191:
118:
424:
429:
572:
24:
577:
576:
578:
575:
437:
404:
700:
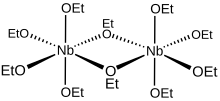
molecule in solution define a pair of octahedra sharing a common edge with the two niobium atoms located at their centres. From a bonding perspective, each niobium centre is surrounded octahedrally by four
779:
The most important reaction of niobium alkoxides is their hydrolysis to produce films and gels of niobium oxides. Although these reactions are complex, they can be described by this simplified equation:
523:
515:
680:
Metal alkoxides rarely adopt monomeric structures, and niobium(V) ethoxide is no exception. Early studies established that niobium alkoxides aggregate in solution as
234:
1133:
519:
373:
475:
346:
688:
established that the methoxide and isopropoxides of niobium adopt bioctahedral structures. From a geometric perspective, the ten ethoxide
593:
1099:
1066:
600:
205:
1126:
75:
985:
U. Schubert "Sol–Gel
Processing of Metal Compounds" Comprehensive Coordination Chemistry II 2003, Pages 629–656 Volume 7.
1482:
723:
more comprehensively represents this dimeric structure, though the simplified formula is commonly used for most purposes.
507:
637:
469:
169:
668:. It is a colorless liquid that dissolves in some organic solvents but hydrolyzes readily. It is mainly used for the
1119:
607:
861:
709:. The oxygen atoms of the bridging ethoxides are each bonded to both niobium centres, and these two ligands are
586:
125:
487:
945:
W. M. Haynes. CRC Handbook of
Chemistry and Physics, 93rd Edition. Physical Constants of Inorganic Compounds.
423:
1002:
958:
857:
511:
1210:
828:
begins above 325 – 350 °C. This can be observed with QMS as an increasing amount of ethanol and
428:
113:
1202:
1156:
495:
1378:
1218:
1190:
1005:; Holloway, C. E. (1968). "Nuclear Magnetic Resonance Studies on Niobium and Tantalum Penta-alkoxides".
736:
451:
416:
1417:
1255:
1242:
1050:
527:
1366:
1346:
1338:
1330:
1322:
1287:
1038:
716:
710:
186:
41:
483:
1477:
1314:
1279:
1263:
1020:
543:
499:
1398:
1354:
1271:
1226:
1142:
1095:
1062:
1042:
681:
95:
921:
1166:
1161:
1087:
1054:
1012:
986:
853:
539:
329:
257:
531:
1390:
1295:
1250:
1185:
1007:
732:
706:
685:
669:
51:
190:
117:
535:
631:
461:
1471:
990:
833:
318:
308:
106:
1084:
Atomic Layer
Deposition of High Permittivity Oxides: Film Growth and In Situ Studies
1024:
551:
503:
157:
702:
619:
1058:
479:
280:
86:
491:
457:
1016:
606:
599:
592:
585:
558:
1091:
298:
144:
126:
23:
829:
689:
1111:
630:
Except where otherwise noted, data are given for materials in their
74:
64:
547:
1115:
174:
864:
process. The decomposition reaction can be summarised as:
570:
856:
are the decomposition products released following an
1410:
1307:
1235:
1178:
1149:
221:InChI=1S/5C2H5O.Nb/c5*1-2-3;/h5*2H2,1H3;/q5*-1;+5
156:
574:
394:−6872.6 ± 1.7 kJ mol
367:−1583.9 ± 2.7 kJ mol
50:
1127:
8:
1043:"Recent Trends in Metal Alkoxide Chemistry"
652:is an metalorganic compound with formula Nb
1407:
1134:
1120:
1112:
189:
116:
94:
15:
29:Skeletal structure of niobium(V) ethoxide
981:
979:
672:of materials containing niobium oxides.
941:
939:
913:
239:
210:
185:
953:
951:
323:203 °C (397 °F; 476 K)
107:
285:318.209 g mol
214:Key: PNCDAXYMWHXGON-UHFFFAOYSA-N
7:
624:36 °C; 97 °F; 309 K
313:5 °C (41 °F; 278 K)
147:
1086:(Thesis). University of Helsinki.
816:The thermal decomposition of Nb(OC
14:
427:
422:
22:
1047:Progress in Inorganic Chemistry
1045:. In Karlin, Kenneth D. (ed.).
634:(at 25 °C , 100 kPa).
991:10.1016/B0-08-043748-6/06213-7
1:
731:This compound is prepared by
1499:
715:to one another within the
1059:10.1002/9780470166475.ch4
1041:; Singh, Anirudh (1997).
