1252:
however, it does not reveal the numerous folding pathways that are possible. A different molecule of the same exact protein may be able to follow marginally different folding pathways, seeking different lower energy intermediates, as long as the same native structure is reached. Different pathways may have different frequencies of utilization depending on the thermodynamic favorability of each pathway. This means that if one pathway is found to be more thermodynamically favorable than another, it is likely to be used more frequently in the pursuit of the native structure. As the protein begins to fold and assume its various conformations, it always seeks a more thermodynamically favorable structure than before and thus continues through the energy funnel. Formation of secondary structures is a strong indication of increased stability within the protein, and only one combination of secondary structures assumed by the polypeptide backbone will have the lowest energy and therefore be present in the native state of the protein. Among the first structures to form once the polypeptide begins to fold are alpha helices and beta turns, where alpha helices can form in as little as 100 nanoseconds and beta turns in 1 microsecond.
530:. Chaperones exist in all cellular compartments and interact with the polypeptide chain in order to allow the native three-dimensional conformation of the protein to form; however, chaperones themselves are not included in the final structure of the protein they are assisting in. Chaperones may assist in folding even when the nascent polypeptide is being synthesized by the ribosome. Molecular chaperones operate by binding to stabilize an otherwise unstable structure of a protein in its folding pathway, but chaperones do not contain the necessary information to know the correct native structure of the protein they are aiding; rather, chaperones work by preventing incorrect folding conformations. In this way, chaperones do not actually increase the rate of individual steps involved in the folding pathway toward the native structure; instead, they work by reducing possible unwanted aggregations of the polypeptide chain that might otherwise slow down the search for the proper intermediate and they provide a more efficient pathway for the polypeptide chain to assume the correct conformations. Chaperones are not to be confused with folding
814:
one must have a suitable solvent for crystallization, obtain a pure protein at supersaturated levels in solution, and precipitate the crystals in solution. Once a protein is crystallized, X-ray beams can be concentrated through the crystal lattice which would diffract the beams or shoot them outwards in various directions. These exiting beams are correlated to the specific three-dimensional configuration of the protein enclosed within. The X-rays specifically interact with the electron clouds surrounding the individual atoms within the protein crystal lattice and produce a discernible diffraction pattern. Only by relating the electron density clouds with the amplitude of the X-rays can this pattern be read and lead to assumptions of the phases or phase angles involved that complicate this method. Without the relation established through a mathematical basis known as
851:
different but discrete protein states, i.e. native state, intermediate states, unfolded state, depends on the denaturant value; therefore, the global fluorescence signal of their equilibrium mixture also depends on this value. One thus obtains a profile relating the global protein signal to the denaturant value. The profile of equilibrium unfolding may enable one to detect and identify intermediates of unfolding. General equations have been developed by Hugues
Bedouelle to obtain the thermodynamic parameters that characterize the unfolding equilibria for homomeric or heteromeric proteins, up to trimers and potentially tetramers, from such profiles. Fluorescence spectroscopy can be combined with fast-mixing devices such as
994:
843:
proteins, at the interface between two protein domains, or at the interface between subunits of oligomeric proteins. In this apolar environment, they have high quantum yields and therefore high fluorescence intensities. Upon disruption of the protein's tertiary or quaternary structure, these side chains become more exposed to the hydrophilic environment of the solvent, and their quantum yields decrease, leading to low fluorescence intensities. For Trp residues, the wavelength of their maximal fluorescence emission also depend on their environment.
1260:
transition state. The transition state can be referred to as a variant or premature form of the native state rather than just another intermediary step. The folding of the transition state is shown to be rate-determining, and even though it exists in a higher energy state than the native fold, it greatly resembles the native structure. Within the transition state, there exists a nucleus around which the protein is able to fold, formed by a process referred to as "nucleation condensation" where the structure begins to collapse onto the nucleus.
1269:
1248:. The description of protein folding by the leveling free-energy landscape is also consistent with the 2nd law of thermodynamics. Physically, thinking of landscapes in terms of visualizable potential or total energy surfaces simply with maxima, saddle points, minima, and funnels, rather like geographic landscapes, is perhaps a little misleading. The relevant description is really a high-dimensional phase space in which manifolds might take a variety of more complicated topological forms.
970:(NMR) is able to collect protein structural data by inducing a magnet field through samples of concentrated protein. In NMR, depending on the chemical environment, certain nuclei will absorb specific radio-frequencies. Because protein structural changes operate on a time scale from ns to ms, NMR is especially equipped to study intermediate structures in timescales of ps to s. Some of the main techniques for studying proteins structure and non-folding protein structural changes include
204:
conformation. The amino acid composition is not as important as the sequence. The essential fact of folding, however, remains that the amino acid sequence of each protein contains the information that specifies both the native structure and the pathway to attain that state. This is not to say that nearly identical amino acid sequences always fold similarly. Conformations differ based on environmental factors as well; similar proteins fold differently based on where they are found.
700:-like structures which can cause degenerative disorders and cell death. The amyloids are fibrillary structures that contain intermolecular hydrogen bonds which are highly insoluble and made from converted protein aggregates. Therefore, the proteasome pathway may not be efficient enough to degrade the misfolded proteins prior to aggregation. Misfolded proteins can interact with one another and form structured aggregates and gain toxicity through intermolecular interactions.
1198:
430:
231:
477:, or the inward folding of the hydrophobic groups. The hydrophobic collapse introduces entropy back to the system via the breaking of the water cages which frees the ordered water molecules. The multitude of hydrophobic groups interacting within the core of the globular folded protein contributes a significant amount to protein stability after folding, because of the vastly accumulated van der Waals forces (specifically
990:. NOE is especially useful because magnetization transfers can be observed between spatially proximal hydrogens are observed. Different NMR experiments have varying degrees of timescale sensitivity that are appropriate for different protein structural changes. NOE can pick up bond vibrations or side chain rotations, however, NOE is too sensitive to pick up protein folding because it occurs at larger timescale.
473:
aqueous environment, the water molecules tend to aggregate around the hydrophobic regions or side chains of the protein, creating water shells of ordered water molecules. An ordering of water molecules around a hydrophobic region increases order in a system and therefore contributes a negative change in entropy (less entropy in the system). The water molecules are fixed in these water cages which drives the
1012:
thermodynamics and kinetics between the excited and ground. Saturation
Transfer measures changes in signal from the ground state as excited states become perturbed. It uses weak radio frequency irradiation to saturate the excited state of a particular nuclei which transfers its saturation to the ground state. This signal is amplified by decreasing the magnetization (and the signal) of the ground state.
330:
457:
1030:, excited intermediates were studied with relaxation dispersion and Saturation transfer. SOD1 had been previously tied to many disease causing mutants which were assumed to be involved in protein aggregation, however the mechanism was still unknown. By using Relaxation Dispersion and Saturation Transfer experiments many excited intermediate states were uncovered misfolding in the SOD1 mutants.
800:
39:
1307:. Because of computational cost, ab initio MD folding simulations with explicit water are limited to peptides and small proteins. MD simulations of larger proteins remain restricted to dynamics of the experimental structure or its high-temperature unfolding. Long-time folding processes (beyond about 1 millisecond), like folding of larger proteins (>150 residues) can be accessed using
1137:(vWF) is a protein with an essential role in blood clot formation process. It discovered – using single molecule optical tweezers measurement – that calcium-bound vWF acts as a shear force sensor in the blood. Shear force leads to unfolding of the A2 domain of vWF, whose refolding rate is dramatically enhanced in the presence of calcium. Recently, it was also shown that the simple src
31:
511:
219:
606:, mechanical forces, and the presence of chemical denaturants can contribute to protein denaturation, as well. These individual factors are categorized together as stresses. Chaperones are shown to exist in increasing concentrations during times of cellular stress and help the proper folding of emerging proteins as well as denatured or misfolded ones.
1221:, meaning that naturally evolved proteins have optimized their folding energy landscapes, and that nature has chosen amino acid sequences so that the folded state of the protein is sufficiently stable. In addition, the acquisition of the folded state had to become a sufficiently fast process. Even though nature has reduced the level of
1341:. The longest published result of a simulation performed using Anton as of 2011 was a 2.936 millisecond simulation of NTL9 at 355 K. Such simulations are currently able to unfold and refold small proteins (<150 amino acids residues) in equilibrium and predict how mutations affect folding kinetics and stability.
1251:
The unfolded polypeptide chain begins at the top of the funnel where it may assume the largest number of unfolded variations and is in its highest energy state. Energy landscapes such as these indicate that there are a large number of initial possibilities, but only a single native state is possible;
588:
to humans, suggesting that they evolved very early and have an important function. Some proteins never fold in cells at all except with the assistance of chaperones which either isolate individual proteins so that their folding is not interrupted by interactions with other proteins or help to unfold
597:
amorphous aggregates. The external factors involved in protein denaturation or disruption of the native state include temperature, external fields (electric, magnetic), molecular crowding, and even the limitation of space (i.e. confinement), which can have a big influence on the folding of proteins.
1259:
for a particular protein is found. The transition state in the energy funnel diagram is the conformation that must be assumed by every molecule of that protein if the protein wishes to finally assume the native structure. No protein may assume the native structure without first passing through the
1011:
phenomenon. This technique exposes the target nuclei to a 90 pulse followed by one or more 180 pulses. As the nuclei refocus, a broad distribution indicates the target nuclei is involved in an intermediate excited state. By looking at
Relaxation dispersion plots the data collect information on the
842:
are high enough to give good fluorescence signals. Both Trp and Tyr are excited by a wavelength of 280 nm, whereas only Trp is excited by a wavelength of 295 nm. Because of their aromatic character, Trp and Tyr residues are often found fully or partially buried in the hydrophobic core of
813:
is one of the more efficient and important methods for attempting to decipher the three dimensional configuration of a folded protein. To be able to conduct X-ray crystallography, the protein under investigation must be located inside a crystal lattice. To place a protein inside a crystal lattice,
750:
or
Vyndaqel (a kinetic stabilizer of tetrameric transthyretin) for the treatment of transthyretin amyloid diseases. This suggests that the process of amyloid fibril formation (and not the fibrils themselves) causes the degeneration of post-mitotic tissue in human amyloid diseases. Misfolding and
550:
because they provide the protein with the aid needed to assume its proper alignments and conformations efficiently enough to become "biologically relevant". This means that the polypeptide chain could theoretically fold into its native structure without the aid of chaperones, as demonstrated by
203:
The primary structure of a protein, its linear amino-acid sequence, determines its native conformation. The specific amino acid residues and their position in the polypeptide chain are the determining factors for which portions of the protein fold closely together and form its three-dimensional
850:
of proteins by measuring the variation in the intensity of fluorescence emission or in the wavelength of maximal emission as functions of a denaturant value. The denaturant can be a chemical molecule (urea, guanidinium hydrochloride), temperature, pH, pressure, etc. The equilibrium between the
695:
that are organized in a supramolecular arrangement known as a cross-β structure. These β-sheet-rich assemblies are very stable, very insoluble, and generally resistant to proteolysis. The structural stability of these fibrillar assemblies is caused by extensive interactions between the protein
472:
Minimizing the number of hydrophobic side-chains exposed to water is an important driving force behind the folding process. The hydrophobic effect is the phenomenon in which the hydrophobic chains of a protein collapse into the core of the protein (away from the hydrophilic environment). In an
288:
The α-Helices and β-Sheets are commonly amphipathic, meaning they have a hydrophilic and a hydrophobic portion. This ability helps in forming tertiary structure of a protein in which folding occurs so that the hydrophilic sides are facing the aqueous environment surrounding the protein and the
1132:
have been used to stretch single protein molecules from their C- and N-termini and unfold them to allow study of the subsequent refolding. The technique allows one to measure folding rates at single-molecule level; for example, optical tweezers have been recently applied to study folding and
464:
462:
460:
458:
320:
Tertiary structure may give way to the formation of quaternary structure in some proteins, which usually involves the "assembly" or "coassembly" of subunits that have already folded; in other words, multiple polypeptide chains could interact to form a fully functional quaternary protein.
463:
269:
to form a spiral shape (refer to figure on the right). The β pleated sheet is a structure that forms with the backbone bending over itself to form the hydrogen bonds (as displayed in the figure to the left). The hydrogen bonds are between the amide hydrogen and carbonyl oxygen of the
1367:
AlphaFold's protein structure prediction results at CASP were described as "transformational" and "astounding". Some researchers noted that the accuracy is not high enough for a third of its predictions, and that it does not reveal the physical mechanism of protein folding for the
274:. There exists anti-parallel β pleated sheets and parallel β pleated sheets where the stability of the hydrogen bonds is stronger in the anti-parallel β sheet as it hydrogen bonds with the ideal 180 degree angle compared to the slanted hydrogen bonds formed by parallel sheets.
1364:, a test that measures the degree of similarity between the structure, predicted by a computational program, and the empirical structure, determined experimentally in a lab. A score of 100 is considered a complete match, within the distance cutoff used for calculating GDT.
696:
monomers, formed by backbone hydrogen bonds between their β-strands. The misfolding of proteins can trigger the further misfolding and accumulation of other proteins into aggregates or oligomers. The increased levels of aggregated proteins in the cell leads to formation of
168:
proteins with lengths of up to a hundred amino acids typically fold in a single step. Time scales of milliseconds are the norm, and the fastest known protein folding reactions are complete within a few microseconds. The folding time scale of a protein depends on its size,
461:
289:
hydrophobic sides are facing the hydrophobic core of the protein. Secondary structure hierarchically gives way to tertiary structure formation. Once the protein's tertiary structure is formed and stabilized by the hydrophobic interactions, there may also be
1051:
by determining the overall size of a monolayer of the protein and its density in real time at sub-Angstrom resolution, although real-time measurement of the kinetics of protein folding are limited to processes that occur slower than ~10 Hz. Similar to
489:
molecule containing a large hydrophobic region. The strength of hydrogen bonds depends on their environment; thus, H-bonds enveloped in a hydrophobic core contribute more than H-bonds exposed to the aqueous environment to the stability of the native state.
1175:
is a thought experiment based on the observation that if a protein were folded by sequential sampling of all possible conformations, it would take an astronomical amount of time to do so, even if the conformations were sampled at a rapid rate (on the
837:
is a highly sensitive method for studying the folding state of proteins. Three amino acids, phenylalanine (Phe), tyrosine (Tyr) and tryptophan (Trp), have intrinsic fluorescence properties, but only Tyr and Trp are used experimentally because their
1170:
In 1969, Cyrus
Levinthal noted that, because of the very large number of degrees of freedom in an unfolded polypeptide chain, the molecule has an astronomical number of possible conformations. An estimate of 3 or 10 was made in one of his papers.
1006:
have become some of the primary techniques for NMR analysis of folding. In addition, both techniques are used to uncover excited intermediate states in the protein folding landscape. To do this, CPMG Relaxation dispersion takes advantage of the
3533:
Johnson SM, Wiseman RL, Sekijima Y, Green NS, Adamski-Werner SL, Kelly JW (December 2005). "Native state kinetic stabilization as a strategy to ameliorate protein misfolding diseases: a focus on the transthyretin amyloidoses".
1161:
Computational studies of protein folding includes three main aspects related to the prediction of protein stability, kinetics, and structure. A 2013 review summarizes the available computational methods for protein folding.
