1074/jbc.M110.177717
2404:10.1073/pnas.1302764110
2097:10.1073/pnas.93.11.5550
1022:Chr 6: 49.43 – 49.46 Mb
450:mucosa of sigmoid colon
4690:Aspartate transaminase
4460:Aspartate transaminase
4283:conversion to cysteine
4253:Adenosylhomocysteinase
4106:Glutathione synthetase
3315:10.1073/pnas.84.5.1421
2811:10.1074/jbc.M600047200
2663:10.1002/anie.201205299
2507:10.1074/jbc.M111809200
1757:
1709:
1600:methylmalonic acidemia
1408:central nervous system
1366:, spanning over 35kb.
1310:methylmalonic aciduria
454:pancreatic ductal cell
350:17 B2|17 19.55 cM
5176:Eadie–Hofstee diagram
5109:Allosteric regulation
4987:Cycloartenol synthase
4455:Asparagine synthetase
3623:Essential amino acids
2457:10.1631/jzus.B1100329
1754:
1742:deoxyadenosyl radical
1707:
1590:Clinical significance
1287:, MCM), mitochondrial
311:Chromosome 17 (mouse)
5186:Lineweaver–Burk plot
4685:Pyruvate carboxylase
4573:Malate dehydrogenase
4126:Glutamine synthetase
3900:Alanine transaminase
3531:10.1093/hmg/6.9.1457
2947:Pediatric Nephrology
687:mitochondrial matrix
500:brown adipose tissue
209:Chromosome 6 (human)
78:List of PDB id codes
51:Available structures
4992:Lanosterol synthase
4835:Alternative oxidase
4639:α-ketoglutaric acid
4266:Methionine synthase
4179:Enoyl-CoA hydratase
4064:alpha-ketoglutarate
3981:Urocanate hydratase
3694:Enoyl-CoA hydratase
3306:1987PNAS...84.1421F
3224:1988PNAS...85.3518L
2395:2013PNAS..11013552M
2088:1996PNAS...93.5550D
1604:autosomal recessive
1475:L-methylmalonyl-CoA
5145:Enzyme superfamily
5078:Enzyme promiscuity
4951:Phosphomannomutase
4946:Phosphoglucomutase
4312:Threonine aldolase
3861:Serine dehydratase
3605:Protein metabolism
3076:10.1007/BF00220536
2185:10.1002/humu.22633
1940:(1 Pt 2): 208–13.
1797:-reliant pathway.
1758:
1710:
1671:nonsense mutations
1667:missense mutations
1441:, the amino acids
863:ENSMUSG00000023921
701:Biological process
680:Cellular component
631:catalytic activity
626:isomerase activity
614:Molecular function
536:intercostal muscle
512:left lobe of liver
474:buccal mucosa cell
5301:
5300:
5010:
5009:
4852:
4851:
4848:
4847:
4698:
4697:
4514:Citric acid cycle
4476:
4475:
4472:
4471:
4468:
4467:
4422:
4421:
4350:
4349:
4139:
4138:
3946:
3945:
3813:
3812:
3704:HMG-CoA reductase
3452:10.1172/JCI117166
3117:10.1172/JCI114972
3037:10.1172/JCI115597
2711:10.1021/bi981375o
2651:Angewandte Chemie
2546:10.1021/bi9903852
2540:(25): 7999–8005.
1682:kidney transplant
1548:methylmalonyl-CoA
1543:
1542:
1358:gene lies on the
1277:
1276:
1273:
1272:
1176:metabolic pathway
1078:
1077:
1074:
1073:
1047:
1046:
1012:
1011:
991:
990:
961:
960:
940:
939:
914:
913:
895:
894:
869:
868:
850:
849:
824:
823:
805:
804:
753:
752:
661:cobalamin binding
636:metal ion binding
598:
597:
594:
593:
556:
555:
543:
542:
481:
480:
379:
378:
278:
277:
105:
104:
101:
100:
61:Ortholog search:
16:(Redirected from
5353:
5318:
5317:
5309:
5181:Hanes–Woolf plot
5124:Enzyme activator
5119:Enzyme inhibitor
5093:Enzyme catalysis
5037:
5030:
5023:
5014:
4879:
4872:
4865:
4856:
4716:
4677:oxaloacetic acid
4589:
4531:Citrate synthase
4503:
4496:
4489:
4480:
4434:
4362:
4156:
3963:
3839:
3824:
3642:
3594:
3587:
3580:
3571:
3543:
3533:
3508:
3498:
3473:
3463:
3430:
3401:
3391:
3366:
3337:
3327:
3317:
3284:
3255:
3245:
3235:
3202:
3173:
3163:
3138:
3128:
3095:
3058:
3048:
3015:
2978:
2940:
2911:
2865:
2864:
2854:
2830:
2824:
2823:
2813:
2804:(26): 17838–44.
2789:
2783:
2782:
2772:
2762:
2753:(49): 38204–13.
2738:
2723:
2722:
2704:
2695:(41): 14386–93.