862:chemical vapor deposition
727:Preparation and reactions
707:bridging ethoxide ligands
686:crystallographic analysis
628:
403:
398:
339:
250:
230:
201:
34:
21:
959:"Niobium(5+) ethanolate"
470:Precautionary statements
922:"ChemSpider CSID:13600"
858:atomic layer deposition
335:N/A; reacts with water
692:oxygen atoms of the Nb
581:
1082:Rahtu, Antti (2002).
1051:John Wiley & Sons
737:niobium pentachloride
580:
303:1.258 g cm
242:CCO(OCC)(OCC)(OCC)OCC
1483:Niobium(V) compounds
1053:. pp. 239–454.
1017:10.1039/J19680000219
563:(fire diamond)
17:Niobium(V) Ethoxide
717:coordination sphere
650:Niobium(V) ethoxide
330:Solubility in water
18:
670:sol-gel processing
638:Infobox references
582:
293:Colourless liquid
16:
1465:
1464:
1461:
1460:
1143:Niobium compounds
646:Chemical compound
644:
643:
452:Hazard statements
170:CompTox Dashboard
76:Interactive image
1490:
1411:Organoniobium(V)
1408:
1136:
1129:
1122:
1113:
1106:
1105:
1079:
1073:
1072:
1049:. Vol. 46.
1039:Mehrotra, Ram C.
1035:
1029:
1028:
999:
993:
983:
974:
973:
971:
969:
963:webbook.nist.gov
955:
946:
943:
934:
933:
931:
929:
918:
854:niobium(V) oxide
609:
602:
595:
588:
573:
553:
549:
545:
541:
537:
533:
529:
525:
521:
517:
513:
509:
505:
501:
497:
493:
489:
485:
481:
477:
463:
459:
431:
426:
390:
363:
340:Thermochemistry
258:Chemical formula
194:
193:
178:
176:
160:
149:
128:
120:
109:
98:
78:
54:
26:
19:
1498:
1497:
1493:
1492:
1491:
1489:
1488:
1487:
1468:
1467:
1466:
1457:
1453:
1449:
1445:
1441:
1433:
1429:
1425:
1421:
1406:
1402:
1394:
1386:
1382:
1374:
1370:
1362:
1358:
1350:
1342:
1334:
1326:
1318:
1303:
1299:
1291:
1283:
1275:
1267:
1259:
1246:
1231:
1222:
1214:
1206:
1198:
1194:
1174:
1170:
1145:
1140:
1110:
1109:
1102:
1081:
1080:
1076:
1069:
1037:
1036:
1032:
1008:J. Chem. Soc. A
1001:
1000:
996:
984:
977:
967:
965:
957:
956:
949:
944:
937:
927:
925:
920:
919:
915:
910:
903:
899:
895:
891:
887:
883:
879:
875:
871:
851:
847:
843:
839:
827:
823:
819:
811:
807:
803:
799:
795:
791:
787:
774:
770:
766:
762:
758:
754:
751:10 NaOEt + Nb
746:
742:
733:salt metathesis
729:
722:
719:. The formula
699:
695:
678:
667:
663:
659:
655:
647:
640:
635:
614:
613:
612:
611:
604:
597:
590:
583:
579:
571:
472:
454:
440:
419:
391:
388:
382:
378:
375:
374:Std enthalpy of
364:
361:
355:
351:
348:
347:Std enthalpy of
332:
274:
270:
266:
260:
246:
243:
238:
237:
226:
223:
222:
216:
215:
209:
208:
197:
179:
172:
163:
150:
138:
101:
81:
68:
57:
44:
30:
27:
12:
11:
5:
1496:
1494:
1486:
1485:
1480:
1470:
1469:
1463:
1462:
1459:
1458:
1456:
1455:
1451:
1447:
1443:
1439:
1435:
1431:
1427:
1423:
1419:
1414:
1412:
1405:
1404:
1400:
1396:
1392:
1388:
1384:
1380:
1376:
1372:
1368:
1364:
1360:
1356:
1352:
1348:
1344:
1340:
1336:
1332:
1328:
1324:
1320:
1316:
1311:
1309:
1305:
1304:
1302:
1301:
1297:
1293:
1289:
1285:
1281:
1277:
1273:
1269:
1265:
1261:
1257:
1253:
1248:
1244:
1239:
1237:
1233:
1232:
1230:
1229:
1224:
1220:
1216:
1212:
1208:
1204:
1200:
1196:
1192:
1188:
1182:
1180:
1176:
1175:
1173:
1172:
1168:
1164:
1159:
1153:
1151:
1147:
1146:
1141:
1139:
1138:
1131:
1124:
1116:
1108:
1107:
1100:
1074:
1067:
1030:
1003:Bradley, D. C.