1068:
The study of protein folding has been greatly advanced in recent years by the development of fast, time-resolved techniques. Experimenters rapidly trigger the folding of a sample of unfolded protein and observe the resulting
416:
Proteins will have limitations on their folding abilities by the restricted bending angles or conformations that are possible. These allowable angles of protein folding are described with a two-dimensional plot known as the
305:
arrangement in a native structure of a protein. Tertiary structure of a protein involves a single polypeptide chain; however, additional interactions of folded polypeptide chains give rise to quaternary structure formation.
1184:
scale). Based upon the observation that proteins fold much faster than this, Levinthal then proposed that a random conformational search does not occur, and the protein must, therefore, fold through a series of meta-stable
742:. It is not completely clear whether the aggregates are the cause or merely a reflection of the loss of protein homeostasis, the balance between synthesis, folding, aggregation and protein turnover. Recently the
493:
In proteins with globular folds, hydrophobic amino acids tend to be interspersed along the primary sequence, rather than randomly distributed or clustered together. However, proteins that have recently been born
690:
if it cannot achieve its normal native state. This can be due to mutations in the amino acid sequence or a disruption of the normal folding process by external factors. The misfolded protein typically contains
997:
Timescale of protein structural changes matched with NMR experiments. For protein folding, CPMG Relaxation
Dispersion (CPMG RD) and chemical exchange saturation transfer (CEST) collect data in the appropriate
440:
Protein folding must be thermodynamically favorable within a cell in order for it to be a spontaneous reaction. Since it is known that protein folding is a spontaneous reaction, then it must assume a negative
80:
The folding of many proteins begins even during the translation of the polypeptide chain. The amino acids interact with each other to produce a well-defined three-dimensional structure, known as the protein's
1236:" landscape allows the protein to fold to the native state through any of a large number of pathways and intermediates, rather than being restricted to a single mechanism. The theory is supported by both
3800:
Ould-Abeih MB, Petit-Topin I, Zidane N, Baron B, Bedouelle H (June 2012). "Multiple folding states and disorder of ribosomal protein SA, a membrane receptor for laminin, anticarcinogens, and pathogens".
4173:
Cross GH, Freeman NJ, Swann MJ (2008). "Dual
Polarization Interferometry: A Real-Time Optical Technique for Measuring (Bio)molecular Orientation, Structure and Function at the Solid/Liquid Interface".
1548:
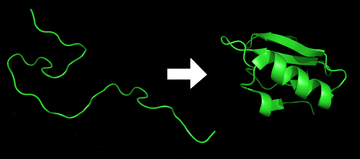
1276:, like the one diagrammed here, to model the possible shapes and folding pathways a protein can take as it condenses from its initial randomly coiled state (left) into its native 3D structure (right).
621:
have been found that grow at temperatures as high as 122 °C, which of course requires that their full complement of vital proteins and protein assemblies be stable at that temperature or above.
572:. Under certain conditions some proteins can refold; however, in many cases, denaturation is irreversible. Cells sometimes protect their proteins against the denaturing influence of heat with
735:. These age onset degenerative diseases are associated with the aggregation of misfolded proteins into insoluble, extracellular aggregates and/or intracellular inclusions including cross-β
1360:, a long-standing structureprediction contest The team achieved a level of accuracy much higher than any other group. It scored above 90% for around two-thirds of the proteins in CASP's
459:
6256:
975:
971:
5047:
Schaefer M, Bartels C, Karplus M (December 1998). "Solution conformations and thermodynamics of structured peptides: molecular dynamics simulation with an implicit solvation model".
771:, where loss of function is the origin of the disorder. While protein replacement therapy has historically been used to correct the latter disorders, an emerging approach is to use
100:
are important. Failure to fold into a native structure generally produces inactive proteins, but in some instances, misfolded proteins have modified or toxic functionality. Several
936:(FT) instruments, provide powerful means for determining protein conformations in solution even for very large protein molecules. Such VCD studies of proteins can be combined with
892:
are chiral, and thus absorb such light. The absorption of this light acts as a marker of the degree of foldedness of the protein ensemble. This technique has been used to measure
6509:
2429:
Deechongkit S, Nguyen H, Powers ET, Dawson PE, Gruebele M, Kelly JW (July 2004). "Context-dependent contributions of backbone hydrogen bonding to beta-sheet folding energetics".
5646:
2343:
Cui D, Ou S, Patel S (December 2014). "Protein-spanning water networks and implications for prediction of protein–protein interactions mediated through hydrophobic effects".
1128:
Single molecule techniques such as optical tweezers and AFM have been used to understand protein folding mechanisms of isolated proteins as well as proteins with chaperones.
979:
153:, and other contexts. Residual structure present, if any, in the supposedly unfolded state may form a folding initiation site and guide the subsequent folding reactions.
5395:
Piana S, Piana S, Sarkar K, Lindorff-Larsen K, Guo M, Gruebele M, Shaw DE (2010). "Computational Design and
Experimental Testing of the Fastest-Folding β-Sheet Protein".
826:
use the presence of a heavy metal ion to diffract the X-rays into a more predictable manner, reducing the number of variables involved and resolving the phase problem.
6054:
561:
Along with its role in aiding native structure formation, chaperones are shown to be involved in various roles such as protein transport, degradation, and even allow
609:
Under some conditions proteins will not fold into their biochemically functional forms. Temperatures above or below the range that cells tend to live in will cause
436:. In the compact fold (to the right), the hydrophobic amino acids (shown as black spheres) collapse toward the center to become shielded from aqueous environment.
3761:"Dimeric tyrosyl-tRNA synthetase from Bacillus stearothermophilus unfolds through a monomeric intermediate. A quantitative analysis under equilibrium conditions"
453:. For a negative delta G to arise and for protein folding to become thermodynamically favorable, then either enthalpy, entropy, or both terms must be favorable.
1228:
A consequence of these evolutionarily selected sequences is that proteins are generally thought to have globally "funneled energy landscapes" (a term coined by
246:
is the first step in the folding process that a protein takes to assume its native structure. Characteristic of secondary structure are the structures known as
6249:
4941:"Transition-state structure as a unifying basis in protein-folding mechanisms: contact order, chain topology, stability, and the extended nucleus mechanism"
265:. Formation of intramolecular hydrogen bonds provides another important contribution to protein stability. α-helices are formed by hydrogen bonding of the
557:; however, this process proves to be too inefficient or too slow to exist in biological systems; therefore, chaperones are necessary for protein folding
3439:
Hammarström P, Wiseman RL, Powers ET, Kelly JW (January 2003). "Prevention of transthyretin amyloid disease by changing protein misfolding energetics".
4635:
5867:
5639:
6242:
1426:
1047:
is a surface-based technique for measuring the optical properties of molecular layers. When used to characterize protein folding, it measures the
6854:
4016:
Vallurupalli P, Bouvignies G, Kay LE (May 2012). "Studying "invisible" excited protein states in slow exchange with a major state conformation".
993:
164:, and must pass through a number of intermediate states, like checkpoints, before the process is complete. On the other hand, very small single-
546:
or interconversion between cis and trans stereoisomers of peptide group. Chaperones are shown to be critical in the process of protein folding
4300:
Park C, Marqusee S (March 2005). "Pulse proteolysis: a simple method for quantitative determination of protein stability and ligand binding".
4856:
4688:
Bryngelson JD, Onuchic JN, Socci ND, Wolynes PG (March 1995). "Funnels, pathways, and the energy landscape of protein folding: a synthesis".
4190:
3613:
3195:"Cell proliferation at 122 degrees C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation"
2163:
2105:
1945:
1639:
1560:
1478:
1431:
4206:
Bu Z, Cook J, Callaway DJ (September 2001). "Dynamic regimes and correlated structural dynamics in native and denatured alpha-lactalbumin".
6152:
6047:
5455:
3970:
Ortega, Gabriel; Pons, Miquel; Millet, Oscar (2013). "Protein
Functional Dynamics in Multiple Timescales as Studied by NMR Spectroscopy".
2185:
Zhang G, Ignatova Z (February 2011). "Folding at the birth of the nascent chain: coordinating translation with co-translational folding".
5431:
1225:
in proteins, some degree of it remains up to now as can be observed in the presence of local minima in the energy landscape of proteins.
5632:
908:(Tm) of the protein. As for fluorescence spectroscopy, circular-dichroism spectroscopy can be combined with fast-mixing devices such as
93:
1149:
Biotin painting enables condition-specific cellular snapshots of (un)folded proteins. Biotin 'painting' shows a bias towards predicted
7072:
1117:
534:
proteins, which catalyze chemical reactions responsible for slow steps in folding pathways. Examples of folding catalysts are protein
2147:
6157:
6147:
3987:
3683:
Bedouelle H (February 2016). "Principles and equations for measuring and interpreting protein stability: From monomer to tetramer".
1150:
1081:. Among the many scientists who have contributed to the development of these techniques are Jeremy Cook, Heinrich Roder, Terry Oas,
499:
5523:
Callaway, Ewen (30 November 2020). "'It will change everything': DeepMind's AI makes gigantic leap in solving protein structures".
3404:
Soto C, Estrada L, Castilla J (March 2006). "Amyloids, prions and the inherent infectious nature of misfolded protein aggregates".
664:
Some proteins have multiple native structures, and change their fold based on some external factors. For example, the KaiB protein
5501:
6137:
2927:
Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU (2013). "Molecular chaperone functions in protein folding and proteostasis".
1044:
1039:
1015:
The main limitations in NMR is that its resolution decreases with proteins that are larger than 25 kDa and is not as detailed as
823:
712:
880:
is one of the most general and basic tools to study protein folding. Circular dichroism spectroscopy measures the absorption of
656:
chaperonin that is absolutely necessary for the folding and assembly in vivo of the bacteriophage T4 major capsid protein gp23.
6265:
6142:
6040:
5895:
3193:
Takai K, Nakamura K, Toki T, Tsunogai U, Miyazaki M, Miyazaki J, Hirayama H, Nakagawa S, Nunoura T, Horikoshi K (August 2008).
1441:
724:
720:
589:
misfolded proteins, allowing them to refold into the correct native structure. This function is crucial to prevent the risk of
7097:
6465:
929:
3720:"Quantitative measurement of protein stability from unfolding equilibria monitored with the fluorescence maximum wavelength"
708:
1206:
1023:
5598:
6678:
6104:
6094:
5860:
1292:
1241:
1630:
Alberts B, Bray D, Hopkin K, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2010). "Protein
Structure and Function".
1019:. Additionally, protein NMR analysis is quite difficult and can propose multiple solutions from the same NMR spectrum.
6623:
6167:
6084:
1416:
562:
243:
213:
134:
7107:
6089:
5655:
4610:
967:
768:
315:
283:
74:
4343:
Mashaghi A, Kramer G, Lamb DC, Mayer MP, Tans SJ (January 2014). "Chaperone action at the single-molecule level".
3054:"Electric field-driven disruption of a native beta-sheet protein conformation and generation of a helix-structure"
6223:
6079:
1963:"The design and characterization of two proteins with 88% sequence identity but different structure and function"
987:
901:
834:
772:
743:
590:
539:
198:
7102:
6563:
5216:"De novo prediction of protein folding pathways and structure using the principle of sequential stabilization"
2270:
565:
exposed to certain external denaturant factors an opportunity to refold into their correct native structures.
92:
The correct three-dimensional structure is essential to function, although some parts of functional proteins
6888:
5853:
2884:
Hartl FU, Bracher A, Hayer-Hartl M (July 2011). "Molecular chaperones in protein folding and proteostasis".
1369:
1349:
1308:
791:
or folding of proteins and observing conformational changes using standard non-crystallographic techniques.
478:
7060:
4666:
4544:
4486:"Direct observation of a force-induced switch in the anisotropic mechanical unfolding pathway of a protein"
3256:
1376:
and great progress towards a decades-old grand challenge of biology, predicting the structure of proteins.
156:
The duration of the folding process varies dramatically depending on the protein of interest. When studied
6929:
6124:
6114:
6063:
2041:
1172:
1003:
732:
716:
354:
350:
904:
of unfolding as well as the protein's m value, or denaturant dependence. A temperature melt measures the
6310:
6132:
5999:
5731:
1396:
1373:
1186:
1134:
1016:
905:
893:
881:
847:
810:
804:
788:
181:
2222:"Macromolecular crowding perturbs protein refolding kinetics: implications for folding inside the cell"
896:
of the protein by measuring the change in this absorption as a function of denaturant concentration or
485:
exists as a driving force in thermodynamics only if there is the presence of an aqueous medium with an
4575:
Compiani M, Capriotti E (December 2013). "Computational and theoretical methods for protein folding".
2121:
5890:
5579:
5532:
5353:
5227:
5168:
4952:
4894:
4813:
4758:
4650:
4497:
4440:
4254:
4127:
4067:
3929:
3642:
3448:
3299:
3206:
3065:
2839:
2783:
2570:
2503:
2438:
2395:
2033:
1974:
1887:
1826:
1588:
1361:
1082:
1048:
474:
433:
374:
366:
346:
255:
58:
5097:
2046:
1814:
1655:
Yagi-Utsumi M, Chandak MS, Yanaka S, Hiranyakorn M, Nakamura T, Kato K, Kuwajima K (November 2020).
6273:
6193:
6162:
5767:
1406:
1386:
1338:
535:
521:
410:
338:
5619:
6919:
6287:
5556:
5506:
5473:
5377:
4918:
4723:
4697:
4325:
3836:
Royer CA (May 2006). "Probing protein folding and conformational transitions with fluorescence".
3515:
3472:
3383:
2993:
2909:
2863:
2807:
2560:
2493:
2462:
2368:
2325:
1860:
1612:
1411:
1296:
1273:
1116:
is routinely used to probe the fraction unfolded under a wide range of solution conditions (e.g.
1074:
1053:
877:
872:
860:
784:
687:
581:
577:
568:
A fully denatured protein lacks both tertiary and secondary structure, and exists as a so-called
515:
495:
482:
235:
5298:
2964:"The denatured state (the other half of the folding equation) and its role in protein stability"
5344:
Lindorff-Larsen K, Piana S, Dror RO, Shaw DE (October 2011). "How fast-folding proteins fold".
6589:
6099:
6071:
5931:
5905:
5787:
5679:
5548:
5412:
5369:
5255:
5196:
5137:
5064:
5029:
4980:
4910:
4862:
4852:
4786:
4715:
4592:
4525:
4466:
4409:
4360:
4317:
4282:
4223:
4186:
4155:
4093:
4033:
3993:
3983:
3949:
3901:
3853:
3818:
3782:
3741:
3700:
3660:
3609:
3551:
3507:
3464:
3421:
3375:
3327:
3268:
3234:
3175:
3140:
3091:
3034:
2985:
2944:
2901:
2855:
2799:
2745:
2696:
2647:
2598:
2529:
2454:
2411:
2360:
2317:
2251:
2202:
2159:
2101:
2071:
2002:
1941:
1903:
1852:
1795:
1756:
1721:
1686:
1635:
1604:
1556:
1525:
1474:
1304:
1268:
949:
937:
933:
913:
815:
669:
668:, acting as a clock for cyanobacteria. It has been estimated that around 0.5–4% of PDB (
618:
442:
418:
101:
86:
4114:
Sekhar A, Rumfeldt JA, Broom HR, Doyle CM, Sobering RE, Meiering EM, Kay LE (November 2016).
3503:
2095:
580:(a type of chaperone), which assist other proteins both in folding and in remaining folded.
