1024:
245:
150:
1286:
both liver and kidney was decreased through the two highest doses. The mice fed for one year with nivalenol (also with the lower doses) were affected with severe leukopenia whereas the mice fed for two years had no differences in count of white blood cells. Also "no histopathological changes including tumours were found in liver, thymus, spleen, kidneys, stomach, adrenal glands, pituitary glands, ovaries, bone marrow, lymph node, brain and small intestines with or without
468:
458:
647:
463:
643:
1152:
1092:
7,8-dihydroxycalonectrin is formed. It further reacts spontaneously to 3,15-acetyl-deoxynivalenol via elimination of a hydrogen and formation of a keto-group at C8. The addition of a hydroxyl group at C4 controlled by TRI13 occurs and is acetylated under the help of TRI7. This yields 3,4,15-triacetylnivalenol (3,4,15-triANIV) from which it is than again the same synthesis as described above.
1128:, this cytokine plays a role in the mobility regulation of mononuclear leukocyte cells. Nivalenol causes CCL2 secretion to be lowered, and thus the mobility of monocytes to be reduced. This explains part of the immunosuppressive nature of nivalenol. Again, this effect is reduced by NF-κB inhibition which shows, that nivalenol and NF-κB interact to influence the cell.
27:
1168:
to be higher than normal. This suggests that nivalenol is present and later degraded in the liver as the liver is responsible for the segregation of cholesterol into the bloodstream. The higher amount of cholesterol in the blood leads then to a higher amount of filtered cholesterol by the kidneys and eventually to an increased concentration in the urea.
648:
1143:. Nivalenol is also known to influence human leukocyte proliferation. It has been shown that nivalenol can change proliferation rates of human leukocytes in a dose dependent manner. Lower concentrations are known to enhance leukocyte proliferation, while higher concentrations decrease proliferation in a dose dependent manner.
1197:
feeding. There were further no nivalenol metabolites found in feces or urine within the first three days. After a week of exposure to 2.5 or 5 mg of nivalenol per kg of body weight twice a day, a microbiological adaptation was seen as nivalenol metabolites (de-epoxidated nivalenol) could be found in feces and urine.
933:
in
Southeast Asia and Afghanistan. The Russian government however refuses to give a statement on these pieces of evidence. Furthermore, it has been shown that samples taken on the location of attacks contain these toxins, while sites that have not been attacked do not show any signs of toxins in them.
1302:
It was found that nivalenol effects the genes of
Chinese hamster V79 (CHO) cells by slightly increased frequencies of chromosomal aberrations and sister chromatid exchange. The DNA was damaged in CHO cells as well as in mice. In mice (given 20 mg nivalenol /kg bw orally or 3.7 mg /kg bw ip)
1171:
The lowered concentration of amides is assumed to be caused in the degradation process of the reactive epoxide group. Therefore, the epoxides are often found to react with amides or amide groups by adding a hydroxyl group at a primary or secondary amine. As a consequence the epoxide group is degraded
1167:
In the urine of tested mice and pigs 80% of the de-epoxidated compound and only 7% of the actual nivalenol were found showing a high metabolising rate of the trichodienes. Thereby a low concentration of nitrogen in low proteins and urea were observed whereas the cholesterol concentration was observed
998:
group, responsible for the reactivity for most parts, is attached at C12 and C13 in the tetrahydropyran. Only the remaining groups at positions C3, C4, C7, C15 vary for the different mycotoxins. In case of nivalenol each of the four remaining groups is a substituted hydroxyl group which add up to the
1091:
at C3, C4 and C15 resulting in the end product nivalenol. A partly alternative synthesis can occur when the catalysts TRI1 and TRI13, TRI7 are used in opposite order. Then the addition of the hydroxyl groups at C7 and C8 controlled by TRI1 are happening with calonectrin as reactant. In this reaction
932:
mycotoxins as biological weapons in
Southeast Asia in a very detailed manner, covering reports of survivors, eyewitnesses, prisoners of war and Soviet informants along with information on the presence of Soviet technicians and laboratories. This led to the conclusion that these toxins have been used
1285:
Female mice were fed with different doses of nivalenol (0, 0.7, 1.4 or 3.5 mg nivalenol /kg bw) for one or two years to investigate whether nivalenol is chronic toxic and/or carcinogenic. Also during this study a decrease in body weight and feed consumption was observed. The absolute weight of
1272:
The subchronic toxicity was tested by feeding mice with a daily dose of 0 to 3.5 mg nivalenol/ kg bw for 4 or 12 weeks. The observations after 4 weeks were reduced body weight and food consumption. The reduction in body weight can be explained by statistical decrease in organ weight in thymus,
1232:
The toxicity of nivalenol in humans is for the most parts unknown yet, but it was investigated in mice, rats and hamster cells. Thereby the toxicity was divided in the following topics: acute/subacute, subchronic, chronic and carcinogenicity, genotoxicity, developmental toxicity studies and studies
1159:
Nivalenol in mice is not only metabolized through the liver but also, for a lesser part through microbial detoxification in the intestines. Thereby especially the epoxide group as most toxic part of the molecule is degraded. This happens by eliminating the oxygen of the epoxide group resulting in a
895:
toxins under which nivalenol (0.03–0.1 mg/kg in 2 of 24 samples), deoxynivalenol (0.34–8.4 mg/kg in 11 of 24 samples) and acetyldeoxynivalenol (0.6–2.4 mg/kg in 4 of 24 samples) were found in rain-damaged wheat used for bread production. There were again no lethal cases and reported
1196:
Toxicity studies in swine that received a dose of 0.05 mg nivalenol/kg body weight twice daily showed no lethal effects. Most nivalenol was secreted with the feces and did not reach the bloodstream despite the fact that there was still nivalenol upstage over the intestines after 16 hours of
1077:
Isotrichodermin is converted to 15-decalonecitrin due to a substitution (encoded by TRI11) of one hydrogen by one hydroxyl at C15 which is then acetylated under help of TRI3. The same substitution and following acetylation reactions occur at C4 again under the control of TRI13 and TRI7. TRI1 in
1328:
in reproduction studies with nivalenol given by oral exposure was stated to be 1.4 mg/kg bw given in the feed throughout gestation and 5 mg/kg bw when given by gavage on days 7–15". Data from other species and on reproductive effects in adult males and females are not provided yet.
1319:
For developmental and reproduction studies pregnant mice were injected with different amounts of purified nivalenol on days 7–15 of gestation and for one additional study with mouldy rice containing nivalenol. The studies showed that the toxin is embryotoxic in mice. No evidence of
1358:
and immunoglobulin production induced by pokeweed, are inhibited by nivalenol. The effects of nivalenol are in the same range as same doses of deoxynivalenol, whereas the T-2 toxin are 100 fold more toxic. An additive effect is gained by combination of nivalenol with T-2 toxin,
646:
1294:) could not be derived from these studies. IARC (1993) concluded that there is inadequate evidence of carcinogenicity of nivalenol in experimental animals. No human data were available. The overall conclusion was that the carcinogenicity was not classifiable (group 3)".
1135:, which are also immunorelevant messenger molecules, nivalenol does inhibit their secretion. Nivalenol also upregulates the expression of proinflammatory genes in macrophages, displaying a mixed effect on different cell types. It does so even at cytotoxic levels.
1180:
Nivalenol did not yet find usage in medical treatments, and therefore it does not have known adverse effects besides the toxic effects described. It is however worth noting that it could be interesting for investigation due to its immunosuppressive effects.
1160:
double carbon-carbon bond between C12 and C13. This double bond is nonpolar and very stable leading to a less reactive form of nivalenol called de-epoxynivalenol. The de-epoxinated nivalenol gained is therefore much less toxic, same as every de-epoxinated
1273:
spleen and kidneys. Whereas the consumption time was less for female mice in comparison to male mice. After 12 weeks the toxin consumption resulted in reduction of relative organ weight in both males and females. Hereby only the liver was affected and no
973:
collected data from 15774 nivalenol occurrences in 18 European countries to be assessed. This led to the establishment of a TDI of 1.2 μg/kg bw per day. Nivalenol was in this studies not found to be genotoxic, but well haematotoxic and immunotoxic.