2681:
2675:
2674:
2646:
2640:
2639:
2629:
2605:
2599:
2598:
2588:
2564:
2558:
2557:
2529:
2520:
2519:
2509:
2485:
2479:
2478:
2468:
2436:
2427:
2426:
2416:
2406:
2373:
2367:
2366:
2356:
2324:
2318:
2317:
2289:
2283:
2282:
2254:
2243:
2242:
2213:
2207:
2206:
2196:
2164:
2155:
2154:
2126:
2120:
2119:
2109:
2099:
2067:
2058:
2057:
2047:
2015:
2009:
2008:
1971:
1958:
1957:
1929:
1920:
1919:
1908:
1902:
1901:
1890:
1884:
1874:
1863:
1853:
1678:liver transplant
1624:metabolic stroke
1518:
1503:
1493:
1471:
1470:
1289:, also known as
1230:
1080:
1060:
1059:
1031:
1024:
1007:
995:
986:
970:
966:RefSeq (protein)
956:
944:
935:
923:
899:
880:
854:
835:
809:
790:
759:
604:
590:
581:
574:
567:
552:
492:
490:Top expressed in
485:
470:secondary oocyte
458:endothelial cell
430:
428:Top expressed in
423:
402:
385:
375:
362:
351:
336:
329:
323:
312:
300:
284:
274:
261:
250:
235:
228:
222:
211:
197:
181:
175:
173:MMUT - orthologs
126:
119:
96:
75:
69:
48:
47:
42:
30:
21:
5361:
5360:
5356:
5355:
5354:
5352:
5351:
5350:
5326:
5325:
5324:
5312:
5304:
5302:
5297:
5209:Oxidoreductases
5195:
5171:Enzyme kinetics
5159:
5155:List of enzymes
5128:
5097:
5068:Catalytic triad
5046:
5041:
5011:
5006:
4997:Lupeol synthase
4972:
4921:
4897:
4883:
4853:
4844:
4823:
4765:
4739:
4709:
4704:
4694:
4670:
4651:
4632:
4578:
4519:
4507:
4477:
4464:
4418:
4346:
4316:
4297:
4227:
4198:
4149:
4135:
4077:
4057:
4028:
4005:Proline oxidase
3990:
3958:α-ketoglutarate
3956:
3942:
3923:
3904:
3885:
3881:Creatine kinase
3831:
3809:
3788:
3764:Arylformamidase
3740:
3664:
3627:
3625:are in Capitals
3615:
3611:and catabolism
3598:
3551:
3546:
3511:
3476:
3433:
3404:
3369:
3340:
3287:
3258:
3218:(10): 3518–21.
3205:
3176:
3141:
3098:
3061:
3018:
2981:
2943:
2914:
2877:
2873:
2871:Further reading
2868:
2845:(50): 31630–4.
2832:
2831:
2827:
2791:
2790:
2786:
2740:
2739:
2726:
2683:
2682:
2678:
2648:
2647:
2643:
2620:(46): 32733–7.
2607:
2606:
2602:
2566:
2565:
2561:
2531:
2530:
2523:
2500:(21): 18523–7.
2487:
2486:
2482:
2438:
2437:
2430:
2389:(33): 13552–7.
2375:
2374:
2370:
2326:
2325:
2321:
2291:
2290:
2286:
2256:
2255:
2246:
2215:
2214:
2210:
2179:(12): 1449–58.
2166:
2165:
2158:
2128:
2127:
2123:
2069:
2068:
2061:
2017:
2016:
2012:
1973:
1972:
1961:
1931:
1930:
1923:
1910:
1909:
1905:
1892:
1891:
1887:
1875:
1866:
1854:
1845:
1841:
1803:
1702:
1680:or a liver and
1592:
1545:
1380:
1372:
1352:
1347:
1302:
1069:View/Edit Mouse
1064:View/Edit Human
1027:
1020:
1017:Location (UCSC)
1003:
982:
978:
952:
931:
844:ENSG00000146085
737:
696:
675:
656:protein binding
651:GTPase activity
586:
576:
575:
548:
539:
534:
530:
528:right ventricle
526:
522:
518:
516:proximal tubule
514:
510:
506:
502:
488:
477:
472:
468:
464:
460:
456:
452:
448:
444:
440:
426:
370:
357:
349:
339:
338:
337:
330:
310:
287:Gene location (
269:
256:
248:
238:
237:
236:
229:
207:
184:Gene location (
135:
122:
115:
84:
62:
28:
23:
22:
15:
12:
11:
5:
5359:
5357:
5349:
5348:
5343:
5341:Human proteins
5338:
5328:
5327:
5323:
5322:
5299:
5298:
5296:
5295:
5282:
5269:
5256:
5243:
5230:
5217:
5203:
5201:
5197:
5196:
5194:
5193:
5188:
5183:
5178:
5173:
5167:
5165:
5161:
5160:
5158:
5157:
5152:
5147:
5142:
5136:
5134:
5133:Classification
5130:
5129:
5127:
5126:
5121:
5116:
5111:
5105:
5103:
5099:
5098:
5096:
5095:
5090:
5085:
5080:
5075:
5070:
5065:
5060:
5054:
5052:
5048:
5047:
5042:
5040:
5039:
5032:
5025:
5017:
5008:
5007:
5005:
5004:
4999:
4994:
4989:
4983:
4981:
4974:
4973:
4971:
4970:
4965:
4964:
4963:
4958:
4948:
4943:
4938:
4932:
4930:
4929:Phosphomutases
4923:
4922:
4920:
4919:
4914:
4908:
4906:
4899:
4898:
4884:
4882:
4881:
4874:
4867:
4859:
4850:
4849:
4846:
4845:
4843:
4842:
4837:
4831:
4829:
4825:
4824:
4822:
4821:
4816:
4811:
4806:
4801:
4796:
4791:
4786:
4781:
4776:
4771:
4763:
4758:
4757:
4752:
4747:
4742:
4737:
4733:
4728:
4722:
4720:
4713:
4700:
4699:
4696:
4695:
4693:
4692:
4687:
4681:
4679:
4672:
4671:
4669:
4668:
4662:
4660:
4653:
4652:
4650:
4649:
4643:
4641:
4634:
4633:
4631:
4630:
4621:(regulated by
4618:
4617:
4598:
4596:
4586:
4580:
4579:
4577:
4576:
4570:
4565:
4554:
4553:
4548:
4543:
4538:
4533:
4527:
4525:
4521:
4520:
4508:
4506:
4505:
4498:
4491:
4483:
4474:
4473:
4470:
4469:
4466:
4465:
4463:
4462:
4457:
4447:
4445:
4431:
4424:
4423:
4420:
4419:
4417:
4416:
4402:
4401:
4396:
4391:
4386:
4381:
4375:
4373:
4359:
4352:
4351:
4348:
4347:
4345:
4344:
4339:
4334:
4328:
4326:
4318:
4317:
4315:
4314:
4308:
4306:
4299:
4298:
4296:
4295:
4290:
4278:
4277:
4272:
4256:
4255:
4250:
4238:
4236:
4229:
4228:
4226:
4225:
4220:
4215:
4209:
4207:
4200:
4199:
4197:
4196:
4191:
4186:
4181:
4176:
4171:
4165:
4163:
4153:
4141:
4140:
4137:
4136:
4134:
4133:
4128:
4114:
4113:
4108:
4103:
4085:
4083:
4079:
4078:
4076:
4075:
4069:
4067:
4059:
4058:
4056:
4055:
4050:
4045:
4039:
4037:
4030:
4029:
4027:
4026:
4021:
4012:
4007:
4001:
3999:
3992:
3991:
3989:
3988:
3983:
3978:
3972:
3970:
3960:
3948:
3947:
3944:
3943:
3941:
3940:
3934:
3932:
3925:
3924:
3922:
3921:
3915:
3913:
3906:
3905:
3903:
3902:
3896:
3894:
3887:
3886:
3884:
3883:
3878:
3864:
3863:
3858:
3852:
3850:
3836:
3821:
3815:
3814:
3811:
3810:
3808:
3807:
3803:
3801:
3790:
3789:
3787:
3786:
3781:
3776:
3771:
3766:
3761:
3751:
3749:
3742:
3741:
3732:
3731:
3726:
3721:
3716:
3711:
3706:
3701:
3696:
3691:
3686:
3681:
3675:
3673:
3666:
3665:
3663:
3662:
3657:
3651:
3649:
3639:
3629:
3628:
3620:
3617:
3616:
3599:
3597:
3596:
3589:
3582:
3574:
3568:
3567:
3557:
3550:
3549:External links
3547:
3545:
3544:
3524:(9): 1457–64.