502:, show the opposite pattern of hydrophobic amino acid clustering along the primary sequence.
180:
Understanding and simulating the protein folding process has been an important challenge for
6849:
6808:
6803:
6778:
6768:
6763:
6753:
6553:
6218:
6009:
5967:
5962:
5957:
5782:
5705:
5540:
5463:
5404:
5361:
5245:
5235:
5186:
5176:
5127:
5056:
5019:
5011:
4970:
4960:
4902:
4844:
4821:
4776:
4766:
4707:
4658:
4611:"Structural Biochemistry/Proteins/Protein Folding - Wikibooks, open books for an open world"
4584:
4555:
4515:
4505:
4456:
4448:
4399:
4391:
4352:
4309:
4272:
4262:
4215:
4178:
4145:
4135:
4083:
4075:
4025:
3975:
3941:
3891:
3845:
3810:
3772:
3731:
3692:
3650:
3603:
3543:
3499:
3456:
3413:
3365:
3317:
3307:
3224:
3214:
3167:
3130:
3122:
3081:
3073:
3024:
2975:
2936:
2893:
2847:
2791:
2735:
2727:
2716:"A Shift in Aggregation Avoidance Strategy Marks a Long-Term Direction to Protein Evolution"
2686:
2678:
2637:
2629:
2588:
2578:
2519:
2511:
2446:
2403:
2352:
2307:
2241:
2233:
2194:
2061:
2051:
1992:
1982:
1895:
1842:
1834:
1787:
1748:
1713:
1676:
1668:
1657:"Residual Structure of Unfolded Ubiquitin as Revealed by Hydrogen/Deuterium-Exchange 2D NMR"
1596:
1515:
1507:
1421:
1401:
1330:
1256:
1210:
1129:
1102:
1078:
1070:
983:
631:
468:
Entropy is decreased as the water molecules become more orderly near the hydrophobic solute.
398:
302:
174:
97:
50:
6824:
6758:
4843:. Progress in Molecular Biology and Translational Science. Vol. 84. pp. 161–202.
2097:
Structure and Mechanism in Protein Science: A Guide to Enzyme Catalysis and Protein Folding
429:
7055:
5915:
5808:
5772:
4243:"Determining biophysical protein stability in lysates by a fast proteolysis assay, FASTpp"
4056:"Relaxation dispersion NMR spectroscopy as a tool for detailed studies of protein folding"
3573:
2940:
1237:
1197:
1086:
764:
610:
120:
4742:
3158:
Ellis RJ (July 2006). "Molecular chaperones: assisting assembly in addition to folding".
1229:
1002:
Because protein folding takes place in about 50 to 3000 s CPMG Relaxation dispersion and
822:" would render predicting the diffraction patterns very difficult. Emerging methods like
728:
5575:
5536:
5357:
5231:
5172:
4956:
4898:
4817:
4762:
4654:
4501:
4444:
4258:
4131:
4071:
3974:. Advances in Protein Chemistry and Structural Biology. Vol. 92. pp. 219–251.
3646:
3490:
Chiti F, Dobson CM (2006). "Protein misfolding, functional amyloid, and human disease".
3452:
3303:
3210:
3069:
2843:
2787:
2574:
2507:
2442:
2399:
2037:
1978:
1891:
1830:
1717:
1592:
230:
6602:
6597:
6188:
6109:
5936:
5813:
5741:
5710:
5669:
5250:
5215:
5191:
5156:
5024:
4999:
4520:
4485:
4461:
4428:
4404:
4379:
4277:
4242:
4150:
4116:"Probing the free energy landscapes of ALS disease mutants of SOD1 by NMR spectroscopy"
4115:
4088:
4055:
3979:
3322:
3287:
3229:
3194:
3135:
3110:
3086:
3053:
2740:
2715:
2691:
2666:
2642:
2617:
2524:
2481:
2386:
Tanford C (June 1978). "The hydrophobic effect and the organization of living matter".
2066:
2021:
1997:
1962:
1704:
Kim PS, Baldwin RL (1990). "Intermediates in the folding reactions of small proteins".
1681:
1656:
1520:
1495:
1245:
1233:
1090:
543:
294:
266:
165:
160:, the slowest folding proteins require many minutes or hours to fold, primarily due to
5083:
4848:
3896:
2515:
2246:
2221:
1752:
7091:
5994:
5989:
5777:
5560:
5477:
5000:"Using simulations to provide the framework for experimental protein folding studies"
4975:
4940:
4781:
4746:
3370:
3353:
2593:
2548:
2152:
1864:
1544:
1462:
1372:
to be considered solved. Nevertheless, it is considered a significant achievement in
1214:
1201:
The energy funnel by which an unfolded polypeptide chain assumes its native structure
839:
819:
394:
382:
378:
342:
290:
262:
258:
170:
150:
124:
5381:
4747:"Protein folding funnels: a kinetic approach to the sequence-structure relationship"
4727:
4329:
3519:
3476:
3387:
2997:
2372:
2329:
617:
turn opaque). Protein thermal stability is far from constant, however; for example,
6958:
6940:
6203:
5695:
4922:
4885:
Dill KA, MacCallum JL (November 2012). "The protein-folding problem, 50 years on".
4804:
Sharma V, Kaila VR, Annila A (2009). "Protein folding as an evolutionary process".
2913:
2867:
2811:
2466:
1616:
1391:
1319:
1315:
1303:. First equilibrium folding simulations were done using implicit solvent model and
1094:
917:
909:
856:
852:
329:
271:
82:
6234:
4267:
4182:
3696:
3029:
3012:
1899:
1878:
Anfinsen CB (July 1973). "Principles that govern the folding of protein chains".
7034:
6902:
6014:
6004:
5736:
5700:
5132:
5115:
4825:
3945:
2980:
2963:
2731:
2682:
2312:
2295:
1436:
1281:
1113:
1098:
1057:
961:
897:
889:
885:
799:
756:
752:
681:
569:
486:
406:
247:
223:
138:
70:
38:
5544:
5220:
Proceedings of the National Academy of Sciences of the United States of America
5161:
Proceedings of the National Academy of Sciences of the United States of America
4945:
Proceedings of the National Academy of Sciences of the United States of America
4751:
Proceedings of the National Academy of Sciences of the United States of America
4490:
Proceedings of the National Academy of Sciences of the United States of America
4120:
Proceedings of the National Academy of Sciences of the United States of America
4079:
3417:
3199:
Proceedings of the National Academy of Sciences of the United States of America
3171:
3126:
2553:
Proceedings of the National Academy of Sciences of the United States of America
2237:
2026:
Proceedings of the National Academy of Sciences of the United States of America
1967:
Proceedings of the National Academy of Sciences of the United States of America
1466:
341:
that is mainly guided by hydrophobic interactions, formation of intramolecular
6864:
6292:
6198:
5941:
5818:
5762:
5746:
5715:
5468:
5408:
5114:
Kmiecik S, Gront D, Kolinski M, Wieteska L, Dawid AE, Kolinski A (July 2016).
5015:
3655:
3630:
3077:
2198:
1791:
1672:
1181:
1177:
1138:
787:, typically, experimental techniques for studying protein folding rely on the
692:
362:
358:
251:
128:
66:
30:
5624:
4662:
3777:
3760:
3736:
3719:
115:
formed by misfolded proteins, the infectious varieties of which are known as
6612:
6558:
6019:
5834:
5803:
5365:
5240:
5181:
4906:
4771:
4510:
4140:
3460:
3312:
3219:
2667:"Gene Birth Contributes to Structural Disorder Encoded by Overlapping Genes"
2633:
2583:
2056:
1987:
1345:
1300:
1287:
1008:
760:
747:
614:
594:
531:
5552:
5416:
5373:
5259:
5200:
5141:
5060:
5033:
4984:
4965:
4914:
4866:
4711:
4596:
4529:
4470:
4413:
4364:
4321:
4286:
4227:
4219:
4159:
4097:
4037:
3997:
3953:
3857:
3822:
3745:
3704:
3664:
3555:
3511:
3468:
3425:
3379:
3331:
3238:
3179:
3144:
3095:
3038:
2948:
2905:
2803:
2749:
2700:
2651:
2533:
2458:
2407:
2364:
2255:
2206:
2075:
2006:
1856:
1799:
1690:
1608:
751:
excessive degradation instead of folding and function leads to a number of
5068:
4790:
4719:
3905:
3879:
3786:
3272:
2989:
2859:
2830:
Hartl FU (June 1996). "Molecular chaperones in cellular protein folding".
2602:
2321:
1907:
1760:
1725:
1529:
524:
are a class of proteins that aid in the correct folding of other proteins
510:
6892:
6525:
6517:
6032:
5972:
5910:
5432:"DeepMind solves 50-year-old 'grand challenge' with protein folding A.I."
2498:
2415:
1353:
652:
particles during infection. Like GroES, gp31 forms a stable complex with
636:
585:
553:
446:
370:
298:
157:
62:
17:
5157:"Characterization of protein-folding pathways by reduced-space modeling"
4702:
4429:"Calcium modulates force sensing by the von Willebrand factor A2 domain"
3354:"Protein-misfolding diseases and chaperone-based therapeutic approaches"
2897:
2795:
2565:
2450:
1847:
1600:
218:
77:. This structure permits the protein to become biologically functional.
6643:
6530:
6490:
6421:
6416:
6411:
6406:
6401:
6356:
6183:
5982:
5977:
5876:
5583:
4452:
3111:"Effects of macromolecular crowding on protein folding and aggregation"
2356:
1838:
736:
697:
626:
599:
526:
450:
386:
161:
142:
112:
109:
105:
54:
4588:
4395:
4356:
4029:
3849:
3814:
3547:
1511:
1333:, a massively parallel supercomputer designed and built around custom
1240:
and experimental studies, and it has been used to improve methods for
6859:
6834:
6829:
6819:
6783:
6773:
6748:
6733:
6728:
6723:
6718:
6713:
6708:
6703:
6698:
6663:
6648:
6495:
6460:
6445:
6440:
6435:
6396:
6391:
6386:
6381:
6376:
6371:
6366:
6361:
6351:
6346:
6341:
6336:
6331:
5319:
5273:
4313:
2851:
1323:
1255:
There exists a saddle point in the energy funnel landscape where the
739:
573:
373:; however, a protein molecule may fold spontaneously during or after
2549:"Evidence for nonrandom hydrophobicity structures in protein chains"
1775:
4560:
1813:
Scalvini, Barbara; Sheikhhassani, Vahid; Mashaghi, Alireza (2021).
445:
value. Gibbs free energy in protein folding is directly related to
301:
residues. These non-covalent and covalent contacts take a specific
7050:
7040:
7030:
7015:
6997:
6992:
6987:
6982:
6914:
6874:
6839:
6813:
6798:
6793:
6788:
6743:
6738:
6693:
6688:
6683:
6653:
6638:
6535:
6504:
6485:
6480:
6475:
6470:
6455:
6450:
6430:
6326:
6319:
6315:
6305:
6300:
1634:(Third ed.). New York, NY: Garland Science. pp. 120–70.
1267:
941:
704:
653:
649:
648:
and able to substitute for it in the assembly of bacteriophage T4
645:
509:
428:
390:
229:
116:
37:
29:
5502:'The game has changed.' AI triumphs at solving protein structures
4484:
Jagannathan B, Elms PJ, Bustamante C, Marqusee S (October 2012).
3880:"Protein structure determination in solution by NMR spectroscopy"
2296:"Forces contributing to the conformational stability of proteins"
123:
are caused by the incorrect folding of some proteins because the
7065:
7045:
7025:
7020:
6970:
6953:
6909:
6897:
6879:
6869:
6668:
6658:
6633:
6628:
6573:
6568:
4545:"Biotinylation by proximity labelling favours unfolded proteins"
2271:"Torsion Angles and the Ramachnadran Plot in Protein Structures"
1739:
Jackson SE (1998). "How do small single-domain proteins fold?".
1357:
1334:
1027:
665:
146:
6238:
6036:
5849:
5628:
2714:
Foy SG, Wilson BA, Bertram J, Cordes MH, Masel J (April 2019).
1295:
can be used for simulating various aspects of protein folding.
6213:
6208:
4839:
Robson B, Vaithilingam A (2008). "Protein Folding Revisited".
1329:
Long continuous-trajectory simulations have been performed on
3255:
Marusich, EI; Kurochkina, LP; Mesyanzhinov, VV (April 1998).
2774:
Dobson CM (December 2003). "Protein folding and misfolding".
1579:
Selkoe DJ (December 2003). "Folding proteins in fatal ways".
1299:(MD) was used in simulations of protein folding and dynamics
613:
proteins to unfold or denature (this is why boiling makes an
85:. This structure is determined by the amino-acid sequence or
2020:
Rose GD, Fleming PJ, Banavar JR, Maritan A (November 2006).
1077:, ultrafast mixing of solutions, photochemical methods, and
640:) appears to be structurally and functionally homologous to
5845:
2220:
van den Berg B, Wain R, Dobson CM, Ellis RJ (August 2000).
1961:
Alexander PA, He Y, Chen Y, Orban J, Bryan PN (July 2007).
1465:, Johnson A, Lewis J, Raff M, Roberts K, Walters P (2002).
1232:) that are largely directed toward the native state. This "
783:
While inferences about protein folding can be made through
603:
402:
137:
of proteins is a process of transition from a folded to an
4643:
Journal de Chimie Physique et de Physico-Chimie Biologique
3574:"Phase Problem in X-ray Crystallography, and Its Solution"
2294:
Pace CN, Shirley BA, McNutt M, Gajiwala K (January 1996).
846:
Fluorescence spectroscopy can be used to characterize the
421:, depicted with psi and phi angles of allowable rotation.
4427:
Jakobi AJ, Mashaghi A, Tans SJ, Huizinga EG (July 2011).
727:, as well as intracellular aggregation diseases such as
1352:(AI) protein structure prediction program developed by
5116:"Coarse-Grained Protein Models and Their Applications"
1496:"The formation and stabilization of protein structure"
409:, the possible presence of cofactors and of molecular
5456:"Structural biology: How proteins got their close-up"
4806:
Physica A: Statistical Mechanics and Its Applications
3109:
van den Berg B, Ellis RJ, Dobson CM (December 1999).
1133:
unfolding of proteins involved in blood coagulation.
715:(mad cow disease), amyloid-related illnesses such as
1314:
Several large-scale computational projects, such as
1209:
of a protein during folding can be visualized as an
779:
Experimental techniques for studying protein folding
775:
to fold mutated proteins to render them functional.
7008:
6939:
6928:
6611:
6588:
6546:
6286:
6272:
6176:
6123:
6070:
5950:
5924:
5883:
5827:
5796:
5755:
5724:
5688:
5662:
5299:"The Folding@home Consortium (FAHC) – Folding@home"
3934:
Progress in Nuclear Magnetic Resonance Spectroscopy
3583:. Macmillan Publishers Ltd, Nature Publishing Group
3286:Porter, Lauren L.; Looger, Loren L. (5 June 2018).
2547:Irbäck A, Peterson C, Potthast F (September 1996).
1774:Kubelka J, Hofrichter J, Eaton WA (February 2004).
932:(VCD) techniques for proteins, currently involving
5496:
5494:
5214:Adhikari AN, Freed KF, Sosnick TR (October 2012).
2482:"On hydrophobicity correlations in protein chains"
2151:
1141:accesses multiple unfolding pathways under force.