87:
1053:
In a further reaction trichotriol was gained through a shift of the C11 hydroxyl group of the isotrichotriol to the C9, similar the double bond shifted from C9=C10 to C10=C11. Trichotriol reacts in a non-enzymatic cyclization reaction to its
1050:(encoded by TRI4). Thereby hydroxyl groups were substituted to the carbon atoms C2, C3 and C11 and one oxygen was added to C12 and C13 facilitating the formation of an epoxide group. This results in the intermediate isotrichotriol.
1337:
Acute toxicity of nivalenol induces bone marrow toxicity and toxicity of lymphoid organs. Long-term exposure may result in erythropenia and leukopenia. In mice it was also observed that nivalenol increased the presence of serum
476:
438:
1204:
and leukopenia already noticed at lowest doses of 0.7 mg/kg of body weight per day. Lethal doses were dependent on the route of administration/intake of nivalenol. As nivalenol is normally taken up with feed the
1422:
1138:
Another mechanism of cytotoxicity of nivalenol is the apoptotic cytotoxicity showing that nivalenol is more toxic than its often co-occurring mycotoxin partner deoxynivalenol, and does so by causing DNA damage and
920:
and to have used them themselves in
Afghanistan. All three compounds could be identified in the vegetation at affected sites, whereas T-2 toxin could also be found in urine and blood samples of victims.
1870:
Sugita-Konishi, Y.; Pestka, J. J. (2001). "Differential upregulation of TNF-alpha, IL-6, and IL-8 production by deoxynivalenol (vomitoxin) and other 8-ketotrichothecenes in a human macrophage model".
827:
species belongs to the most prevalent mycotoxin producing fungi in the temperate regions of the northern hemisphere, therefore making them a considerable risk for the food crop production industry.
664:
928:" as described by witnesses. The toxins were delivered as a cloud of yellow dust or droplets. An article by L. R. Ember published in 1984 in Chemical Engineering News describes the use of
1193:
species it is often found in infected wheat and grain. As unprocessed wheat and grain product are often used as feed for livestock animals these are at a higher risk of nivalenol intake.
939:
There was a number of ways in which trichothecenes were weaponized, such as dispersion as aerosol, smoke, droplets or dust from aircraft, missiles, handheld devices or artillery.
746:
1433:
904:
infected cereals and is mainly via the route of digestion of uncontrolled wheat or other grains that are further processed or does enter the food chain via another route.
1256:
in intestine, in acute toxicity also lymphoid organs are included. Nivalenol given over time periods of 24 days in lower doses (ca. 3,5 mg/kg bw) showed significant
1991:
Sundstøl
Eriksen, G.; Pettersson, H.; Lundh, T. (2004). "Comparative cytotoxicity of deoxynivalenol, nivalenol, their acetylated derivatives and de-epoxy metabolites".
1913:
Minervini, F.; et al. (2004). "Toxicity and apoptosis induced by the mycotoxins nivalenol, deoxynivalenol and fumonisin B1 in a human erythroleukemia cell line".
1311:
changes were found upon histopathological examination. It can be concluded that an adequate evaluation of the genotoxicity is not allowed based on the available data.
1108:
that can be found in almost all human cells, and regulates the expression of its target genes by binding to specific motifs on the genomic DNA on regulatory elements.
1003:
group is capable of reacting with a proton promoting polarity and reactivity as well. But altogether the epoxide group is crucial for the reactivity of the molecule.
649:
936:
Even though it remains questionable if all witness reports are reliable sources of evidence, the symptoms recorded are typical for intoxication with trichothecenes.
924:
The best documented use of trichothecenes in warfare is the yellow rain controversy, a number of attacks in
Southeastern Asia, Laos and Afghanistan which used a "
291:
982:
Nivalenol as part of the family of mycotoxins has the common structure which all members of this toxin family have. This includes the basic structure of a
268:
InChI=1S/C15H20O7/c1-6-3-7-14(4-16,11(20)8(6)17)13(2)10(19)9(18)12(22-7)15(13)5-21-15/h3,7,9-12,16,18-20H,4-5H2,1-2H3/t7-,9-,10-,11-,12-,13-,14-,15+/m1/s1
863:
infected grains (scrabby grain disease) were reported in Japan, Korea and India. There have been no reports of lethal cases and only mild symptoms like
1084:
further catalyzes the addition of a fourth OH-group at C8 and a fifth OH-group at C7 at which then the hydrogen is eliminated and a keto group forms.