3509:
3474:
3431:
3402:
3382:(7): 2708–12.
3367:
3338:
3285:
3267:(2): 198–205.
3256:
3203:
3174:
3139:
3096:
3064:Human Genetics
3059:
3016:
2979:
2953:(8): 1209–14.
2941:
2912:
2880:Human Mutation
2874:
2872:
2869:
2867:
2866:
2825:
2784:
2724:
2702:10.1.1.608.304
2676:
2657:(40): 9974–6.
2641:
2600:
2559:
2521:
2480:
2428:
2368:
2319:
2284:
2244:
2208:
2173:Human Mutation
2156:
2121:
2082:(11): 5550–5.
2059:
2030:(1–2): 34–43.
2010:
1959:
1921:
1903:
1885:
1864:
1842:
1840:
1837:
1836:
1835:
1830:
1825:
1820:
1817:
1812:
1809:
1802:
1799:
1756:from PDB 4REQ.
1701:
1698:
1684:to combat the
1620:cardiomyopathy
1612:hyperammonemia
1591:
1588:
1541:
1540:
1538:
1535:
1531:
1530:
1526:
1525:
1523:
1520:
1519:
1511:
1510:
1508:
1505:
1504:
1497:
1494:
1486:
1485:
1480:
1477:
1433:and odd-chain
1379:
1376:
1371:
1368:
1351:
1348:
1346:
1343:
1300:
1275:
1274:
1271:
1270:
1265:
1259:
1258:
1253:
1247:
1246:
1241:
1235:
1234:
1226:
1225:
1216:
1210:
1209:
1198:
1191:
1190:
1185:
1179:
1178:
1173:
1167:
1166:
1161:
1155:
1154:
1149:
1143:
1142:
1137:
1131:
1130:
1125:
1119:
1118:
1114:
1113:
1108:
1102:
1101:
1096:
1090:
1089:
1085:
1084:
1076:
1075:
1072:
1071:
1066:
1056:
1055:
1049:
1048:
1045:
1044:
1042:
1040:
1033:
1032:
1025:
1018:
1014:
1013:
1010:
1009:
999:
998:
992:
989:
988:
974:
973:
967:
963:
962:
959:
958:
948:
947:
941:
938:
937:
927:
926:
920:
916:
915:
912:
911:
903:
902:
896:
893:
892:
884:
883:
877:
871:
870:
867:
866:
858:
857:
851:
848:
847:
839:
838:
832:
826:
825:
822:
821:
813:
812:
806:
803:
802:
794:
793:
787:
781:
780:
775:
770:
766:
765:
755:
754:
751:
750:
739:
738:
736:
735:
730:
725:
720:
715:
710:
704:
702:
698:
697:
695:
694:
689:
683:
681:
677:
676:
674:
673:
668:
663:
658:
653:
648:
643:
638:
633:
628:
623:
617:
615:
611:
610:
600:
599:
596:
595:
592:
591:
583:
582:
564:
558:
557:
554:
553:
545:
544:
541:
540:
538:
537:
533:
529:
525:
521:
517:
513:
509:
505:
501:
497:
494:
493:
482:
479:
478:
476:
475:
471:
467:
466:jejunal mucosa
463:
459:
455:
451:
447:
443:
439:
435:
432:
431:
419:
418:
410:
399:
393:
392:
389:RNA expression
381:
380:
377:
376:
368:
364:
363:
355:
352:
347:
341:
340:
331:
324:
318:
314:
313:
308:
302:
301:
293:
292:
280:
279:
276:
275:
267:
263:
262:
254:
251:
246:
240:
239:
230:
223:
217:
213:
212:
205:
199:
198:
190:
189:
177:
176:
133:
129:
128:
120:
112:
111:
107:
106:
103:
102:
99:
98:
80:
79:
71:
70:
59:
53:
52:
44:
43:
35:
34:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
5358:
5347:
5344:
5342:
5339:
5337:
5334:
5333:
5331:
5321:
5316:
5311:
5307:
5293:
5289:
5288:
5283:
5280:
5276:
5275:
5270:
5267:
5263:
5262:
5257:
5254:
5250:
5249:
5244:
5241:
5237:
5236:
5231:
5228:
5224:
5223:
5218:
5215:
5211:
5210:
5205:
5204:
5202:
5198:
5192:
5189:
5187:
5184:
5182:
5179:
5177:
5174:
5172:
5169:
5168:
5166:
5162:
5156:
5153:
5151:
5150:Enzyme family
5148:
5146:
5143:
5141:
5138:
5137:
5135:
5131:
5125:
5122:
5120:
5117:
5115:
5114:Cooperativity
5112:
5110:
5107:
5106:
5104:
5100:
5094:
5091:
5089:
5086:
5084:
5081:
5079:
5076:
5074:
5073:Oxyanion hole
5071:
5069:
5066:
5064:
5061:
5059:
5056:
5055:
5053:
5049:
5045:
5038:
5033:
5031:
5026:
5024:
5019:
5018:
5015:
5003:
5000:
4998:
4995:
4993:
4990:
4988:
4985:
4984:
4982:
4979:
4975:
4969:
4966:
4962:
4959:
4957:
4954:
4953:
4952:
4949:
4947:
4944:
4942:
4939:
4937:
4934:
4933:
4931:
4928:
4924:
4918:
4915:
4913:
4910:
4909:
4907:
4904:
4900:
4895:
4891:
4887:
4880:
4875:
4873:
4868:
4866:
4861:
4860:
4857:
4841:
4838:
4836:
4833:
4832:
4830:
4826:
4820:
4817:
4815:
4812:
4810:
4807:
4805:
4802:
4800:
4797:
4795:
4792:
4790:
4787:
4785:
4782:
4780:
4777:
4775:
4772:
4770:
4766:
4760:
4759:
4756:
4753:
4751:
4748:
4746:
4743:
4741:
4734:
4732:
4729:
4727:
4724:
4723:
4721:
4717:
4714:
4712:
4707:
4703:Mitochondrial
4701:
4691:
4688:
4686:
4683:
4682:
4680:
4678:
4673:
4667:
4664:
4663:
4661:
4659:
4654:
4648:
4645:
4644:
4642:
4640:
4635:
4628:
4624:
4620:
4619:
4615:
4611:
4607:
4603:
4600:
4599:
4597:
4595:
4590:
4587:
4585:
4581:
4574:
4571:
4569:
4566:
4563:
4559:
4556:
4555:
4552:
4549:
4547:
4544:
4542:
4539:
4537:
4534:
4532:
4529:
4528:
4526:
4522:
4518:
4515:
4511:
4504:
4499:
4497:
4492:
4490:
4485:
4484:
4481:
4461:
4458:
4456:
4452:
4449:
4448:
4446:
4443:
4439:
4435:
4432:
4430:
4425:
4415:
4411:
4407:
4404:
4403:
4400:
4397:
4395:
4392:
4390:
4387:
4385:
4382:
4380:
4377:
4376:
4374:
4371:
4367:
4366:PHENYLALANINE
4363:
4360:
4358:
4353:
4343:
4340:
4338:
4335:
4333:
4330:
4329:
4327:
4324:
4319:
4313:
4310:
4309:
4307:
4304:
4300:
4294:
4291:
4289:
4286:
4284:
4280:
4279:
4276:
4273:
4271:
4267:
4264:
4262:
4258:
4257:
4254:
4251:
4249:
4246:
4244:
4240:
4239:
4237:
4234:
4230:
4224:
4221:
4219:
4216:
4214:
4211:
4210:
4208:
4205:
4201:
4195:
4192:
4190:
4187:
4185:
4182:
4180:
4177:
4175:
4172:
4170:
4167:
4166:
4164:
4161:
4157:
4154:
4152:
4147:
4146:propionyl-CoA
4142:
4132:
4129:
4127:
4123:
4119:
4116:
4115:
4112:
4109:
4107:
4104:
4102:
4098:
4094:
4090:
4087:
4086:
4084:
4080:
4074:
4071:
4070:
4068:
4065:
4060:
4054:
4051:
4049:
4046:
4044:
4041:
4040:
4038:
4035:
4031:
4025:
4022:
4020:
4016:
4013:
4011:
4008:
4006:
4003:
4002:
4000:
3997:
3993:
3987:
3984:
3982:
3979:
3977:
3974:
3973:
3971:
3968:
3964:
3961:
3959:
3954:
3949:
3939:
3936:
3935:
3933:
3930:
3926:
3920:
3917:
3916:
3914:
3911:
3907:
3901:
3898:
3897:
3895:
3892:
3888:
3882:
3879:
3877:
3873:
3869:
3866:
3865:
3862:
3859:
3857:
3854:
3853:
3851:
3848:
3844:
3840:
3837:
3835:
3830:
3825:
3822:
3820:
3816:
3805:
3804:
3802:
3799:
3795:
3794:PHENYLALANINE
3791:
3785:
3782:
3780:
3777:
3775:
3772:
3770:
3767:
3765:
3762:
3760:
3756:
3753:
3752:
3750:
3747:
3743:
3739:
3737:
3730:
3727:
3725:
3722:
3720:
3717:
3715:
3712:
3710:
3707:
3705:
3702:
3700:
3699:HMG-CoA lyase
3697:
3695:
3692:
3690:
3687:
3685:
3682:
3680:
3677:
3676:
3674:
3671:
3667:
3661:
3658:
3656:
3653:
3652:
3650:
3647:
3643:
3640:
3638:
3634:
3630:
3626:
3624:
3618:
3614:
3610:
3606:
3602:
3595:
3590:
3588:
3583:
3581:
3576:
3575:
3572:
3565:
3561:
3558:
3556:
3553:
3552:
3548:
3541:
3537:
3532:
3527:
3523:
3519:
3515:
3510:
3506:
3502:
3497:
3492:
3488:
3484:
3480:
3475:
3471:
3467:
3462:
3457:
3453:
3449:
3446:(4): 1812–9.
3445:
3441:
3437:
3432:
3428:
3424:
3420:
3416:
3413:(2): 815–23.
3412:
3408:
3403:
3399:
3395:
3390:
3385:
3381:
3377:
3373:
3368:
3364:
3360:
3356:
3352:
3348:
3344:
3339:
3335:
3331:
3326:
3321:
3316:
3311:
3307:
3303:
3300:(5): 1421–4.