1056:, the stimulus for folding can be a denaturant or
855:, to measure protein folding kinetics, generate a
27:Change of a linear protein chain to a 3D structure
3347:
3345:
3343:
3341:
3013:"Molecular chaperones in protein quality control"
254:that fold rapidly because they are stabilized by
5576:"CASP14 #s just came out and they're astounding"
3678:
3676:
3674:
2616:Wilson BA, Foy SG, Neme R, Masel J (June 2017).
676:Protein misfolding and neurodegenerative disease
108:are believed to result from the accumulation of
5518:
5516:
4054:Neudecker P, Lundström P, Kay LE (March 2009).
3292:Proceedings of the National Academy of Sciences
3288:"Extant fold-switching proteins are widespread"
3250:
3248:
956:Protein nuclear magnetic resonance spectroscopy
238:displaying hydrogen bonding within the backbone
4934:
4932:
3567:
3565:
2769:
2767:
2765:
2763:
2761:
2759:
2089:
2087:
2085:
584:have been found in all species examined, from
6250:
6048:
5861:
5640:
4683:
4681:
4679:
3017:Journal of Biochemistry and Molecular Biology
2879:
2877:
2825:
2823:
2821:
1471:Molecular Biology of the Cell; Fourth Edition
8:
5084:"Fragment-based Protein Folding Simulations"
4880:
4878:
4876:
4841:Molecular Biology of Protein Folding, Part B
3718:Monsellier E, Bedouelle H (September 2005).
3399:
3397:
2022:"A backbone-based theory of protein folding"
1931:
1929:
1927:
1925:
1923:
1921:
1919:
1917:
1064:Studies of folding with high time resolution
944:data for protein solutions in heavy water (D
4543:Minde DP, Ramakrishna M, Lilley KS (2018).
4380:"Protein folding and unfolding under force"
4378:Jagannathan B, Marqusee S (November 2013).
3724:Protein Engineering, Design & Selection
3605:Principles of Protein X-Ray Crystallography
1815:"Topological principles of protein folding"
1574:
1572:
1238:computational simulations of model proteins
6936:
6283:
6257:
6243:
6235:
6055:
6041:
6033:
5868:
5854:
5846:
5647:
5633:
5625:
924:Vibrational circular dichroism of proteins
5467:
5249:
5239:
5190:
5180:
5131:
5023:
4974:
4964:
4780:
4770:
4701:
4636:"Are there pathways for protein folding?"
4559:
4519:
4509:
4460:
4403:
4276:
4266:
4241:Minde DP, Maurice MM, Rüdiger SG (2012).
4149:
4139:
4087:
3895:
3776:
3735:
3654:
3608:. Springer Science & Business Media.
3369:
3321:
3311:
3257:"Chaperones in bacteriophage T4 assembly"
3228:
3218:
3134:
3085:
3028:
2979:
2739:
2690:
2641:
2592:
2582:
2564:
2523:
2497:
2311:
2245:
2065:
2055:
2045:
1996:
1986:
1846:
1680:
1519:
333:All forms of protein structure summarized
4018:Journal of the American Chemical Society
3504:10.1146/annurev.biochem.75.101304.123901
1473:. New York and London: Garland Science.
1344:In 2020 a team of researchers that used
1196:
1157:Computational studies of protein folding
1022:In a study focused on the folding of an
992:
798:
703:Aggregated proteins are associated with
455:
361:of the protein begins to fold while the
328:
217:
5004:Archives of Biochemistry and Biophysics
1454:
1427:Protein misfolding cyclic amplification
3972:Dynamics of Proteins and Nucleic Acids
3928:Zhuravleva A, Korzhnev DM (May 2017).
2480:Irbäck A, Sandelin E (November 2000).
634:, and the phage encoded gp31 protein (
551:protein folding experiments conducted
365:portion of the protein is still being
353:. The process of folding often begins
4109:
4107:
4049:
4047:
4011:
4009:
4007:
3965:
3963:
3923:
3921:
3919:
3917:
3915:
3873:
3871:
3869:
3867:
2941:10.1146/annurev-biochem-060208-092442
2187:Current Opinion in Structural Biology
1780:Current Opinion in Structural Biology
1467:"The Shape and Structure of Proteins"
1432:Protein structure prediction software
1213:. According to Joseph Bryngelson and
1004:chemical exchange saturation transfer
542:that may be involved in formation of
7:
5454:Stoddart, Charlotte (1 March 2022).
3052:Ojeda-May P, Garcia ME (July 2010).
2665:Willis S, Masel J (September 2018).
5574:@MoAlQuraishi (November 30, 2020).
5155:Kmiecik S, Kolinski A (July 2007).
4998:Rizzuti B, Daggett V (March 2013).
4175:Handbook of Biosensors and Biochips
3884:The Journal of Biological Chemistry
3765:The Journal of Biological Chemistry
3352:Chaudhuri TK, Paul S (April 2006).
1819:Physical Chemistry Chemical Physics
1776:"The protein folding 'speed limit'"
1718:10.1146/annurev.bi.59.070190.003215
1549:"3. Protein Structure and Function"
1193:Energy landscape of protein folding
1079:laser temperature jump spectroscopy
385:", the process also depends on the
7073:Prokaryotic ubiquitin-like protein
5597:Balls, Phillip (9 December 2020).
3980:10.1016/b978-0-12-411636-8.00006-7
3759:Park YC, Bedouelle H (July 1998).
1936:Voet D, Voet JG, Pratt CW (2016).
1124:Single-molecule force spectroscopy
1118:fast parallel proteolysis (FASTpp)
884:. In proteins, structures such as
25:
5599:"Behind the screens of AlphaFold"
1151:Intrinsically disordered proteins
1073:. Fast techniques in use include
900:. A denaturant melt measures the
325:Driving forces of protein folding
3635:Acta Crystallographica Section D
3371:10.1111/j.1742-4658.2006.05181.x
1555:. San Francisco: W. H. Freeman.
1219:principle of minimal frustration
1045:Dual polarisation interferometry
1040:Dual-polarization interferometry
1034:Dual-polarization interferometry
928:The more recent developments of
824:multiple isomorphous replacement
713:bovine spongiform encephalopathy
666:switches fold throughout the day
261:, as was first characterized by
131:for certain protein structures.
34:Protein before and after folding
5896:Post-translational modification
5680:Structure determination methods
1442:Time-resolved mass spectrometry
721:familial amyloid cardiomyopathy
6603:Mitochondrial targeting signal
6266:Posttranslational modification
5620:Human Proteome Folding Project
3406:Trends in Biochemical Sciences
3160:Trends in Biochemical Sciences
2622:Nature Ecology & Evolution
930:vibrational circular dichroism
686:A protein is considered to be
514:Example of a small eukaryotic
1:
4849:10.1016/S0079-6603(08)00405-4
3897:10.1016/S0021-9258(18)45665-7
3581:Encyclopedia of Life Sciences
3536:Accounts of Chemical Research
3492:Annual Review of Biochemistry
2929:Annual Review of Biochemistry
2516:10.1016/S0006-3495(00)76472-1
1753:10.1016/S1359-0278(98)00033-9
1706:Annual Review of Biochemistry
1291:techniques for computational
1089:, Brian Dyer, William Eaton,
1024:amyotrophic lateral sclerosis
316:Protein quarternary structure
6679:Ubiquitin-conjugating enzyme
5086:. University College London.
5049:Journal of Molecular Biology
4268:10.1371/journal.pone.0046147
4208:Journal of Molecular Biology
4183:10.1002/9780470061565.hbb055
3878:Wüthrich K (December 1990).
3697:10.1016/j.biochi.2015.11.013
3030:10.5483/BMBRep.2005.38.3.259
1900:10.1126/science.181.4096.223
1293:protein structure prediction
1242:protein structure prediction
6967:E2 SUMO-conjugating enzyme
6624:Ubiquitin-activating enzyme
5133:10.1021/acs.chemrev.6b00163
4939:Fersht AR (February 2000).
4826:10.1016/j.physa.2008.12.004
3946:10.1016/j.pnmrs.2016.10.002
2981:10.1096/fasebj.10.1.8566543
2732:10.1534/genetics.118.301719
2683:10.1534/genetics.118.301249
2313:10.1096/fasebj.10.1.8566551
2146:Pratt C, Cornely K (2004).
1417:Potential energy of protein
1264:Modeling of protein folding
940:data for protein crystals,
912:to measure protein folding
707:-related illnesses such as
214:Protein secondary structure
75:three-dimensional structure
69:, changes from an unstable
7124:
6950:E1 SUMO-activating enzyme
5937:Protein structural domains
5656:Protein tertiary structure
5545:10.1038/d41586-020-03348-4
4080:10.1016/j.bpj.2008.12.3907
3418:10.1016/j.tibs.2006.01.002
3172:10.1016/j.tibs.2006.05.001
2962:Shortle D (January 1996).
1938:Principles of Biochemistry
1362:global distance test (GDT)
1326:, target protein folding.
1037:
968:nuclear magnetic resonance
959:
882:circularly polarized light
870:
769:lysosomal storage diseases
679:
619:hyperthermophilic bacteria
540:peptidyl-prolyl isomerases
313:
284:Protein tertiary structure
281:
211:
196:
188:Process of protein folding
42:Results of protein folding
6224:Nucleic acid double helix
5469:10.1146/knowable-022822-1
5430:Shead, Sam (2020-11-30).
5409:10.1016/j.jmb.2010.10.023
5016:10.1016/j.abb.2012.12.015
3656:10.1107/S0907444903017815
3078:10.1016/j.bpj.2010.04.040
2275:www.proteinstructures.com
2199:10.1016/j.sbi.2010.10.008
1940:(Fifth ed.). Wiley.
1792:10.1016/j.sbi.2004.01.013
1673:10.1016/j.bpj.2020.10.003
1494:Anfinsen CB (July 1972).
835:Fluorescence spectroscopy
830:Fluorescence spectroscopy
773:pharmaceutical chaperones
744:European Medicines Agency
709:Creutzfeldt–Jakob disease
672:) proteins switch folds.
199:Protein primary structure
6564:Survival of motor neuron
3930:"Protein folding by NMR"
3778:10.1074/jbc.273.29.18052
3261:Biochemistry. Biokhimiia
3127:10.1093/emboj/18.24.6927
2238:10.1093/emboj/19.15.3870
906:denaturation temperature
500:intrinsically disordered
479:London Dispersion forces
397:), the concentration of
6930:Ubiquitin-like proteins
6889:Deubiquitinating enzyme
5366:10.1126/science.1208351
5241:10.1073/pnas.1209000109
5182:10.1073/pnas.0702265104
5101:(by Molecular Dynamics)
4907:10.1126/science.1219021
4772:10.1073/pnas.89.18.8721
4511:10.1073/pnas.1201800109
4141:10.1073/pnas.1611418113
3602:Drenth J (2007-04-05).
3461:10.1126/science.1079589
3313:10.1073/pnas.1800168115
3220:10.1073/pnas.0712334105
3011:Lee S, Tsai FT (2005).
2634:10.1038/s41559-017-0146
2584:10.1073/pnas.93.18.9533
2057:10.1073/pnas.0606843103
1988:10.1073/pnas.0700922104
1500:The Biochemical Journal
1370:protein folding problem
1350:artificial intelligence
1165:
598:High concentrations of
349:, and it is opposed by
6064:Biomolecular structure
5061:10.1006/jmbi.1998.2172
4966:10.1073/pnas.97.4.1525
4741:Leopold PE, Montal M,
4712:10.1002/prot.340210302
4663:10.1051/jcp/1968650044
4220:10.1006/jmbi.2001.5006
3737:10.1093/protein/gzi046
2408:10.1126/science.653353
2154:Essential Biochemistry
1632:Essential cell biology
1543:Berg JM, Tymoczko JL,
1277:
1217:, proteins follow the
1202:
999:
807:
518:
469:
437:
351:conformational entropy
334:
239:
227:
184:since the late 1960s.
43:
35:
7098:Biochemical reactions
6000:Photoreceptor protein
5732:Immunoglobulin domain
4433:Nature Communications
1397:Denaturation midpoint
1374:computational biology
1337:and interconnects by
1309:coarse-grained models
1271:
1200:
1135:von Willebrand factor
1017:X-ray crystallography
996:
894:equilibrium unfolding
848:equilibrium unfolding
811:X-ray crystallography
805:X-ray crystallography
802:
795:X-ray crystallography
513:
467:
432:
332:
233:
221:
182:computational biology
162:proline isomerization
127:does not produce the
65:as a linear chain of
41:
33:
5891:Protein biosynthesis
4634:Levinthal C (1968).
2618:"De Novo Gene Birth"
1741:Folding & Design
1105:and Lars Konermann.
950:quantum computations
746:approved the use of
536:disulfide isomerases
522:Molecular chaperones
475:hydrophobic collapse
434:Hydrophobic collapse
381:may be regarded as "
347:van der Waals forces
310:Quaternary structure
73:into a more ordered
6288:Heat shock proteins
6194:Protein engineering
5768:Leucine-rich repeat
5537:2020Natur.588..203C
5500:Robert F. Service,
5358:2011Sci...334..517L
5232:2012PNAS..10917442A
5173:2007PNAS..10412330K
4957:2000PNAS...97.1525F
4899:2012Sci...338.1042D
4818:2009PhyA..388..851S
4763:1992PNAS...89.8721L
4655:1968JCP....65...44L
4502:2012PNAS..10917820J
4445:2011NatCo...2..385J
4259:2012PLoSO...746147M
4132:2016PNAS..113E6939S
4126:(45): E6939–E6945.
4072:2009BpJ....96.2045N
4060:Biophysical Journal
3647:2003AcCrD..59.1881T
3631:"The phase problem"
3453:2003Sci...299..713H
3304:2018PNAS..115.5968P
3211:2008PNAS..10510949T
3070:2010BpJ....99..595O
3058:Biophysical Journal
2898:10.1038/nature10317
2844:1996Natur.381..571H
2796:10.1038/nature02261
2788:2003Natur.426..884D
2575:1996PNAS...93.9533I
2508:2000BpJ....79.2252I
2486:Biophysical Journal
2451:10.1038/nature02611
2443:2004Natur.430..101D
2400:1978Sci...200.1012T
2122:"Protein Structure"
2038:2006PNAS..10316623R
1979:2007PNAS..10411963A
1892:1973Sci...181..223A
1831:2021PCCP...2321316S
1825:(37): 21316–21328.
1661:Biophysical Journal
1601:10.1038/nature02264
1593:2003Natur.426..900S
1407:Folding (chemistry)
1339:D. E. Shaw Research
1274:Markov state models
1207:configuration space
1187:intermediate states
1173:Levinthal's paradox
1166:Levinthal's paradox
986:(T1 & T2), and
733:Parkinson's disease
717:Alzheimer's disease
582:Heat shock proteins
578:heat shock proteins
498:, which tend to be
339:spontaneous process
297:formed between two
244:secondary structure
208:Secondary structure
94:may remain unfolded
5510:, 30 November 2020
5278:boinc.bakerlab.org
4745:(September 1992).