582:
278:
InChI=1/C15H20O6/c1-7-3-9-14(5-16,11(19)10(7)18)13(2)4-8(17)12(21-9)15(13)6-20-15/h3,8-9,11-12,16-17,19H,4-6H2,1-2H3/t8-,9-,11-,12-,13-,14-,15+/m1/s1
916:
have been used as biological warfare agents in Laos and
Cambodia as well as in Afghanistan. The Soviet Union has been alleged to have provided the
1605:
Venkataramana, M.; Chandranayaka, S.; Prakash, H. S.; Niranja, R. (2014). "na, S. (2014). Mycotoxins
Relevant to Biowarfare and Their Detection".
1212:
of oral administration which is 38.9 mg/kg of body weight per day in mice and 19.5 mg/kg per day in rats can be used as standard. The LD
671:
1031:
The synthesis of nivalenol is a 16 step process. It can differ in step 11 to step 14 depending on the order in which the reaction controlling
538:
1245:
of nivalenol was found to be 38.9 mg/kg bw in mice whereas the intraperitoneal, subcutaneous and intravenous routes of exposure gave
830:
The fungi are abundant in various agricultural products (cereal crops) and their further processed products (malt, beer and bread). "The
1948:
Taranu, I.; et al. (2010). "Comparative aspects of in vitro proliferation of human and porcine lymphocytes exposed to mycotoxins".
1011:
Nivalenol, deoxynivalenol]] and T2-toxin are the three structural and similar synthesized mycotoxins naturally appearing in fungi (e.g.
1252:
values of 5–10 mg/kg bw. In mice already within 3 days the most deaths occurred after oral exposure through marked congestion and
1700:
1290:". The lowest doses (0.7 mg nivalenol /kg bw) inhibited the growth and caused leukopenia. "A no observable adverse effect level (
999:
reactivity in presence of hydrophilic compounds or subgroups respectively thanks to their polar characteristics. In acidic medium the
1589:
259:
888:
In the same period two outbreaks involving over 100 cases were reported in India and China. These outbreaks were also non-lethal.
2074:
970:
1100:
Nivalenol causes a change in a number of different biological pathways. The most well known and probably important, is the
566:
790:
1120:. When treated with an NF-κB inhibitor, IL-8 secretion was lowered. Another important factor influenced by nivalenol is
1116:, which are important controller molecules of the immune system. Nivalenol induced the secretion of IL-8, a mediator of
948:
574:
532:
202:
223:
1039:
is used as starting compound for the synthesis of nivalenol. Its cyclization reaction to trichodiene is catalyzed by
614:
467:
770:
757:
415:
1488:"Scientific Opinion on risks for animal and public health related to the presence of nivalenol in food and feed".
2142:
2102:
1217:
1079:
657:
1549:"Scientific Opinion on risks for animal and public health related to the presence of nivalenol in food and feed"
900:, diarrhea, bloody stool and vomiting. These cases show that the main emerging danger of nivalenol comes from
694:
542:
457:
1074:(encoded by TRI101) catalyzes the acetylation of the C3 OH-group of isotrichodermol forming isotrichdermin.
1043:
cyclase trichodiene synthase (Tri5). This reaction is followed by several oxidation reactions catalyzed by
145:
2127:
1221:
990:
ring connected at C6 and C11. Additionally an ethyl-group connects the tetrahydropyran at C2 and C5 and a
952:
838:
708:
490:
462:
450:
1023:
1307:
and colon was damaged. The DNA of the thymus and liver was not effected. In organs with DNA damage no
891:
A well documented and acute outbreak in India in 1987 affected around 50,000 thousand people. Several
606:
1512:
Hedman, R.; Pettersson, H.; Lindberg, J.E. (2009). "Absorption and metabolism of nivalenol in pigs".
1164:, and can be segregated into the urine without having much toxic effects anymore (nearly non-toxic).