3299:
3295:
3291:
3286:
3282:
3278:
3274:
3270:
3266:
3262:
3257:
3253:
3249:
3244:
3239:
3234:
3229:
3225:
3221:
3217:
3213:
3209:
3204:
3200:
3196:
3192:
3188:
3184:
3180:
3175:
3171:
3167:
3162:
3157:
3154:(5): 808–14.
3153:
3149:
3145:
3140:
3136:
3132:
3127:
3122:
3118:
3114:
3110:
3106:
3102:
3097:
3093:
3089:
3085:
3081:
3077:
3073:
3070:(3): 259–64.
3069:
3065:
3060:
3056:
3052:
3047:
3042:
3038:
3034:
3031:(2): 385–91.
3030:
3026:
3022:
3017:
3013:
3009:
3005:
3001:
2997:
2993:
2990:(4): 501–13.
2989:
2985:
2980:
2976:
2972:
2968:
2964:
2960:
2956:
2952:
2948:
2942:
2938:
2934:
2930:
2926:
2922:
2918:
2913:
2909:
2905:
2901:
2897:
2893:
2889:
2885:
2881:
2876:
2875:
2870:
2862:
2858:
2853:
2848:
2844:
2840:
2836:
2829:
2826:
2821:
2817:
2812:
2807:
2803:
2799:
2795:
2788:
2785:
2780:
2776:
2771:
2766:
2761:
2756:
2752:
2748:
2744:
2737:
2735:
2733:
2731:
2729:
2725:
2720:
2716:
2712:
2708:
2703:
2698:
2694:
2690:
2686:
2680:
2677:
2672:
2668:
2664:
2660:
2656:
2652:
2645:
2642:
2637:
2633:
2628:
2623:
2619:
2615:
2611:
2604:
2601:
2596:
2592:
2587:
2582:
2579:(6): 711–20.
2578:
2574:
2570:
2563:
2560:
2555:
2551:
2547:
2543:
2539:
2535:
2528:
2526:
2522:
2517:
2513:
2508:
2503:
2499:
2495:
2491:
2484:
2481:
2476:
2472:
2467:
2462:
2458:
2454:
2451:(6): 423–37.
2450:
2446:
2442:
2435:
2433:
2429:
2424:
2420:
2415:
2410:
2405:
2400:
2396:
2392:
2388:
2384:
2380:
2372:
2369:
2364:
2360:
2355:
2350:
2346:
2342:
2338:
2334:
2330:
2323:
2320:
2315:
2311:
2307:
2303:
2299:
2295:
2288:
2285:
2280:
2276:
2272:
2268:
2264:
2260:
2253:
2251:
2249:
2245:
2240:
2236:
2232:
2228:
2225:(4): 419–23.
2224:
2220:
2212:
2209:
2204:
2200:
2195:
2190:
2186:
2182:
2178:
2174:
2170:
2163:
2161:
2157:
2152:
2148:
2144:
2140:
2137:(4): 317–25.
2136:
2132:
2125:
2122:
2117:
2113:
2108:
2103:
2098:
2093:
2089:
2085:
2081:
2077:
2073:
2066:
2064:
2060:
2055:
2051:
2046:
2041:
2037:
2033:
2029:
2025:
2021:
2014:
2011:
2006:
2002:
1998:
1994:
1990:
1986:
1983:(2): 578–87.
1982:
1978:
1970:
1968:
1966:
1964:
1960:
1955:
1951:
1947:
1943:
1939:
1935:
1928:
1926:
1922:
1917:
1913:
1907:
1904:
1899:
1895:
1889:
1886:
1882:
1878:
1873:
1871:
1869:
1865:
1861:
1857:
1852:
1850:
1848:
1844:
1838:
1834:
1831:
1829:
1826:
1824:
1821:
1818:
1816:
1813:
1810:
1808:
1805:
1804:
1800:
1798:
1796:
1792:
1788:
1784:
1780:
1779:
1773:
1771:
1767:
1763:
1753:
1749:
1747:
1743:
1739:
1735:
1730:
1725:
1723:
1719:
1715:
1706:
1699:
1697:
1695:
1690:
1687:
1686:renal disease
1683:
1679:
1674:
1672:
1668:
1664:
1660:
1656:
1652:
1648:
1644:
1640:
1635:
1633:
1629:
1625:
1621:
1617:
1613:
1609:
1605:
1601:
1597:
1589:
1587:
1585:
1581:
1577:
1573:
1569:
1565:
1561:
1557:
1553:
1552:propionyl-CoA
1549:
1539:
1533:
1532:
1527:
1521:
1517:
1512:
1506:
1502:
1492:
1487:
1484:
1481:
1476:
1473:
1472:
1469:
1466:
1464:
1460:
1456:
1452:
1448:
1444:
1440:
1436:
1432:
1428:
1424:
1421:, as well as
1420:
1416:
1413:
1409:
1405:
1401:
1397:
1393:
1389:
1385:
1377:
1375:
1369:
1367:
1365:
1361:
1357:
1349:
1344:
1342:
1340:
1336:
1332:
1328:
1325:
1321:
1318:
1313:
1311:
1307:
1303:
1296:
1292:
1288:
1286:
1283:
1269:
1266:
1264:
1260:
1257:
1254:
1252:
1248:
1245:
1242:
1240:
1236:
1231:
1227:
1224:
1220:
1217:
1215:
1214:Gene Ontology
1211:
1208:
1205:
1202:
1199:
1196:
1192:
1189:
1186:
1184:
1180:
1177:
1174:
1172:
1168:
1165:
1162:
1160:
1156:
1153:
1152:NiceZyme view
1150:
1148:
1144:
1141:
1138:
1136:
1132:
1129:
1126:
1124:
1120:
1115:
1112:
1109:
1107:
1103:
1100:
1097:
1095:
1091:
1086:
1081:
1070:
1065:
1061:
1057:
1054:
1050:
1043:
1041:
1038:
1034:
1030:
1026:
1023:
1019:
1015:
1008:
1006:
1000:
996:
993:
987:
985:
981:
975:
971:
968:
964:
957:
955:
949:
945:
942:
936:
934:
928:
924:
921:
919:RefSeq (mRNA)
917:
910:
909:
904:
900:
897:
891:
890:
885:
881:
878:
876:
872:
865:
864:
859:
855:
852:
846:
845:
840:
836:
833:
831:
827:
820:
819:
814:
810:
807:
801:
800:
795:
791:
788:
786:
782:
779:
776:
774:
771:
767:
764:
760:
756:
749:
745:
740:
734:
731:
729:
726:
724:
721:
719:
716:
714:
711:
709:
706:
705:
703:
700:
699:
693:
692:mitochondrion
690:
688:
685:
684:
682:
679:
678:
672:
669:
667:
664:
662:
659:
657:
654:
652:
649:
647:
644:
642:
639:
637:
634:
632:
629:
627:
624:
622:
619:
618:
616:
613:
612:
609:
608:Gene ontology
605:
601:
589:
584:
580:
573:
568:
565:
563:
559:
551:
546:
535:
531:
527:
523:
520:parotid gland
519:
515:
511:
507:
503:
499:
498:
495:
491:
486:
483:
473:
469:
465:
461:
457:
453:
449:
446:kidney tubule
445:
441:
437:
436:
433:
429:
424:
421:
420:
417:
415:
411:
409:
408:
404:
403:
400:
398:
394:
390:
386:
382:
374:
369:
365:
361:
356:
346:
342:
335:
328:
322:
315:
307:
303:
299:
294:
290:
285:
281:
273:
268:
264:
260:
255:
245:
241:
234:
227:
221:
214:
210:
204:
200:
196:
191:
187:
182:
178:
174:
170:
166:
162:
158:
154:
150:
146:
142:
138:
130:
125:
118:
113:
108:
97:
95:
91:
87:
81:
76:
73:
72:
68:
65:
58:
54:
49:
45:
41:
36:
31:
19:
5287:Translocases
5284:
5271:
5258:
5245:
5232:
5222:Transferases
5219:
5206:
5063:Binding site
5001:
4980:Other groups
4761:
4750:Cytochrome c
4665:
4658:succinyl-CoA
4451:Asparaginase
4429:oxaloacetate
4341:
4323:succinyl-CoA
4281:
4259:
4241:
4151:succinyl-CoA
3769:Kynureninase
3733:
3621:
3521:
3517:
3489:(1): 42–50.
3486:
3482:
3443:
3439:
3410:
3406:
3379:
3375:
3349:(4): 396–8.
3346:
3342:
3297:
3293:
3264:
3260:
3215:
3211:
3185:(4): 710–6.
3182:
3178:
3151:
3147:
3111:(1): 203–7.
3108:
3104:
3067:
3063:
3028:
3024:
2987:
2983:
2950:
2946:
2920:
2916:
2883:
2879:
2842:
2838:
2828:
2801:
2797:
2787:
2750:
2746:
2692:
2689:Biochemistry
2688:
2679:
2654:
2650:
2644:
2617:
2613:
2603:
2576:
2572:
2562:
2537:
2534:Biochemistry
2533:
2497:
2493:
2483:
2448:
2444:
2386:
2382:
2371:
2339:(3): 617–9.
2336:
2332:
2322:
2300:(4): 753–7.
2297:
2293:
2287:
2265:(1): 443–7.
2262:
2258:
2222:
2218:
2211:
2176:
2172:
2134:
2130:
2124:
2079:
2075:
2027:
2023:
2013:
1980:
1977:Neuroscience
1976:
1937:
1933:
1915:
1906:
1897:
1888:
1801:Interactions
1790:
1782:
1776:
1774:
1759:
1736:-containing
1726:
1718:free radical
1711:
1691:
1675:
1662:
1658:
1654:
1650:
1646:
1642:
1638:
1636:
1628:pancreatitis
1616:pancytopenia
1608:ketoacidosis
1593:
1580:succinyl-CoA
1544:
1483:Succinyl-CoA
1467:
1381:
1373:
1355:
1353:
1314:
1305:
1294:
1290:
1279:
1278:
1140:BRENDA entry
1002:
977:
951:
930:
906:
887:
861:
842:
816:
797:
777:
772:
532:ciliary body
508:human kidney
504:right kidney
412:
405:
132:External IDs
83:
5058:Active site
4905:Acyl Groups
4767:synthesis:
4584:Anaplerotic
4131:Glutaminase
4097:glutathione
3806:(see below)
2923:: 269–313.
1811:Vitamin B12
1738:corrin ring
1576:cholesterol
1459:cholesterol
1439:fatty acids
1435:fatty acids
1331:amino acids
1297:gene. This
1128:IntEnz view
1088:Identifiers
984:NP_000246.2
371:41,272,879
358:41,245,576
270:49,463,253
257:49,430,360
110:Identifiers
5330:Categories
5261:Isomerases
5235:Hydrolases
5102:Regulation
4762:Coenzyme Q
4736:Coenzyme Q
4594:acetyl-CoA
4510:Metabolism
4438:asparagine
4414:Tyrosinase
4233:METHIONINE
4204:ISOLEUCINE
4053:Agmatinase
3746:TRYPTOPHAN
3637:acetyl-CoA
3601:Metabolism
2886:(1): 1–6.