4453:10.1038/ncomms1385
2357:10.1002/prot.24683
2128:. Nature Education
1839:10.1039/D1CP03390E
1412:Phi value analysis
1297:Molecular dynamics
1278:
1272:Folding@home uses
1203:
1075:neutron scattering
1054:circular dichroism
1000:
878:Circular dichroism
873:Circular dichroism
867:Circular dichroism
861:Phi value analysis
808:
644:chaperone protein
611:thermally unstable
563:denatured proteins
519:
516:heat shock protein
483:hydrophobic effect
470:
438:
425:Hydrophobic effect
383:folding themselves
355:co-translationally
335:
278:Tertiary structure
240:
236:beta pleated sheet
228:
96:, indicating that
44:
36:
7108:Protein structure
7085:
7084:
7081:
7080:
6590:Protein targeting
6584:
6583:
6232:
6231:
6030:
6029:
5932:Protein structure
5906:Protein targeting
5843:
5842:
5788:Trefoil knot fold
5670:Structural domain
5531:(7837): 203–204.
5460:Knowable Magazine
5098:"Protein folding"
4858:978-0-12-374595-8
4589:10.1021/bi4001529
4396:10.1002/bip.22321
4357:10.1021/cr400326k
4192:978-0-470-01905-4
4030:10.1021/ja3001419
3850:10.1021/cr0404390
3815:10.1021/bi300335r
3629:Taylor G (2003).
3615:978-0-387-33746-3
3572:Cowtan K (2001).
3548:10.1021/ar020073i
3298:(23): 5968–5973.
2165:978-0-471-39387-0
2107:978-0-7167-3268-6
2094:Fersht A (1999).
1947:978-1-118-91840-1
1641:978-0-8153-4454-4
1562:978-0-7167-4684-3
1512:10.1042/bj1280737
1480:978-0-8153-3218-3
1305:umbrella sampling
1026:involved protein
938:X-ray diffraction
934:Fourier transform
816:Fourier transform
789:gradual unfolding
755:diseases such as
670:Protein Data Bank
465:
443:Gibbs free energy
419:Ramachandran plot
295:disulfide bridges
234:An anti-parallel
193:Primary structure
102:neurodegenerative
87:primary structure
16:(Redirected from
7115:
6937:
6850:Ubiquitin ligase
6616:(ubiquitylation)
6554:Alpha crystallin
6284:
6259:
6252:
6245:
6236:
6219:Structural motif
6057:
6050:
6043:
6034:
6010:Phycobiliprotein
5968:Globular protein
5963:Membrane protein
5958:List of proteins
5870:
5863:
5856:
5847:
5828:Irregular folds:
5783:Thioredoxin fold
5706:Homeodomain fold
5649:
5642:
5635:
5626:
5607:
5606:
5594:
5588:
5587:
5571:
5565:
5564:
5520:
5511:
5498:
5489:
5488:
5486:
5484:
5471:
5451:
5445:
5444:
5442:
5441:
5427:
5421:
5420:
5392:
5386:
5385:
5352:(6055): 517–20.
5341:
5335:
5334:
5332:
5330:
5316:
5310:
5309:
5307:
5305:
5295:
5289:
5288:
5286:
5284:
5270:
5264:
5263:
5253:
5243:
5211:
5205:
5204:
5194:
5184:
5152:
5146:
5145:
5135:
5126:(14): 7898–936.
5120:Chemical Reviews
5111:
5105:
5104:
5102:
5094:
5088:
5087:
5079:
5073:
5072:
5044:
5038:
5037:
5027:
4995:
4989:
4988:
4978:
4968:
4936:
4927:
4926:
4893:(6110): 1042–6.
4882:
4871:
4870:
4836:
4830:
4829:
4801:
4795:
4794:
4784:
4774:
4738:
4732:
4731:
4705:
4685:
4674:
4673:
4671:
4665:. Archived from
4640:
4631:
4625:
4624:
4622:
4621:
4615:en.wikibooks.org
4607:
4601:
4600:
4572:
4566:
4565:
4563:
4549:
4540:
4534:
4533:
4523:
4513:
4481:
4475:
4474:
4464:
4424:
4418:
4417:
4407:
4375:
4369:
4368:
4345:Chemical Reviews
4340:
4334:
4333:
4314:10.1038/nmeth740
4297:
4291:
4290:
4280:
4270:
4238:
4232:
4231:
4203:
4197:
4196:
4170:
4164:
4163:
4153:
4143:
4111:
4102:
4101:
4091:
4051:
4042:
4041:
4013:
4002:
4001:
3967:
3958:
3957:
3925:
3910:
3909:
3899:
3890:(36): 22059–62.
3875:
3862:
3861:
3838:Chemical Reviews
3833:
3827:
3826:
3797:
3791:
3790:
3780:
3756:
3750:
3749:
3739:
3715:
3709:
3708:
3680:
3669:
3668:
3658:
3626:
3620:
3619:
3599:
3593:
3592:
3590:
3588:
3578:
3569:
3560:
3559:
3530:
3524:
3523:
3487:
3481:
3480:
3436:
3430:
3429:
3401:
3392:
3391:
3373:
3358:The FEBS Journal
3349:
3336:
3335:
3325:
3315:
3283:
3277:
3276:
3252:
3243:
3242:
3232:
3222:
3205:(31): 10949–54.
3190:
3184:
3183:
3155:
3149:
3148:
3138:
3115:The EMBO Journal
3106:
3100:
3099:
3089:
3049:
3043:
3042:
3032:
3008:
3002:
3001:
2983:
2959:
2953:
2952:
2924:
2918:
2917:
2892:(7356): 324–32.
2881:
2872:
2871:
2852:10.1038/381571a0
2827:
2816:
2815:
2782:(6968): 884–90.
2771:
2754:
2753:
2743:
2726:(4): 1345–1355.
2711:
2705:
2704:
2694:
2662:
2656:
2655:
2645:
2613:
2607:
2606:
2596:
2586:
2568:
2544:
2538:
2537:
2527:
2501:
2499:cond-mat/0010390
2477:
2471:
2470:
2426:
2420:
2419:
2394:(4345): 1012–8.
2383:
2377:
2376:
2340:
2334:
2333:
2315:
2291:
2285:
2284:
2282:
2281:
2269:Al-Karadaghi S.
2266:
2260:
2259:
2249:
2226:The EMBO Journal
2217:
2211:
2210:
2182:
2176:
2175:
2173:
2172:
2157:
2148:"Thermodynamics"
2143:
2137:
2136:
2134:
2133:
2118:
2112:
2111:
2091:
2080:
2079:
2069:
2059:
2049:
2032:(45): 16623–33.
2017:
2011:
2010:
2000:
1990:
1958:
1952:
1951:
1933:
1912:
1911:
1886:(4096): 223–30.
1875:
1869:
1868:
1850:
1810:
1804:
1803:
1771:
1765:
1764:
1736:
1730:
1729:
1701:
1695:
1694:
1684:
1652:
1646:
1645:
1627:
1621:
1620:
1576:
1567:
1566:
1540:
1534:
1533:
1523:
1491:
1485:
1484:
1459:
1422:Protein dynamics
1402:Downhill folding
1387:Anfinsen's dogma
1356:placed first in
1257:transition state
1211:energy landscape
1130:Optical tweezers
916:and to generate
785:mutation studies
639:
632:bacteriophage T4
630:is the host for
466:
291:covalent bonding
226:spiral formation
175:circuit topology
158:outside the cell
141:. It happens in
98:protein dynamics
51:physical process
21:
7123:
7122:
7118:
7117:
7116:
7114:
7113:
7112:
7103:Protein folding
7088:
7087:
7086:
7077:
7004:
6979:E3 SUMO ligase
6943:
6932:
6924:
6615:
6607:
6580:
6542:
6521:
6513:
6291:
6279:protein folding
6277:
6268:
6263:
6233:
6228:
6172:
6119:
6066:
6061:
6031:
6026:
5990:Fibrous protein
5946:
5920:
5916:Protein methods
5901:Protein folding
5879:
5874:
5844:
5839:
5823:
5809:Ferredoxin fold
5792:
5773:Flavodoxin fold
5751:
5720:
5684:
5675:Protein folding
5658:
5653:
5616:
5611:
5610:
5603:Chemistry World
5596:
5595:
5591:
5573:
5572:
5568:
5522:
5521:
5514:
5499:
5492:
5482:
5480:
5453:
5452:
5448:
5439:
5437:
5429:
5428:
5424:
5394:
5393:
5389:
5343:
5342:
5338:
5328:
5326:
5318:
5317:
5313:
5303:
5301:
5297:
5296:
5292:
5282:
5280:
5272:
5271:
5267:
5226:(43): 17442–7.
5213:
5212:
5208:
5167:(30): 12330–5.
5154:
5153:
5149:
5113:
5112:
5108:
5100:
5096:
5095:
5091:
5081:
5080:
5076:
5046:
5045:
5041:
5010:(1–2): 128–35.
4997:
4996:
4992:
4938:
4937:
4930:
4884:
4883:
4874:
4859:
4838:
4837:
4833:
4803:
4802:
4798:
4740:
4739:
4735:
4703:chem-ph/9411008
4687:
4686:
4677:
4669:
4638:
4633:
4632:
4628:
4619:
4617:
4609:
4608:
4604:
4583:(48): 8601–24.
4574:
4573:
4569:
4547:
4542:
4541:
4537:
4496:(44): 17820–5.
4483:
4482:
4478:
4426:
4425:
4421:
4377:
4376:
4372:
4342:
4341:
4337:
4299:
4298:
4294:
4240:
4239:
4235:
4205:
4204:
4200:
4193:
4172:
4171:
4167:
4113:
4112:
4105:
4053:
4052:
4045:
4024:(19): 8148–61.
4015:
4014:
4005:
3990:
3969:
3968:
3961:
3927:
3926:
3913:
3877:
3876:
3865:
3835:
3834:
3830:
3809:(24): 4807–21.
3799:
3798:
3794:
3771:(29): 18052–9.
3758:
3757:
3753:
3717:
3716:
3712:
3682:
3681:
3672:
3641:(11): 1881–90.
3628:
3627:
3623:
3616:
3601:
3600:
3596:
3586:
3584:
3576:
3571:
3570:
3563:
3532:
3531:
3527:
3489:
3488:
3484:
3447:(5607): 713–6.
3438:
3437:
3433:
3403:
3402:
3395:
3351:
3350:
3339:
3285:
3284:
3280:
3254:
3253:
3246:
3192:
3191:
3187:
3157:
3156:
3152:
3121:(24): 6927–33.
3108:
3107:
3103:
3051:
3050:
3046:
3010:
3009:
3005:
2961:
2960:
2956:
2926:
2925:
2921:
2883:
2882:
2875:
2838:(6583): 571–9.
2829:
2828:
2819:
2773:
2772:
2757:
2713:
2712:
2708:
2664:
2663:
2659:
2628:(6): 0146–146.
2615:
2614:
2610:
2566:chem-ph/9512004
2546:
2545:
2541:
2479:
2478:
2474:
2437:(6995): 101–5.
2428:
2427:
2423:
2385:
2384:
2380:
2351:(12): 3312–26.
2342:
2341:
2337:
2293:
2292:
2288:
2279:
2277:
2268:
2267:
2263:
2219:
2218:
2214:
2184:
2183:
2179:
2170:
2168:
2166:
2145:
2144:
2140:
2131:
2129:
2120:
2119:
2115:
2108:
2093:
2092:
2083:
2047:10.1.1.630.5487
2019:
2018:
2014:
1973:(29): 11963–8.
1960:
1959:
1955:
1948:
1935:
1934:
1915:
1877:
1876:
1872:
1812:
1811:
1807:
1773:
1772:
1768:
1738:
1737:
1733:
1703:
1702:
1698:
1667:(10): 2029–38.
1654:
1653:
1649:
1642:
1629:
1628:
1624:
1587:(6968): 900–4.
1578:
1577:
1570:
1563:
1542:
1541:
1537:
1493:
1492:
1488:
1481:
1461:
1460:
1456:
1451:
1446:
1382:
1266:
1195:
1168:
1159:
1147:
1145:Biotin painting
1126:
1111:
1087:Martin Gruebele
1066:
1042:
1036:
984:time relaxation
964:
958:
947:
926:
875:
869:
832:
797:
781:
765:cystic fibrosis
684:
678:
662:
635:
544:disulfide bonds
508:
456:
427:
327:
318:
312:
293:in the form of
286:
280:
242:Formation of a
216:
210:
201:
195:
190:
151:proteinopathies
47:Protein folding
28:
23:
22:
15:
12:
11:
5:
7121:
7119:
7111:
7110:
7105:
7100:
7090:
7089:
7083:
7082:
7079:
7078:
7076:
7075:
7069:
7068:
7063:
7058:
7053:
7048:
7043:
7038:
7028:
7023:
7018:
7012:
7010:
7006:
7005:
7003:
7002:
7001:
7000:
6995:
6990:
6985:
6976:
6975:
6974:
6973:
6964:
6963:
6962:
6961:
6956:
6947:
6945:
6934:
6926:
6925:
6923:
6922:
6917:
6912:
6906:
6905:
6900:
6895:
6885:
6884:
6883:
6882:
6877:
6872:
6867:
6862:
6857:
6845:
6844:
6843:
6842:
6837:
6832:
6827:
6822:
6817:
6811:
6806:
6801:
6796:
6791:
6786:
6781:
6776:
6771:
6766:
6761:
6756:
6751:
6746:
6741:
6736:
6731:
6726:
6721:
6716:
6711:
6706:
6701:
6696:
6691:
6686:
6674:
6673:
6672:
6671:
6666:
6661:
6656:
6651:
6646:
6641:
6636:
6631:
6619:
6617:
6609:
6608:
6606:
6605:
6600:
6598:Signal peptide
6594:
6592:
6586:
6585:
6582:
6581:
6579:
6578:
6577:
6576:
6571:
6561:
6556:
6550:
6548:
6544:
6543:
6541:
6540:
6539:
6538:
6533:
6528:
6523:
6519:
6515:
6511:
6501:
6500:
6499:
6498:
6493:
6488:
6483:
6478:
6473:
6468:
6463:
6458:
6453:
6448:
6443:
6438:
6427:
6426:
6425:
6424:
6419:
6414:
6409:
6404:
6399:
6394:
6389:
6384:
6379:
6374:
6369:
6364:
6359:
6354:
6349:
6344:
6339:
6334:
6323:
6322:
6313:
6308:
6303:
6297:
6295:
6281:
6270:
6269:
6264:
6262:
6261:
6254:
6247:
6239:
6230:
6229:
6227:
6226:
6221:
6216:
6211:
6206:
6201:
6196:
6191:
6189:Protein domain
6186:
6180:
6178:
6174:
6173:
6171:
6170:
6168:Thermodynamics
6165:
6160:
6155:
6150:
6145:
6140:
6135:
6129:
6127:
6121:
6120:
6118:
6117:
6115:Thermodynamics
6112:
6107:
6102:
6097:
6092:
6087:
6082:
6076:
6074:
6068:
6067:
6062:
6060:
6059:
6052:
6045:
6037:
6028:
6027:
6025:
6024:
6023:
6022:
6017:
6012:
6002:
5997:
5992:
5987:
5986:
5985:
5980:
5975:
5965:
5960:
5954:
5952:
5948:
5947:
5945:
5944:
5939:
5934:
5928:
5926:
5922:
5921:
5919:
5918:
5913:
5908:
5903:
5898:
5893:
5887:
5885:
5881:
5880:
5875:
5873:
5872:
5865:
5858:
5850:
5841:
5840:
5838:
5837:
5831:
5829:
5825:
5824:
5822:
5821:
5816:
5814:Ribonuclease A
5811:
5806:
5800:
5798:
5794:
5793:
5791:
5790:
5785:
5780:
5775:
5770:
5765:
5759:
5757:
5753:
5752:
5750:
5749:
5744:
5742:Beta-propeller
5739:
5734:
5728:
5726:
5722:
5721:
5719:
5718:
5713:
5711:Alpha solenoid
5708:
5703:
5698:
5692:
5690:
5686:
5685:
5683:
5682:
5677:
5672:
5666:
5664:
5660:
5659:
5654:
5652:
5651:
5644:
5637:
5629:
5623:
5622:
5615:
5614:External links
5612:
5609:
5608:
5589:
5582:) – via
5566:
5512:
5490:
5446:
5422:
5387:
5336:
5311:
5290:
5274:"Rosetta@home"
5265:
5206:
5147:
5106:
5089:
5074:
5039:
4990:
4928:
4872:
4857:
4831:
4796:
4757:(18): 8721–5.