994:
975:
947:
935:
912:
911:
909:
906:
905:
904:
901:
897:
893:
889:
885:
881:
877:
873:
869:
849:
845:
841:
837:
825:
821:
817:
814:
813:
809:
805:
801:
797:
793:
789:
785:
777:
776:
772:
768:
764:
760:
756:
752:
744:
740:
728:
725:
720:
697:
693:
684:. Subsequent
677:
674:
665:
661:
657:
653:
645:
642:
641:
636:
632:standard state
629:
626:
625:
622:
616:
615:
605:
598:
591:
584:
569:
568:
567:
566:
564:
555:
554:
524:P305+P351+P338
516:P303+P361+P353
512:P301+P330+P331
473:
468:
465:
464:
455:
450:
447:
446:
441:
436:
433:
432:
420:
415:
412:
411:
401:
400:
396:
395:
392:
386:
380:
372:
369:
368:
365:
359:
353:
345:
342:
341:
337:
336:
333:
328:
325:
324:
321:
315:
314:
311:
305:
304:
301:
295:
294:
291:
287:
286:
283:
277:
276:
272:
268:
264:
261:
256:
253:
252:
248:
247:
245:
244:
241:
233:
232:
231:
228:
227:
225:
224:
220:
219:
217:
213:
212:
204:
203:
202:
199:
198:
196:
195:
182:
180:
168:
165:
164:
162:
161:
153:
151:
143:
140:
139:
137:
136:
132:
130:
122:
121:
111:
103:
102:
100:
99:
91:
89:
83:
82:
80:
79:
71:
69:
62:
59:
58:
56:
55:
47:
45:
40:
37:
36:
32:
31:
28:
13:
10:
9:
6:
4:
3:
2:
1495:
1484:
1481:
1479:
1476:
1475:
1473:
1454:
1436:
1434:
1416:
1415:
1413:
1409:
1403:
1397:
1395:
1389:
1387:
1377:
1375:
1365:
1363:
1353:
1351:
1345:
1343:
1337:
1335:
1329:
1327:
1321:
1319:
1313:
1312:
1310:
1306:
1300:
1294:
1292:
1286:
1284:
1278:
1276:
1270:
1268:
1262:
1260:
1254:
1252:
1249:
1247:
1241:
1240:
1238:
1234:
1228:
1225:
1223:
1217:
1215:
1209:
1207:
1201:
1199:
1189:
1187:
1184:
1183:
1181:
1177:
1171:
1165:
1163:
1160:
1158:
1155:
1154:
1152:
1148:
1144:
1137:
1132:
1130:
1125:
1123:
1118:
1117:
1114:
1103:
1101:952-10-0646-3
1097:
1093:
1089:
1085:
1078:
1075:
1070:
1068:9780470167045
1064:
1060:
1056:
1052:
1048:
1044:
1040:
1034:
1031:
1026:
1022:
1018:
1014:
1010:
1009:
1004:
998:
995:
992:
988:
982:
980:
976:
964:
960:
954:
952:
948:
942:
940:
936:
923:
917:
914:
907:
867:
866:
865:
863:
859:
855:
835:
834:Diethyl ether
831:
783:
782:
781:
750:
749:
748:
738:
734:
726:
724:
718:
714:
713:
708:
704:
691:
687:
683:
675:
673:
671:
651:
639:
633:
627:
623:
621:
618:
617:
610:
603:
596:
589:
565:
562:
561:
557:
556:
474:
471:
467:
466:
456:
453:
449:
448:
445:
442:
439:
435:
434:
430:
425:
421:
418:
414:
413:
409:
407:
402:
397:
393:
385:
377:
371:
370:
366:
358:
350:
344:
343:
338:
334:
331:
327:
326:
322:
320:
319:Boiling point
317:
316:
312:
310:
309:Melting point
307:
306:
302:
300:
297:
296:
292:
289:
288:
284:
282:
279:
278:
262:
259:
255:
254:
249:
240:
236:
229:
218:
211:
207:
200:
192:
188:
187:DTXSID5062924
184:
183:
181:
171:
167:
166:
159:
155:
154:
152:
146:
142:
141:
134:
133:
131:
129:
124:
123:
119:
115:
112:
110:
108:ECHA InfoCard
105:
104:
97:
93:
92:
90:
88:
85:
84:
77:
73:
72:
70:
66:
61:
60:
53:
49:
48:
46:
43:
39:
38:
33:
25:
20:
1437:
1179:Niobium(III)
1083:
1077:
1046:
1033:
1006:
997:
968:November 15,
966:. Retrieved
962:
926:. Retrieved
924:. ChemSpider
916:
815:
778:
730:
711:
679:
649:
648:
559:
443:
405:
383:
356:
35:Identifiers
1236:Niobium(IV)
1150:Niobium(II)
1092:10138/21065
1011:: 219–223.