1883:, May 2017
1862:, May 2017
1839:References
1819:CoA-esters
1568:methionine
1556:isoleucine
1451:methionine
1447:isoleucine
1423:methionine
1415:isoleucine
1360:chromosome
1339:holoenzyme
1335:N-terminus
1197:structures
1164:KEGG entry
1111:9023-90-9
718:metabolism
416:(ortholog)
153:HomoloGene
5346:EC 5.4.99
5140:EC number
4886:Isomerase
4536:Aconitase
4442:aspartate
4303:THREONINE
4122:glutamine
4118:glutamate
4093:glutamate
3967:HISTIDINE
3953:glutamate
3929:threonine
3609:synthesis
2697:CiteSeerX
2573:Structure
1787:apoenzyme
1762:Glutamate
1746:histidine
1729:isomerase
1722:homolytic
1700:Mechanism
1564:threonine
1455:threonine
1427:threonine
1345:Structure
1299:vitamin B
1117:Databases
1005:NP_032676
980:NP_000246
954:NM_008650
933:NM_000255
763:Orthologs
161:GeneCards
5164:Kinetics
5088:Cofactor
5051:Activity
4568:Fumarase
4406:tyrosine
4370:tyrosine
4357:fumarate
4089:cysteine
4034:arginine
3910:cysteine
3872:creatine
3829:pyruvate
3798:tyrosine
3343:Genomics
3261:Genomics
3179:Genomics
2975:24610554
2967:17401587
2908:41661834
2820:16641088
2779:20876572
2685:Thomä NH
2671:22945861
2636:10551831
2554:10387043
2516:11893736
2475:22661206
2423:23898205
2363:23046887
2314:23041189
2279:21138732
2239:22727635
2203:25125334
2151:15781192
2054:16182581
2005:34612963
1997:19699272
1954:26449400
1879:–
1858:–
1770:tyrosine
1378:Function
1285:5.4.99.2
1268:proteins
1256:articles
1244:articles
1201:RCSB PDB
1099:5.4.99.2
1053:Wikidata
742:Sources:
5320:Biology
5274:Ligases
5044:Enzymes
4890:mutases
4740:(CoQ10)
4719:Primary
4575:and ETC
4517:enzymes
4410:melanin
4019:ALDH4A1
3996:proline
3891:alanine
3868:glycine
3843:glycine
3834:citrate
3670:LEUCINE
3613:enzymes
3540:9285782
3505:7912889
3496:1918235
3470:7909321
3427:6124211
3398:6102092
3363:2907507
3334:2881300
3302:Bibcode
3281:2567699
3252:2453061
3220:Bibcode
3199:1980486
3170:1977311
3161:1683687
3135:1670635
3092:5624280
3084:1351030
3055:1346616
3012:1232083
2937:9242908
2900:8990001
2861:7989334
2770:2992254
2719:9772164
2595:9655823
2466:3370288
2414:3746875
2391:Bibcode
2354:3522145
2194:4441004
2116:8643613
2084:Bibcode
2045:2657357
1881:Ensembl
1860:Ensembl
1572:thymine
1534:
1529:
1496:
1431:thymine
1392:ovaries
1370:Protein
1223:QuickGO
1188:profile
1171:MetaCyc
1106:CAS no.
875:UniProt
830:Ensembl
769:Species
748:QuickGO
391:pattern
117:Aliases
5306:Portal
5248:Lyases
4978:5.4.99
4809:COQ10B
4804:COQ10A
4160:VALINE
3847:serine
3646:LYSINE
3566:(MeSH)
3538:
3503:
3493:
3468:
3461:294249
3458:
3425:
3396:
3361:
3332:
3325:304442
3322:
3279:
3250:
3243:280243
3240:
3197:
3168:
3158:
3133:
3126:295026
3123:
3090:
3082:
3053:
3046:442864
3043:
3010:
3002:
2973:
2965:
2935:
2906:
2898:
2859:
2818:
2777:
2767:
2717:
2699:
2669:
2634:
2593:
2552:
2514:
2473:
2463:
2421:
2411:
2361:
2351:
2312:
2277:
2237:
2201:
2191:
2149:
2114:
2104:
2052:
2042:
2003:
1995:
1952:
1833:MMADHC
1828:MMACHC
1766:ribose
1734:cobalt
1694:murine
1669:while
1630:, and
1614:, and
1560:valine
1457:, and
1453:, and
1443:valine
1419:valine
1404:spleen
1400:muscle
1384:kidney
1341:form.
1327:kidney
1251:PubMed
1233:Search
1219:AmiGO
1207:PDBsum
1147:ExPASy
1135:BRENDA
1123:IntEnz
1094:EC no.
1039:search
1037:PubMed
908:P16332
889:P22033
785:Entrez
562:BioGPS
442:oocyte
249:6p12.3
141:609058
5200:Types
4927:5.4.2
4903:5.4.1
4828:Other
4819:PDSS2
4814:PDSS1
4524:Cycle
4082:Other
4024:PYCR1
3734:(See
3088:S2CID
3008:S2CID
3004:24458
2971:S2CID
2904:S2CID
2107:39284
2001:S2CID
1396:brain
1388:heart
1364:exons
1324:sheep
1320:liver
1183:PRIAM
818:17850
778:Mouse
773:Human
744:Amigo
462:liver
414:Mouse
407:Human
354:Start
289:Mouse
253:Start
186:Human
157:20097
149:97239
5292:list
5285:EC7
5279:list
5272:EC6
5266:list
5259:EC5
5253:list
5246:EC4
5240:list
5233:EC3
5227:list
5220:EC2
5214:list
5207:EC1
4961:PMM2
4956:PMM1
4896:5.4)
4799:COQ9
4794:COQ7
4789:COQ6
4784:COQ5
4779:COQ4
4774:COQ3
4769:COQ2
4625:and
4562:SDHA
4325:→TCA
4066:→TCA
3536:PMID
3501:PMID
3466:PMID
3423:PMID
3394:PMID
3359:PMID
3330:PMID
3277:PMID
3248:PMID
3195:PMID
3166:PMID
3131:PMID
3080:PMID
3051:PMID
3000:PMID
2963:PMID
2933:PMID
2896:PMID
2857:PMID
2816:PMID
2775:PMID
2715:PMID
2667:PMID
2632:PMID
2591:PMID
2550:PMID
2512:PMID
2471:PMID
2419:PMID
2359:PMID
2310:PMID
2275:PMID
2235:PMID
2199:PMID
2147:PMID
2112:PMID
2050:PMID
1993:PMID
1950:PMID
1934:Gene
1823:MMAB
1815:MeaB
1807:MMAA
1791:MMAA
1783:MMAA
1778:MMAA
1692:The
1665:are
1647:MMAA
1643:MMAA
1417:and
1354:The
1350:Gene
1322:and
1263:NCBI
1204:PDBe
1159:KEGG
799:4594
397:Bgee
345:Band
306:Chr.