4733:
4675:
4672:on 2009-09-02.
4626:
4602:
4567:
4561:10.1101/274761
4535:
4476:
4419:
4370:
4335:
4302:Nature Methods
4292:
4253:(10): e46147.
4233:
4198:
4191:
4165:
4103:
4066:(6): 2045–54.
4043:
4003:
3988:
3959:
3911:
3863:
3844:(5): 1769–84.
3828:
3792:
3751:
3710:
3670:
3621:
3614:
3594:
3561:
3542:(12): 911–21.
3525:
3482:
3431:
3393:
3364:(7): 1331–49.
3337:
3278:
3267:(4): 399–406.
3244:
3185:
3166:(7): 395–401.
3150:
3101:
3044:
3003:
2954:
2919:
2873:
2817:
2755:
2706:
2677:(1): 303–313.
2657:
2608:
2559:(18): 9533–8.
2539:
2472:
2421:
2378:
2335:
2286:
2261:
2232:(15): 3870–5.
2212:
2177:
2164:
2138:
2113:
2106:
2081:
2012:
1953:
1946:
1913:
1870:
1805:
1766:
1731:
1696:
1647:
1640:
1622:
1568:
1561:
1535:
1486:
1479:
1453:
1452:
1450:
1447:
1445:
1444:
1439:
1434:
1429:
1424:
1419:
1414:
1409:
1404:
1399:
1394:
1389:
1383:
1381:
1378:
1265:
1262:
1234:folding funnel
1194:
1191:
1167:
1164:
1158:
1155:
1146:
1143:
1125:
1122:
1110:
1107:
1091:Sheena Radford
1065:
1062:
1038:Main article:
1035:
1032:
960:Main article:
957:
954:
945:
925:
922:
871:Main article:
868:
865:
840:quantum yields
831:
828:
796:
793:
780:
777:
725:polyneuropathy
680:Main article:
677:
674:
661:
660:Fold switching
658:
624:The bacterium
602:, extremes of
507:
504:
426:
423:
379:macromolecules
377:. While these
357:, so that the
343:hydrogen bonds
326:
323:
314:Main article:
311:
308:
282:Main article:
279:
276:
259:hydrogen bonds
256:intramolecular
212:Main article:
209:
206:
197:Main article:
194:
191:
189:
186:
139:unfolded state
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
7120:
7109:
7106:
7104:
7101:
7099:
7096:
7095:
7093:
7074:
7071:
7070:
7067:
7064:
7062:
7059:
7057:
7054:
7052:
7049:
7047:
7044:
7042:
7039:
7036:
7032:
7029:
7027:
7024:
7022:
7019:
7017:
7014:
7013:
7011:
7007:
6999:
6996:
6994:
6991:
6989:
6986:
6984:
6981:
6980:
6978:
6977:
6972:
6969:
6968:
6966:
6965:
6960:
6957:
6955:
6952:
6951:
6949:
6948:
6946:
6944:(SUMOylation)
6942:
6938:
6935:
6931:
6927:
6921:
6918:
6916:
6913:
6911:
6908:
6907:
6904:
6901:
6899:
6896:
6894:
6890:
6887:
6886:
6881:
6878:
6876:
6873:
6871:
6868:
6866:
6863:
6861:
6858:
6856:
6853:
6852:
6851:
6847:
6846:
6841:
6838:
6836:
6833:
6831:
6828:
6826:
6823:
6821:
6818:
6815:
6812:
6810:
6807:
6805:
6802:
6800:
6797:
6795:
6792:
6790:
6787:
6785:
6782:
6780:
6777:
6775:
6772:
6770:
6767:
6765:
6762:
6760:
6757:
6755:
6752:
6750:
6747:
6745:
6742:
6740:
6737:
6735:
6732:
6730:
6727:
6725:
6722:
6720:
6717:
6715:
6712:
6710:
6707:
6705:
6702:
6700:
6697:
6695:
6692:
6690:
6687:
6685:
6682:
6681:
6680:
6676:
6675:
6670:
6667:
6665:
6662:
6660:
6657:
6655:
6652:
6650:
6647:
6645:
6642:
6640:
6637:
6635:
6632:
6630:
6627:
6626:
6625:
6621:
6620:
6618:
6614:
6610:
6604:
6601:
6599:
6596:
6595:
6593:
6591:
6587:
6575:
6572:
6570:
6567:
6566:
6565:
6562:
6560:
6557:
6555:
6552:
6551:
6549:
6545:
6537:
6534:
6532:
6529:
6527:
6524:
6522:
6516:
6514:
6508:
6507:
6506:
6503:
6502:
6497:
6494:
6492:
6489:
6487:
6484:
6482:
6479:
6477:
6474:
6472:
6469:
6467:
6464:
6462:
6459:
6457:
6454:
6452:
6449:
6447:
6444:
6442:
6439:
6437:
6434:
6433:
6432:
6429:
6428:
6423:
6420:
6418:
6415:
6413:
6410:
6408:
6405:
6403:
6400:
6398:
6395:
6393:
6390:
6388:
6385:
6383:
6380:
6378:
6375:
6373:
6370:
6368:
6365:
6363:
6360:
6358:
6355:
6353:
6350:
6348:
6345:
6343:
6340:
6338:
6335:
6333:
6330:
6329:
6328:
6325:
6324:
6321:
6317:
6314:
6312:
6309:
6307:
6304:
6302:
6299:
6298:
6296:
6294:
6289:
6285:
6282:
6280:
6275:
6271:
6267:
6260:
6255:
6253:
6248:
6246:
6241:
6240:
6237:
6225:
6222:
6220:
6217:
6215:
6212:
6210:
6207:
6205:
6202:
6200:
6197:
6195:
6192:
6190:
6187:
6185:
6182:
6181:
6179:
6175:
6169:
6166:
6164:
6161:
6159:
6156:
6154:
6153:Determination
6151:
6149:
6146:
6144:
6141:
6139:
6136:
6134:
6131:
6130:
6128:
6126:
6122:
6116:
6113:
6111:
6108:
6106:
6103:
6101:
6100:Determination
6098:
6096:
6093:
6091:
6088:
6086:
6083:
6081:
6078:
6077:
6075:
6073:
6069:
6065:
6058:
6053:
6051:
6046:
6044:
6039:
6038:
6035:
6021:
6018:
6016:
6013:
6011:
6008:
6007:
6006:
6003:
6001:
5998:
5996:
5995:Chromoprotein
5993:
5991:
5988:
5984:
5981:
5979:
5976:
5974:
5971:
5970:
5969:
5966:
5964:
5961:
5959:
5956:
5955:
5953:
5949:
5943:
5940:
5938:
5935:
5933:
5930:
5929:
5927:
5923:
5917:
5914:
5912:
5909:
5907:
5904:
5902:
5899:
5897:
5894:
5892:
5889:
5888:
5886:
5882:
5878:
5871:
5866:
5864:
5859:
5857:
5852:
5851:
5848:
5836:
5833:
5832:
5830:
5826:
5820:
5819:SH2-like fold
5817:
5815:
5812:
5810:
5807:
5805:
5802:
5801:
5799:
5795:
5789:
5786:
5784:
5781:
5779:
5778:Rossmann fold
5776:
5774:
5771:
5769:
5766:
5764:
5761:
5760:
5758:
5754:
5748:
5745:
5743:
5740:
5738:
5735:
5733:
5730:
5729:
5727:
5723:
5717:
5714:
5712:
5709:
5707:
5704:
5702:
5699:
5697:
5694:
5693:
5691:
5687:
5681:
5678:
5676:
5673:
5671:
5668:
5667:
5665:
5661:
5657:
5650:
5645:
5643:
5638:
5636:
5631:
5630:
5627:
5621:
5618:
5617:
5613:
5604:
5600:
5593:
5590:
5585:
5581:
5577:
5570:
5567:
5562:
5558:
5554:
5550:
5546:
5542:
5538:
5534:
5530:
5526:
5519:
5517:
5513:
5509:
5508:
5503:
5497:
5495:
5491:
5479:
5475:
5470:
5465:
5461:
5457:
5450:
5447:
5436:
5433:
5426:
5423:
5418:
5414:
5410:
5406:
5402:
5398:
5391:
5388:
5383:
5379:
5375:
5371:
5367:
5363:
5359:
5355:
5351:
5347:
5340:
5337:
5325:
5321:
5315:
5312:
5300:
5294:
5291:
5279:
5275:
5269:
5266:
5261:
5257:
5252:
5247:
5242:
5237:
5233:
5229:
5225:
5221:
5217:
5210:
5207:
5202:
5198:
5193:
5188:
5183:
5178:
5174:
5170:
5166:
5162:
5158:
5151:
5148:
5143:
5139:
5134:
5129:
5125:
5121:
5117:
5110:
5107:
5099:
5093:
5090:
5085:
5078:
5075:
5070:
5066:
5062:
5058:
5055:(3): 835–48.
5054:
5050:
5043:
5040:
5035:
5031:
5026:
5021:
5017:
5013:
5009:
5005:
5001:
4994:
4991:
4986:
4982:
4977:
4972:
4967:
4962:
4958:
4954:
4951:(4): 1525–9.
4950:
4946:
4942:
4935:
4933:
4929:
4924:
4920:
4916:
4912:
4908:
4904:
4900:
4896:
4892:
4888:
4881:
4879:
4877:
4873:
4868:
4864:
4860:
4854:
4850:
4846:
4842:
4835:
4832:
4827:
4823:
4819:
4815:
4812:(6): 851–62.
4811:
4807:
4800:
4797:
4792:
4788:
4783:
4778:
4773:
4768:
4764:
4760:
4756:
4752:
4748:
4744:
4737:
4734:
4729:
4725:
4721:
4717:
4713:
4709:
4704:
4699:
4696:(3): 167–95.
4695:
4691:
4684:
4682:
4680:
4676:
4668:
4664:
4660:
4656:
4652:
4648:
4644:
4637:
4630:
4627:
4616:
4612:
4606:
4603:
4598:
4594:
4590:
4586:
4582:
4578:
4571:
4568:
4562:
4557:
4553:
4546:
4539:
4536:
4531:
4527:
4522:
4517:
4512:
4507:
4503:
4499:
4495:
4491:
4487:
4480:
4477:
4472:
4468:
4463:
4458:
4454:
4450:
4446:
4442:
4438:
4434:
4430:
4423:
4420:
4415:
4411:
4406:
4401:
4397:
4393:
4390:(11): 860–9.
4389:
4385:
4381:
4374:
4371:
4366:
4362:
4358:
4354:
4351:(1): 660–76.
4350:
4346:
4339:
4336:
4331:
4327:
4323:
4319:
4315:
4311:
4308:(3): 207–12.
4307:
4303:
4296:
4293:
4288:
4284:
4279:
4274:
4269:
4264:
4260:
4256:
4252:
4248:
4244:
4237:
4234:
4229:
4225:
4221:
4217:
4214:(4): 865–73.
4213:
4209:
4202:
4199:
4194:
4188:
4184:
4180:
4176:
4169:
4166:
4161:
4157:
4152:
4147:
4142:
4137:
4133:
4129:
4125:
4121:
4117:
4110:
4108:
4104:
4099:
4095:
4090:
4085:
4081:
4077:
4073:
4069:
4065:
4061:
4057:
4050:
4048:
4044:
4039:
4035:
4031:
4027:
4023:
4019:
4012:
4010:
4008:
4004:
3999:
3995:
3991:
3989:9780124116368
3985:
3981:
3977:
3973:
3966:
3964:
3960:
3955:
3951:
3947:
3943:
3939:
3935:
3931:
3924:
3922:
3920:
3918:
3916:
3912:
3907:
3903:
3898:
3893:
3889:
3885:
3881:
3874:
3872:
3870:
3868:
3864:
3859:
3855:
3851:
3847:
3843:
3839:
3832:
3829:
3824:
3820:
3816:
3812:
3808:
3804:
3796:
3793:
3788:
3784:
3779:
3774:
3770:
3766:
3762:
3755:
3752:
3747:
3743:
3738:
3733:
3730:(9): 445–56.
3729:
3725:
3721:
3714:
3711:
3706:
3702:
3698:
3694:
3690:
3686:
3679:
3677:
3675:
3671:
3666:
3662:
3657:
3652:
3648:
3644:
3640:
3636:
3632:
3625:
3622:
3617:
3611:
3607:
3606:
3598:
3595:
3582:
3575:
3568:
3566:
3562:
3557:
3553:
3549:
3545:
3541:
3537:
3529:
3526:
3521:
3517:
3513:
3509:
3505:
3501:
3497:
3493:
3486:
3483:
3478:
3474:
3470:
3466:
3462:
3458:
3454:
3450:
3446:
3442:
3435:
3432:
3427:
3423:
3419:
3415:
3411:
3407:
3400:
3398:
3394:
3389:
3385:
3381:
3377:
3372:
3367:
3363:
3359:
3355:
3348:
3346:
3344:
3342:
3338:
3333:
3329:
3324:
3319:
3314:
3309:
3305:
3301:
3297:
3293:
3289:
3282:
3279:
3274:
3270:
3266:
3262:
3258:
3251:
3249:
3245:
3240:
3236:
3231:
3226:
3221:
3216:
3212:
3208:
3204:
3200:
3196:
3189:
3186:
3181:
3177:
3173:
3169:
3165:
3161:
3154:
3151:
3146:
3142:
3137:
3132:
3128:
3124:
3120:
3116:
3112:
3105:
3102:
3097:
3093:
3088:
3083:
3079:
3075:
3071:
3067:
3063:
3059:
3055:
3048:
3045:
3040:
3036:
3031:
3026:
3023:(3): 259–65.
3022:
3018:
3014:
3007:
3004:
2999:
2995:
2991:
2987:
2982:
2977:
2973:
2969:
2968:FASEB Journal
2965:
2958:
2955:
2950:
2946:
2942:
2938:
2934:
2930:
2923:
2920:
2915:
2911:
2907:
2903:
2899:
2895:
2891:
2887:
2880:
2878:
2874:
2869:
2865:
2861:
2857:
2853:
2849:
2845:
2841:
2837:
2833:
2826:
2824:
2822:
2818:
2813:
2809:
2805:
2801:
2797:
2793:
2789:
2785:
2781:
2777:
2770:
2768:
2766:
2764:
2762:
2760:
2756:
2751:
2747:
2742:
2737:
2733:
2729:
2725:
2721:
2717:
2710:
2707:
2702:
2698:
2693:
2688:
2684:
2680:
2676:
2672:
2668:
2661:
2658:
2653:
2649:
2644:
2639:
2635:
2631:
2627:
2623:
2619:
2612:
2609:
2604:
2600:
2595:
2590:
2585:
2580:
2576:
2572:
2567:
2562:
2558:
2554:
2550:
2543:
2540:
2535:
2531:
2526:
2521:
2517:
2513:
2509:
2505:
2500:
2495:
2492:(5): 2252–8.