1161:
1105:
1032:
590:
586:
2132:
1351:
558:
240:
53:
1350:
in cultured human lymphocytes, proliferation of human male and female lymphocytes stimulated with
955:
of 0–0.7 μg/kg bw per day was issued after evaluation of the general toxicity as well as the
1973:
1895:
1246:
1201:
578:
546:
2107:
1458:
562:
2137:
2122:
2008:
1965:
1930:
1887:
1852:
1803:
1768:
1696:
1666:
1585:
1529:
1071:
834:
species invade and grow on crops, and may produce nivalenol under moist and cool conditions".
2035:
2000:
1957:
1922:
1879:
1842:
1834:
1795:
1758:
1750:
1656:
1646:
1610:
1560:
1521:
1287:
960:
550:
394:
314:
2026:
Pettersson, H.; Hedman, R. (1997). "Toxicity and metabolism of nivalenol in farm animals".
966:
In 2010 the
Japanese Food Safety Commission (FSCJ) issued a t-TDI of 0.4 μg/kg bw per day.
594:
211:
127:
63:
2147:
1343:
1067:
1044:
987:
876:
598:
244:
149:
610:
1847:
1822:
1763:
1738:
1661:
1634:
1321:
1274:
897:
882:
784:
107:
1926:
2116:
1347:
1088:
1047:
1036:
929:
857:
In the period of 1946 to 1963, several cases of intoxication due to the ingestion of
837:
In pigs, the symptoms observed after nivalenol exposure are "feed refusal, vomiting,
814:
383:
373:
138:
2097:
1977:
1717:
622:
2075:"Opinion of the Scientific Committee on Food on Fusarium Toxins Part 41: Nivalenol"
1614:
1257:
1117:
1059:
554:
1899:
496:
191:
1961:
1253:
1063:
983:
925:
734:
683:
1883:
1799:
1151:
2103:
https://www.sigmaaldrich.com/catalog/product/sigma/n7769?lang=en®ion=NL
2004:
1525:
1261:
1233:
on reproduction, immunotoxicity/hematotoxycity and effects on nervous system.
991:
405:
342:
118:
1565:
1548:
516:
1140:
1132:
956:
917:
913:
846:
810:
570:
512:
2012:
1969:
1934:
1891:
1856:
1807:
1772:
1670:
1386:
1342:, "accompanied by immunopathological changes in kidneys analogous to human
1838:
1754:
1651:
1633:
McCormick, S. P.; Stanley, A. M.; Stover, N. A.; Alexander, N. J. (2011).
1533:
508:
1547:
EFSA CONTAM Panel (EFSA Panel on
Contaminants in the Food Chain) (2013).
1355:
1308:
1113:
1101:
868:
859:
842:
819:
670:
663:
656:
629:
2039:
1304:
1040:
995:
872:
363:
178:
1200:
In rats and mice nivalenol showed to be toxic with adverse effects of
26:
1058:
isotrichodermol. In the reaction the hydroxyl group on the C2 of the
1055:
1000:
864:
1172:
and less nitrogen is present for the synthesis of proteins or urea.
783:
Except where otherwise noted, data are given for materials in their
504:
166:
1737:
Deshmaneand, S. L.; Kremlev, S.; Amini, S.; Sawaya, B. E. (2009).
1325:
1291:
1121:
524:
500:
98:
86:
76:
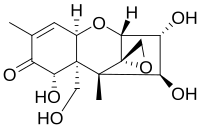
41:(3α,4β,7α)-12,13-epoxy-3,4,7,15-tetrahydroxy-trichothec-9-en-8-one
1070:
ring. The shifted OH-group at C9 is lost during the reaction. An
1786:
Nagashima, H.; et al. (2012). "Environ Toxicol Pharmacol".
1206:
1131:
It was shown that while deoxynivalenol induces the secretion of
1125:
618:
520:
157:
1339:
1112:
tests have shown, that nivalenol can change the expression of
2108:
https://pubchem.ncbi.nlm.nih.gov/compound/430146#section=Top
1087:
In a last step an esterase controlled by TRI8 catalyzes the
947:
In 2000 a scientific opinion on nivalenol was issued by the
602:
228:
1823:"Monocyte chemoattractant protein-1 (MCP-1): an overview"
1739:"Monocyte Chemoattractant Protein-1 (MCP-1): An Overview"
641:
1367:
About the nervous system no data has been provided yet.