244:Band
203:Chr.
165:MMUT
137:OMIM
124:MMUT
94:3BIC
90:2XIQ
86:2XIJ
67:RCSB
64:PDBe
33:MMUT
4675:to
4656:to
4637:to
4592:to
3526:doi
3491:PMC
3456:PMC
3448:doi
3415:doi
3411:214
3384:doi
3380:255
3351:doi
3320:PMC
3310:doi
3269:doi
3238:PMC
3228:doi
3187:doi
3156:PMC
3121:PMC
3113:doi
3072:doi
3041:PMC
3033:doi
2992:doi
2955:doi
2925:doi
2888:doi
2847:doi
2843:269
2806:doi
2802:281
2765:PMC
2755:doi
2751:285
2707:doi
2659:doi
2622:doi
2618:274
2581:doi
2542:doi
2502:doi
2498:277
2461:PMC
2453:doi
2409:PMC
2399:doi
2387:110
2349:PMC
2341:doi
2337:107
2302:doi
2298:427
2267:doi
2263:404
2227:doi
2223:106
2189:PMC
2181:doi
2139:doi
2102:PMC
2092:doi
2040:PMC
2032:doi
1985:doi
1981:164
1942:doi
1938:576
1795:GTP
1663:MUT
1659:MUT
1655:MUT
1651:MUT
1639:MUT
1356:MUT
1317:rat
1306:MUT
1295:MUT
1239:PMC
1195:PDB
367:End
266:End
169:OMA
145:MGI
57:PDB
5332::
4894:EC
4888::
4764:10
4738:10
4614:E3
4612:,
4610:E2
4608:,
4606:E1
4512::
4427:G→
4412::
4355:G→
4144:G→
4124::
4099::
3951:G→
3874::
3827:G→
3607:,
3603::
3534:.
3520:.
3516:.
3499:.
3487:55
3485:.
3481:.
3464:.
3454:.
3444:93
3442:.
3438:.
3421:.
3409:.
3392:.
3378:.
3374:.
3357:.
3345:.
3328:.
3318:.
3308:.
3298:84
3296:.
3292:.
3275:.
3263:.
3246:.
3236:.
3226:.
3216:85
3214:.
3210:.
3193:.
3181:.
3164:.
3152:47
3150:.
3146:.
3129:.
3119:.
3109:87
3107:.
3103:.
3086:.
3078:.
3068:89
3066:.
3049:.
3039:.
3029:89
3027:.
3023:.
3006:.
2998:.
2988:31
2986:.
2969:.
2961:.
2951:22
2949:.
2931:.
2921:66
2919:.
2902:.
2894:.
2882:.
2855:.
2841:.
2837:.
2814:.
2800:.
2796:.
2773:.
2763:.
2749:.
2745:.
2727:^
2713:.
2705:.
2693:37
2691:.
2665:.
2655:51
2653:.
2630:.
2616:.
2612:.
2589:.
2575:.
2571:.
2548:.
2538:38
2536:.
2524:^
2510:.
2496:.
2492:.
2469:.
2459:.
2449:13
2447:.
2443:.
2431:^
2417:.
2407:.
2397:.
2385:.
2381:.
2357:.
2347:.
2335:.
2331:.
2308:.
2296:.
2273:.
2261:.
2247:^
2233:.
2221:.
2197:.
2187:.
2177:35
2175:.
2171:.
2159:^
2145:.
2135:84
2133:.
2110:.
2100:.
2090:.
2080:93
2078:.
2074:.
2062:^
2048:.
2038:.
2028:86
2026:.
2022:.
1999:.
1991:.
1979:.
1962:^
1948:.
1936:.
1924:^
1914:.
1896:.
1867:^
1846:^
1714:Co
1634:.
1626:,
1622:,
1610:,
1586:.
1574:,
1570:,
1566:,
1562:,
1558:,
1465:.
1449:,
1445:,
1429:,
1425:,
1398:,
1394:,
1390:,
1312:.
1301:12
1282:EC
1221:/
746:/
373:bp
360:bp
272:bp
259:bp
167:;
163::
159:;
155::
151:;
147::
143:;
139::
92:,
88:,
5308::
5294:)
5290:(
5281:)
5277:(
5268:)
5264:(
5255:)
5251:(
5242:)
5238:(
5229:)
5225:(
5216:)
5212:(
5036:e
5029:t
5022:v
4892:(
4878:e
4871:t
4864:v
4708:/
4629:)
4616:)
4604:(
4564:)
4560:(
4502:e
4495:t
4488:v
4453:/
4444:→
4440:→
4408:→
4372:→
4368:→
4321:→
4305:→
4285::
4268:/
4263::
4245::
4235:→
4206:→
4162:→
4148:→
4120:→
4095:→
4091:+
4062:→
4036:→
4017:/
3998:→
3969:→
3955:→
3931:→
3912:→
3893:→
3870:→
3849:→
3845:→
3832:→
3819:G
3800:→
3796:→
3757:/
3748:→
3672:→
3648:→
3635:→
3633:K
3593:e
3586:t
3579:v
3542:.
3528::
3522:6
3507:.
3472:.
3450::
3429:.
3417::
3400:.
3386::
3365:.
3353::
3347:3
3336:.
3312::
3304::
3283:.
3271::
3265:4
3254:.
3230::
3222::
3201:.
3189::
3183:8
3172:.
3137:.
3115::
3094:.
3074::
3057:.
3035::
3014:.
2994::
2977:.
2957::
2939:.
2927::
2910:.
2890::
2884:9
2863:.
2849::
2822:.
2808::
2781:.
2757::
2721:.
2709::
2673:.
2661::
2638:.
2624::
2597:.
2583::
2577:6
2556:.
2544::
2518:.
2504::
2477:.
2455::
2425:.
2401::
2393::
2365:.
2343::
2316:.
2304::
2281:.
2269::
2241:.
2229::
2205:.
2183::
2153:.
2141::
2118:.
2094::
2086::
2056:.
2034::
2007:.
1987::
1956:.
1944::
1918:.
1900:.
291:)
188:)
171::
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.