2491:
2487:
2483:
2476:
2473:
2468:
2464:
2460:
2456:
2452:
2448:
2444:
2440:
2436:
2432:
2425:
2422:
2417:
2413:
2409:
2405:
2401:
2397:
2393:
2389:
2382:
2379:
2374:
2370:
2366:
2362:
2358:
2354:
2350:
2346:
2339:
2336:
2331:
2327:
2323:
2319:
2314:
2309:
2305:
2301:
2300:FASEB Journal
2297:
2290:
2287:
2276:
2272:
2265:
2262:
2257:
2253:
2248:
2243:
2239:
2235:
2231:
2227:
2223:
2216:
2213:
2208:
2204:
2200:
2196:
2192:
2188:
2181:
2178:
2167:
2161:
2156:
2155:
2149:
2142:
2139:
2127:
2123:
2117:
2114:
2109:
2103:
2100:. Macmillan.
2099:
2098:
2090:
2088:
2086:
2082:
2077:
2073:
2068:
2063:
2058:
2053:
2048:
2043:
2039:
2035:
2031:
2027:
2023:
2016:
2013:
2008:
2004:
1999:
1994:
1989:
1984:
1980:
1976:
1972:
1968:
1964:
1957:
1954:
1949:
1943:
1939:
1932:
1930:
1928:
1926:
1924:
1922:
1920:
1918:
1914:
1909:
1905:
1901:
1897:
1893:
1889:
1885:
1881:
1874:
1871:
1866:
1862:
1858:
1854:
1849:
1844:
1840:
1836:
1832:
1828:
1824:
1820:
1816:
1809:
1806:
1801:
1797:
1793:
1789:
1785:
1781:
1777:
1770:
1767:
1762:
1758:
1754:
1750:
1747:(4): R81-91.
1746:
1742:
1735:
1732:
1727:
1723:
1719:
1715:
1711:
1707:
1700:
1697:
1692:
1688:
1683:
1678:
1674:
1670:
1666:
1662:
1658:
1651:
1648:
1643:
1637:
1633:
1626:
1623:
1618:
1614:
1610:
1606:
1602:
1598:
1594:
1590:
1586:
1582:
1575:
1573:
1569:
1564:
1558:
1554:
1550:
1546:
1539:
1536:
1531:
1527:
1522:
1517:
1513:
1509:
1506:(4): 737–49.
1505:
1501:
1497:
1490:
1487:
1482:
1476:
1472:
1468:
1464:
1458:
1455:
1448:
1443:
1440:
1438:
1435:
1433:
1430:
1428:
1425:
1423:
1420:
1418:
1415:
1413:
1410:
1408:
1405:
1403:
1400:
1398:
1395:
1393:
1390:
1388:
1385:
1384:
1379:
1377:
1375:
1371:
1365:
1363:
1359:
1355:
1351:
1347:
1342:
1340:
1336:
1332:
1327:
1325:
1321:
1317:
1312:
1310:
1306:
1302:
1298:
1294:
1290:
1289:
1284:
1283:
1275:
1270:
1263:
1261:
1258:
1253:
1249:
1247:
1243:
1239:
1235:
1231:
1226:
1224:
1220:
1216:
1215:Peter Wolynes
1212:
1208:
1199:
1192:
1190:
1188:
1183:
1179:
1174:
1163:
1156:
1154:
1152:
1144:
1142:
1140:
1136:
1131:
1123:
1121:
1119:
1115:
1108:
1106:
1104:
1103:Bengt Nölting
1100:
1096:
1092:
1088:
1084:
1080:
1076:
1072:
1063:
1061:
1059:
1055:
1050:
1046:
1041:
1033:
1031:
1029:
1025:
1020:
1018:
1013:
1010:
1005:
995:
991:
989:
985:
981:
977:
973:
969:
963:
955:
953:
951:
943:
939:
935:
931:
923:
921:
919:
918:chevron plots
915:
911:
907:
903:
899:
895:
891:
887:
886:alpha helices
883:
879:
874:
866:
864:
862:
859:and derive a
858:
854:
849:
844:
841:
836:
829:
827:
825:
821:
820:phase problem
817:
812:
806:
801:
794:
792:
790:
786:
778:
776:
774:
770:
766:
762:
758:
754:
749:
745:
741:
738:
734:
730:
726:
722:
718:
714:
710:
706:
701:
699:
694:
689:
683:
675:
673:
671:
667:
659:
657:
655:
651:
647:
643:
638:
633:
629:
628:
622:
620:
616:
612:
607:
605:
601:
596:
592:
591:precipitation
587:
583:
579:
575:
571:
566:
564:
560:
556:
555:
549:
545:
541:
537:
533:
529:
528:
523:
517:
512:
505:
503:
501:
497:
491:
488:
484:
480:
476:
454:
452:
448:
444:
435:
431:
424:
422:
420:
414:
412:
408:
404:
400:
396:
395:lipid bilayer
392:
388:
384:
380:
376:
372:
368:
364:
360:
356:
352:
348:
344:
340:
337:Folding is a
331:
324:
322:
317:
309:
307:
304:
300:
296:
292:
285:
277:
275:
273:
268:
264:
263:Linus Pauling
260:
257:
253:
249:
248:alpha helices
245:
237:
232:
225:
220:
215:
207:
205:
200:
192:
187:
185:
183:
178:
176:
172:
171:contact order
167:
163:
159:
154:
152:
148:
144:
140:
136:
132:
130:
126:
125:immune system
122:
118:
114:
111:
107:
103:
99:
95:
90:
88:
84:
78:
76:
72:
68:
64:
60:
56:
52:
48:
40:
32:
19:
6941:SUMO protein
6278:
6204:Nucleic acid
6125:Nucleic acid
5900:
5725:All-β folds:
5696:Helix bundle
5689:All-α folds:
5674:
5602:
5592:
5569:
5528:
5524:
5505:
5481:. Retrieved
5459:
5449:
5438:. Retrieved
5434:
5425:
5403:(1): 43–48.
5400:
5397:J. Mol. Biol
5396:
5390:
5349:
5345:
5339:
5327:. Retrieved
5323:
5314:
5302:. Retrieved
5293:
5281:. Retrieved
5277:
5268:
5223:
5219:
5209:
5164:
5160:
5150:
5123:
5119:
5109:
5092:
5077:
5052:
5048:
5042:
5007:
5003:
4993:
4948:
4944:
4890:
4886:
4840:
4834:
4809:
4805:
4799:
4754:
4750:
4736:
4693:
4689:
4667:the original
4646:
4642:
4629:
4618:. Retrieved
4614:
4605:
4580:
4577:Biochemistry
4576:
4570:
4551:
4538:
4493:
4489:
4479:
4436:
4432:
4422:
4387:
4383:
4373:
4348:
4344:
4338:
4305:
4301:
4295:
4250:
4246:
4236:
4211:
4207:
4201:
4174:
4168:
4123:
4119:
4063:
4059:
4021:
4017:
3971:
3937:
3933:
3887:
3883:
3841:
3837:
3831:
3806:
3803:Biochemistry
3802:
3795:
3768:
3764:
3754:
3727:
3723:
3713:
3688:
3684:
3638:
3634:
3624:
3604:
3597:
3585:. Retrieved
3580:
3539:
3535:
3528:
3495:
3491:
3485:
3444:
3440:
3434:
3412:(3): 150–5.
3409:
3405:
3361:
3357:
3295:
3291:
3281:
3264:
3260:
3202:
3198:
3188:
3163:
3159:
3153:
3118:
3114:
3104:
3064:(2): 595–9.
3061:
3057:
3047:
3020:
3016:
3006:
2974:(1): 27–34.
2971:
2967:
2957:
2932:
2928:
2922:
2889:
2885:
2835:
2831:
2779:
2775:
2723:
2719:
2709:
2674:
2670:
2660:
2625:
2621:
2611:
2556:
2552:
2542:
2489:
2485:
2475:
2434:
2430:
2424:
2391:
2387:
2381:
2348:
2344:
2338:
2306:(1): 75–83.
2303:
2299:
2289:
2278:. Retrieved
2274:
2264:
2229:
2225:
2215:
2193:(1): 25–31.
2190:
2186:
2180:
2169:. Retrieved
2153:
2141:
2130:. Retrieved
2125:
2116:
2096:
2029:
2025:
2015:
1970:
1966:
1956:
1937:
1883:
1879:
1873:
1848:1887/3277889
1822:
1818:
1808:
1786:(1): 76–88.
1783:
1779:
1769:
1744:
1740:
1734:
1709:
1705:
1699:
1664:
1660:
1650:
1631:
1625:
1584:
1580:
1553:Biochemistry
1552:
1538:
1503:
1499:
1489:
1470:
1457:
1392:Chevron plot
1366:
1343:
1328:
1320:Folding@home
1316:Rosetta@home
1313:
1286:
1280:
1279:
1254:
1250:
1230:José Onuchic
1227:
1222:
1218:
1204:
1169:
1160:
1148:
1127:
1112:
1095:Chris Dobson
1067:
1049:conformation
1043:
1021:
1014:
1001:
965:
927:
910:stopped flow
876:
857:chevron plot
853:stopped flow
845:
833:
809:
782:
759:-associated
729:Huntington's
702:
685:
663:
641:
625:
623:
608:
567:
558:
552:
547:
525:
520:
492:
471:
439:
415:
375:biosynthesis
336:
319:
287:
272:peptide bond
241:
202:
179:
155:
135:Denaturation
133:
91:
83:native state
79:
46:
45:
7035:neddylation
6301:Hsp10/GroES
6293:Chaperonins
6015:Phytochrome
6005:Biliprotein
5737:Beta barrel
5701:Globin fold
4384:Biopolymers
3587:November 3,
1437:Proteopathy
1223:frustration
1114:Proteolysis
1109:Proteolysis
1099:Alan Fersht
1058:temperature
962:Protein NMR
902:free energy
898:temperature
890:beta sheets
757:antitrypsin
753:proteopathy
682:Proteopathy
570:random coil
487:amphiphilic
407:temperature
367:synthesized
303:topological
252:beta sheets
224:alpha helix
71:random coil
67:amino acids
53:by which a
7092:Categories
6327:Hsp40/DnaJ
6274:Chaperones
6199:Proteasome
6158:Prediction
6148:Quaternary
6105:Prediction
6095:Quaternary
5942:Proteasome
5925:Structures
5797:α+β folds:
5763:TIM barrel
5756:α/β folds:
5747:Beta helix
5716:Death fold
5440:2020-11-30
4743:Onuchic JN
4620:2016-11-05
3498:: 333–66.
2935:: 323–55.
2280:2016-11-26
2171:2016-11-26
2132:2016-11-26
1712:: 631–60.
1449:References
1182:picosecond
1178:nanosecond
1139:SH3 domain
1083:Harry Gray
998:timescale.
506:Chaperones
411:chaperones
363:C-terminal
359:N-terminus
129:antibodies
104:and other
6613:Ubiquitin
6559:Clusterin
6138:Secondary
6085:Secondary
6020:Lipocalin
5884:Processes
5835:Conotoxin
5804:DNA clamp
5561:227243204
5478:247206999
5082:Jones D.
4649:: 44–45.
3940:: 52–77.
3691:: 29–37.
3685:Biochimie
2158:. Wiley.
2042:CiteSeerX
1865:237583577
1463:Alberts B
1346:AlphaFold
1301:in silico
1288:ab initio
1009:spin echo
803:Steps of
761:emphysema
748:Tafamidis
688:misfolded
615:egg white
595:insoluble
576:known as
121:allergies
59:synthesis
18:Misfolded
6893:Ataxin 3
6177:See also
6143:Tertiary
6090:Tertiary
5973:Globulin
5911:Proteome
5877:Proteins
5553:33257889
5483:25 March
5417:20974152
5382:27988268
5374:22034434
5329:14 March
5320:"Foldit"
5304:14 March
5283:14 March
5260:23045636
5201:17636132
5142:27333362
5034:23266569
4985:10677494
4915:23180855
4867:19121702
4728:13838095
4690:Proteins
4597:24187909
4530:22949695
4471:21750539
4414:23784721
4365:24001118
4330:21364478
4322:15782190
4287:23056252
4247:PLOS ONE
4228:11575938
4160:27791136
4098:19289032
4038:22554188
3998:23954103
3954:28552172
3858:16683754
3823:22640394
3746:16087653
3705:26607240
3665:14573942
3556:16359163
3520:23797549
3512:16756495
3477:30829998
3469:12560553
3426:16473510
3388:23370420
3380:16689923
3332:29784778
3239:18664583
3180:16716593
3145:10601015
3096:20643079
3039:15943899
2998:24066207
2949:23746257
2906:21776078
2804:14685248
2750:30692195
2720:Genetics
2701:30026186
2671:Genetics
2652:28642936
2534:11053106
2459:15229605
2373:27113763
2365:25204743
2345:Proteins
2330:20021399
2256:10921869
2207:21111607
2126:Scitable
2076:17075053
2007:17609385
1857:34545868
1800:15102453
1691:33142107
1609:14685251
1547:(2002).
1545:Stryer L
1380:See also
1354:DeepMind
1071:dynamics
966:Protein
914:kinetics
767:and the
693:β-sheets
586:bacteria
559:in vivo.
554:in vitro
532:catalyst
447:enthalpy
371:ribosome
299:cysteine
267:backbone
106:diseases
63:ribosome
57:, after
6816:(CDC34)
6184:Protein
6133:Primary
6080:Primary
6072:Protein
5983:Albumin
5978:Edestin
5663:General
5584:Twitter
5533:Bibcode
5507:Science
5354:Bibcode
5346:Science
5324:fold.it
5251:3491489
5228:Bibcode
5192:1941469
5169:Bibcode
5069:9826519
5025:4084838
4953:Bibcode
4923:5756068
4895:Bibcode
4887:Science
4814:Bibcode
4791:1528885
4759:Bibcode
4720:7784423
4651:Bibcode
4552:bioRxiv
4521:3497811
4498:Bibcode
4462:3144584
4441:Bibcode
4439:: 385.