928:17 November
703:monodentate
620:Flash point
438:Signal word
290:Appearance
251:Properties
114:100.019.814
1472:Categories
1308:Niobium(V)
908:References
832:released.
812:+ 10 HOEt
804:O → Nb
775:+ 10 NaCl
417:Pictograms
376:combustion
281:Molar mass
87:ChemSpider
63:3D model (
42:CAS Number
1478:Ethoxides
676:Structure
544:P403+P235
540:P370+P378
520:P304+P340
408:labelling
349:formation
135:221-795-2
127:EC Number
52:3236-82-6
1025:98638647
705:and two
560:NFPA 704
399:Hazards
96:21241198
892:+ 5 O(C
800:+ 5 H
739:(Et = C
299:Density
275:
145:PubChem
1379:NbO(NO
1367:Nb(ClO
1098:
1065:
1023:
852:, and
830:ethane
759:→ Nb
690:ligand
682:dimers
444:Danger
235:SMILES
158:520571
1391:LiNbO
1347:NbOCl
1021:S2CID
735:from
696:(OEt)
206:InChI
65:JSmol
1430:NbCl
1399:KNbO
1323:NbCl
1315:NbBr
1288:NbSe
1280:NbSe
1243:NbCl
1219:NbCl
1203:NbBr
1096:ISBN
1063:ISBN
970:2012
930:2012
884:→ Nb
552:P501
548:P405
536:P363
532:P321
528:P310
508:P280
504:P264
500:P260
496:P243
492:P242
488:P241
484:P240
480:P233
476:P210
462:H314
458:H226
1442:(OC
1339:NbI
1331:NbF
1296:NbO
1272:NbS
1264:NbI
1256:NbF
1251:NbC
1227:NbP
1211:NbF
1186:NbN
1167:NbB
1162:NbO
1157:NbS
1088:hdl
1055:doi
1013:doi
987:doi
872:(OC
860:or
836:, C
788:(OC
763:(OC
747:):
712:cis
656:(OC
608:COR
406:GHS
387:298
360:298
271:NbO
175:EPA
148:CID
1474::
1452:10
1438:Nb
1418:(C
1355:Nb
1191:Nb
1094:.
1061:.
1019:.
978:^
961:.
950:^
938:^
882:10
868:Nb
844:OC
798:10
784:Nb
773:10
757:10
755:Cl
698:10
666:10
550:,
546:,
542:,
538:,
534:,
530:,
526:,
522:,
518:,
514:,
510:,
506:,
502:,
498:,
494:,
490:,
486:,
482:,
478:,
460:,
410::
379:(Δ
352:(Δ
269:25
265:10
1450:)
1448:5
1446:H
1444:2
1440:2
1432:2
1428:2
1426:)
1424:5
1422:H
1420:5
1401:3
1393:3
1385:3
1383:)
1381:3
1373:5
1371:)
1369:4
1361:5
1359:O
1357:2
1349:3
1341:5
1333:5
1325:5
1317:5
1298:2
1290:3
1282:2
1274:2
1266:4
1258:4
1245:4
1221:3
1213:3
1205:3
1197:3
1195:S
1193:2
1169:2
1135:e
1128:t
1121:v
1104:.
1090::
1071:.
1057::
1027:.
1015::
989::
972:.
932:.
902:2
900:)
898:5
896:H
894:2
890:5
888:O
886:2
880:)
878:5
876:H
874:2
870:2
850:5
848:H
846:2
842:5
840:H
838:2
826:5
824:)
822:5
820:H
818:2
810:5
808:O
806:2
802:2
796:)
794:5
792:H
790:2
786:2
771:)
769:5
767:H
765:2
761:2
753:2
745:5
743:H
741:2
721:2
694:2
664:)
662:5
660:H
658:2
654:2
601:1
594:1
587:1
389:)
384:H
381:c
362:)
357:H
354:f
273:5
267:H
263:C
177:)
173:(
67:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.