4405:4065244
4278:3463568
4255:Bibcode
4151:5111666
4128:Bibcode
4089:2717354
4068:Bibcode
3906:2266107
3787:9660761
3643:Bibcode
3449:Bibcode
3441:Science
3323:6003340
3300:Bibcode
3273:9556522
3230:2490668
3207:Bibcode
3136:1171756
3087:2905109
3066:Bibcode
2990:8566543
2914:4337671
2868:4347271
2860:8637592
2840:Bibcode
2812:1036192
2784:Bibcode
2741:6456324
2692:6116962
2643:5476217
2603:8790365
2571:Bibcode
2525:1301114
2504:Bibcode
2467:4315026
2439:Bibcode
2396:Bibcode
2388:Science
2322:8566551
2067:1636505
2034:Bibcode
1998:1906725
1975:Bibcode
1908:4124164
1888:Bibcode
1880:Science
1827:Bibcode
1761:9710577
1726:2197986
1682:7732725
1617:6451881
1589:Bibcode
1530:4565129
1521:1173893
1282:De novo
978:,
948:O), or
818:, the "
740:fibrils
737:amyloid
698:amyloid
642:E. coli
627:E. coli
600:solutes
574:enzymes
548:in vivo
527:in vivo
496:de novo
481:). The
451:entropy
387:solvent
369:by the
143:cooking
119:. Many
113:fibrils
110:amyloid
55:protein
49:is the
6860:Cullin
6163:Design
6110:Design
5559:
5551:
5525:Nature
5476:
5415:
5380:
5372:
5258:
5248:
5199:
5189:
5140:
5067:
5032:
5022:
4983:
4973:
4921:
4913:
4865:
4855:
4789:
4779:
4726:
4718:
4595:
4528:
4518:
4469:
4459:
4412:
4402:
4363:
4328:
4320:
4285:
4275:
4226:
4189:
4158:
4148:
4096:
4086:
4036:
3996:
3986:
3952:
3904:
3856:
3821:
3785:
3744:
3703:
3663:
3612:
3554:
3518:
3510:
3475:
3467:
3424:
3386:
3378:
3330:
3320:
3271:
3237:
3227:
3178:
3143:
3133:
3094:
3084:
3037:
2996:
2988:
2947:
2912:
2904:
2886:Nature
2866:
2858:
2832:Nature
2810:
2802:
2776:Nature
2748:
2738:
2699:
2689:
2650:
2640:
2601:
2591:
2532:
2522:
2465:
2457:
2431:Nature
2416:653353
2414:
2371:
2363:
2328:
2320:
2254:
2247:306593
2244:
2205:
2162:
2104:
2074:
2064:
2044:
2005:
1995:
1944:
1906:
1863:
1855:
1798:
1759:
1724:
1689:
1679:
1638:
1615:
1607:
1581:Nature
1559:
1528:
1518:
1477:
1324:Foldit
1246:design
637:P17313
405:, the
401:, the
173:, and
166:domain
117:prions
7051:ATG12
7041:FAT10
7031:NEDD8
7016:ISG15
7009:Other
6998:PIAS4
6993:PIAS3
6988:PIAS2
6983:PIAS1
6933:(UBL)
6915:BIRC6
6875:FANCL
6547:Other
6536:TRAP1
6505:Hsp90
6431:Hsp70
6320:GroEL
6316:HSP60
6311:Hsp47
6306:Hsp27
5951:Types
5580:Tweet
5557:S2CID
5474:S2CID
5378:S2CID
4976:26468
4919:S2CID
4782:49992
4724:S2CID
4698:arXiv
4670:(PDF)
4639:(PDF)
4548:(PDF)
4326:S2CID
3577:(PDF)
3516:S2CID
3473:S2CID
3384:S2CID
2994:S2CID
2910:S2CID
2864:S2CID
2808:S2CID
2594:38463
2561:arXiv
2494:arXiv
2463:S2CID
2369:S2CID
2326:S2CID
1861:S2CID
1613:S2CID
1348:, an
1335:ASICs
1331:Anton
976:TOCSY
942:FT-IR
705:prion
654:GroEL
650:virus
646:GroES
593:into
399:salts
391:water
147:burns
61:by a
7066:UBL5
7056:FUB1
7046:ATG8
7026:UFM1
7021:URM1
6971:UBC9
6959:SAE2
6954:SAE1
6920:UFC1
6910:ATG3
6903:CYLD
6898:USP6
6880:UBR1
6870:MDM2
6669:SAE1
6664:NAE1
6659:ATG7
6654:UBA7
6649:UBA6
6644:UBA5
6639:UBA3
6634:UBA2
6629:UBA1
6574:SMN2
6569:SMN1
5549:PMID
5485:2022
5435:CNBC
5413:PMID
5370:PMID
5331:2023
5306:2023
5285:2023
5256:PMID
5197:PMID
5138:PMID
5065:PMID
5030:PMID
4981:PMID
4911:PMID
4863:PMID
4853:ISBN
4787:PMID
4716:PMID
4593:PMID
4526:PMID
4467:PMID
4410:PMID
4361:PMID
4318:PMID
4283:PMID
4224:PMID
4187:ISBN
4156:PMID
4094:PMID
4034:PMID
3994:PMID
3984:ISBN
3950:PMID
3902:PMID
3854:PMID
3819:PMID
3783:PMID
3742:PMID
3701:PMID
3661:PMID
3610:ISBN
3589:2016
3552:PMID
3508:PMID
3465:PMID
3422:PMID
3376:PMID
3328:PMID
3269:PMID
3235:PMID
3176:PMID
3141:PMID
3092:PMID
3035:PMID
2986:PMID
2945:PMID
2902:PMID
2856:PMID
2800:PMID
2746:PMID
2697:PMID
2648:PMID
2599:PMID
2530:PMID
2455:PMID
2412:PMID
2361:PMID
2318:PMID
2252:PMID
2203:PMID
2160:ISBN
2102:ISBN
2072:PMID
2003:PMID
1942:ISBN
1904:PMID
1853:PMID
1796:PMID
1757:PMID
1722:PMID
1687:PMID
1636:ISBN
1605:PMID
1557:ISBN
1526:PMID
1475:ISBN
1358:CASP
1322:and
1244:and
1205:The
1028:SOD1
980:HSQC
972:COSY
888:and
731:and
719:and
538:and
449:and
250:and
222:The
7061:MUB
6865:CBL
6855:VHL
6848:E3
6677:E2
6622:E1
6491:12A
6422:C19
6417:C14
6412:C13
6407:C11
6402:C10
6357:B11
6214:RNA
6209:DNA
5541:doi
5529:588
5464:doi
5405:doi
5401:405
5362:doi
5350:334
5246:PMC
5236:doi
5224:109
5187:PMC
5177:doi
5165:104
5128:doi
5124:116
5057:doi
5053:284
5020:PMC
5012:doi
5008:531
4971:PMC
4961:doi
4903:doi
4891:338
4845:doi
4822:doi
4810:388
4777:PMC
4767:doi
4708:doi
4659:doi
4585:doi
4556:doi
4516:PMC
4506:doi
4494:109
4457:PMC
4449:doi
4400:PMC
4392:doi
4353:doi
4349:114
4310:doi
4273:PMC
4263:doi
4216:doi
4212:312
4179:doi
4146:PMC
4136:doi
4124:113
4084:PMC
4076:doi
4026:doi
4022:134
3976:doi
3942:doi
3938:100
3892:doi
3888:265
3846:doi
3842:106
3811:doi
3773:doi
3769:273
3732:doi
3693:doi
3689:121
3651:doi
3544:doi
3500:doi
3457:doi
3445:299
3414:doi
3366:doi
3362:273
3318:PMC
3308:doi
3296:115
3225:PMC
3215:doi
3203:105
3168:doi
3131:PMC
3123:doi
3082:PMC
3074:doi
3025:doi
2976:doi
2937:doi
2894:doi
2890:475
2848:doi
2836:381
2792:doi
2780:426
2736:PMC
2728:doi
2724:211
2687:PMC
2679:doi
2675:210
2638:PMC
2630:doi
2589:PMC
2579:doi
2520:PMC
2512:doi
2447:doi
2435:430
2404:doi
2392:200
2353:doi
2308:doi
2242:PMC
2234:doi
2195:doi
2062:PMC
2052:doi
2030:103
1993:PMC
1983:doi
1971:104
1896:doi
1884:181
1843:hdl
1835:doi
1788:doi
1749:doi
1714:doi
1677:PMC
1669:doi
1665:119
1597:doi
1585:426
1516:PMC
1508:doi
1504:128
1285:or
1180:or
988:NOE
723:or
393:or
7094::
6891::
6835:V2
6830:V1
6820:R2
6814:R1
6809:Q2
6804:Q1
6784:L6
6779:L4
6774:L3
6769:L2
6764:L1
6754:J2
6749:J1
6734:G2
6729:G1
6724:E3
6719:E2
6714:E1
6709:D3
6704:D2
6699:D1
6531:ER
6496:14
6461:4L
6446:1L
6441:1B
6436:1A
6397:C7
6392:C6
6387:C5
6382:C3
6377:C1
6372:B9
6367:B6
6362:B4
6352:B2
6347:B1
6342:A3
6337:A2
6332:A1
5601:.
5555:.
5547:.
5539:.
5527:.
5515:^
5504:,
5493:^
5472:.
5462:.
5458:.
5411:.
5399:.
5376:.
5368:.
5360:.
5348:.
5322:.
5276:.
5254:.
5244:.
5234:.
5222:.
5218:.
5195:.
5185:.
5175:.
5163:.
5159:.
5136:.
5122:.
5118:.
5063:.
5051:.
5028:.
5018:.
5006:.
5002:.
4979:.
4969:.
4959:.
4949:97
4947:.
4943:.
4931:^
4917:.
4909:.
4901:.
4889:.
4875:^
4861:.
4851:.
4820:.
4808:.
4785:.
4775:.
4765:.
4755:89
4753:.
4749:.
4722:.
4714:.
4706:.
4694:21
4692:.
4678:^
4657:.
4647:65
4645:.
4641:.
4613:.
4591:.
4581:52
4579:.
4554:.
4550:.
4524:.
4514:.
4504:.
4492:.
4488:.
4465:.
4455:.
4447:.
4435:.
4431:.
4408:.
4398:.
4388:99
4386:.
4382:.
4359:.
4347:.
4324:.
4316:.
4304:.
4281:.
4271:.
4261:.
4249:.
4245:.
4222:.
4210:.
4185:.
4177:.
4154:.
4144:.
4134:.
4122:.
4118:.
4106:^
4092:.
4082:.
4074:.
4064:96
4062:.
4058:.
4046:^
4032:.
4020:.
4006:^
3992:.
3982:.
3962:^
3948:.
3936:.
3932:.
3914:^
3900:.
3886:.
3882:.
3866:^
3852:.
3840:.
3817:.
3807:51
3805:.
3781:.
3767:.
3763:.
3740:.
3728:18
3726:.
3722:.
3699:.
3687:.
3673:^
3659:.
3649:.
3639:59
3637:.
3633:.
3579:.
3564:^
3550:.
3540:38
3538:.
3514:.
3506:.
3496:75
3494:.
3471:.
3463:.
3455:.
3443:.
3420:.
3410:31
3408:.
3396:^
3382:.
3374:.
3360:.
3356:.
3340:^
3326:.
3316:.
3306:.
3294:.
3290:.
3265:63
3263:.
3259:.
3247:^
3233:.
3223:.
3213:.
3201:.
3197:.
3174:.
3164:31
3162:.
3139:.
3129:.
3119:18
3117:.
3113:.
3090:.
3080:.
3072:.
3062:99
3060:.
3056:.
3033:.
3021:38
3019:.
3015:.
2992:.
2984:.
2972:10
2970:.
2966:.
2943:.
2933:82
2931:.
2908:.
2900:.
2888:.
2876:^
2862:.
2854:.
2846:.
2834:.
2820:^
2806:.
2798:.
2790:.
2778:.
2758:^
2744:.
2734:.
2722:.
2718:.
2695:.
2685:.
2673:.
2669:.
2646:.
2636:.
2624:.
2620:.
2597:.
2587:.
2577:.
2569:.
2557:93
2555:.
2551:.
2528:.
2518:.
2510:.
2502:.
2490:79
2488:.
2484:.
2461:.
2453:.
2445:.
2433:.
2410:.
2402:.
2390:.
2367:.
2359:.
2349:82
2347:.
2324:.
2316:.
2304:10
2302:.
2298:.
2273:.
2250:.
2240:.
2230:19
2228:.
2224:.
2201:.
2191:21
2189:.
2150:.
2124:.
2084:^
2070:.
2060:.
2050:.
2040:.
2028:.
2024:.
2001:.
1991:.
1981:.
1969:.
1965:.
1916:^
1902:.
1894:.
1882:.
1859:.
1851:.
1841:.
1833:.
1823:23
1821:.
1817:.
1794:.
1784:14
1782:.
1778:.
1755:.
1743:.
1720:.
1710:59
1708:.
1685:.
1675:.
1663:.
1659:.
1611:.
1603:.
1595:.
1583:.
1571:^
1551:.
1524:.
1514:.
1502:.
1498:.
1469:.
1318:,
1311:.
1189:.
1153:.
1120:.
1101:,
1097:,
1093:,
1085:,
1060:.
982:,
974:,
952:.
920:.
863:.
763:,
711:,
604:pH
413:.
403:pH
345:,
177:.
149:,
145:,
89:.
7037:)
7033:(
6840:Z
6825:S
6799:O
6794:N
6789:M
6759:K
6744:I
6739:H
6694:C
6689:B
6684:A
6526:β
6520:2
6518:α
6512:1
6510:α
6486:9
6481:8
6476:7
6471:6
6466:5
6456:4
6451:2
6318:/
6290:/
6276:/
6258:e
6251:t
6244:v
6056:e
6049:t
6042:v
5869:e
5862:t
5855:v
5648:e
5641:t
5634:v
5605:.
5586:.
5578:(
5563:.
5543::
5535::
5487:.
5466::
5443:.
5419:.
5407::
5384:.
5364::
5356::
5333:.
5308:.
5287:.
5262:.
5238::
5230::
5203:.
5179::
5171::
5144:.
5130::
5103:.
5071:.
5059::
5036:.
5014::
4987:.
4963::
4955::
4925:.
4905::
4897::
4869:.
4847::
4828:.
4824::
4816::
4793:.
4769::
4761::
4730:.
4710::
4700::
4661::
4653::
4623:.
4599:.
4587::
4564:.
4558::
4532:.
4508::
4500::
4473:.
4451::
4443::
4437:2
4416:.
4394::
4367:.
4355::
4332:.
4312::
4306:2
4289:.
4265::
4257::
4251:7
4230:.
4218::
4195:.
4181::
4162:.
4138::
4130::
4100:.
4078::
4070::
4040:.
4028::
4000:.
3978::
3956:.
3944::
3908:.
3894::
3860:.
3848::
3825:.
3813::
3789:.
3775::
3748:.
3734::
3707:.
3695::
3667:.
3653::
3645::
3618:.
3591:.
3558:.
3546::
3522:.
3502::
3479:.
3459::
3451::
3428:.
3416::
3390:.
3368::
3334:.
3310::
3302::
3275:.
3241:.
3217::
3209::
3182:.
3170::
3147:.
3125::
3098:.
3076::
3068::
3041:.
3027::
3000:.
2978::
2951:.
2939::
2916:.
2896::
2870:.
2850::
2842::
2814:.
2794::
2786::
2752:.
2730::
2703:.
2681::
2654:.
2632::
2626:1
2605:.
2581::
2573::
2563::
2536:.
2514::
2506::
2496::
2469:.
2449::
2441::
2418:.
2406::
2398::
2375:.
2355::
2332:.
2310::
2283:.
2258:.
2236::
2209:.
2197::
2174:.
2135:.
2110:.
2078:.
2054::
2036::
2009:.
1985::
1977::
1950:.
1910:.
1898::
1890::
1867:.
1845::
1837::
1829::
1802:.
1790::
1763:.
1751::
1745:3
1728:.
1716::
1693:.
1671::
1644:.
1619:.
1599::
1591::
1565:.
1532:.
1510::
1483:.
946:2
389:(
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.