908:
Weaponization and other instances of nivalenol poisoning
1695:. United States Government Printing. pp. 662–664.
1691:
Sidell, F. R.; Takafuji, E. T.; Franz, D. R. (1997).
817:
group. In nature it is mainly found in fungi of the
1635:"Trichothecenes: From Simple to Complex Mycotoxins"
1693:Medical Aspects of Chemical and Biological Warfare
1315:Developmental toxicity and studies on reproduction
1027:Synthesis pathways of nivalenol and deoxynivalenol
741:19.5 mg/kg (rats, oral), 38.9 mg/kg (mouse, oral)
378:222–223 °C (432–433 °F; 495–496 K)
2098:http://ccinfoweb2.ccohs.ca/hsdb/records/3517.html
1582:Handbook of Toxicology of Chemical Warfare Agents
881:could be isolated, which hints at a nivalenol or
994:group is attached at the cyclohexene at C8. The
963:. This t-TDI was reaffirmed by the SCF in 2002.
190:
1189:As nivalenol is a mycotoxic product of certain
645:
62:
1035:TRI1, TRI13 and TRI7 are catalyzing. Farnesyl
1827:Journal of Interferon & Cytokine Research
1743:Journal of Interferon & Cytokine Research
1490:European Food Safety Authority (EFSA) Journal
8:
1224:is between 7 and 7.5 mg/kg bw per day.
1359:4,15-diacetoxyscirpenol or deoxynivalenol.
845:and immunological dysfunction", as well as
243:
148:
126:
18:
1846:
1788:Environmental Toxicology and Pharmacology
1762:
1660:
1650:
1564:
1303:the DNA of kidney, bone marrow, stomach,
210:
1381:
1379:
1150:
1022:
912:Nivalenol as well as deoxynivalenol and
1375:
296:
264:
239:
2069:
1718:"HSDB: Hazardous Substances Data Bank"
1228:Toxicity, indications and side effects
943:Safety guidelines in the food industry
849:, resulting in a low leukocyte count.
702:525 °C (977 °F; 798 K)
139:
2067:
2065:
2063:
2061:
2059:
2057:
2055:
2053:
2051:
2049:
1821:Deshmane, S. L.; et al. (2009).
1686:
1684:
1682:
1680:
1417:
1415:
1413:
1411:
1409:
1407:
971:European Food Safety Authority (EFSA)
271:Key: UKOTXHQERFPCBU-XBXCNEFVSA-N
106:
7:
1628:
1626:
1624:
1584:. Academic Press. pp. 353–369.
1507:
1505:
1503:
1483:
1481:
1479:
1281:Chronic toxicity and carcinogenicity
299:CC1=C2O3(O)(O)(C)(34CO4)2(CO)(O)C1=O
688:5 °C (41 °F; 278 K)
181:
165:
1607:Biological Toxins and Bioterrorism
949:Scientific Committee on Food (SCF)
410:soluble in polar organic solvents
14:
1716:US National Library of Medicine.
466:
461:
456:
326:
25:
2034:(3). Akadémiai Kiadó: 423–427.
1185:Effects on animals and efficacy
787:(at 25 °C , 100 kPa).
2028:Cereal Research Communications
1615:10.1007/978-94-007-6645-7_32-1
1333:Immunotoxicity/haematotoxicity
1155:Structure of de-epoxynivalenol
953:tolerable daily intake (t-TDI)
332:
320:
1:
1927:10.1016/S0887-2333(03)00130-9
751:(US health exposure limits):
2082:Scientific Committee on Food
1993:Food and Chemical Toxicology
1962:10.1080/1745039X.2010.492140
723:or concentration (LD, LC):
2164:
1884:10.1080/152873901753246223
1800:10.1016/j.etap.2012.07.008
1580:Gupta, R. C., ed. (2015).
969:Between 2001 and 2011 the
2005:10.1016/j.fct.2003.11.006
1526:10.1080/17450399709386115
1514:Archiv für Tierernaehrung
1363:Effects on nervous system
875:pain. In these incidents
841:and dermal irritation or
781:
745:
719:
437:
432:
307:
287:
255:
46:
38:
33:
24:
1872:Toxicol Environ Health A
1566:10.2903/j.efsa.2013.3262
1062:binds to the C11 of the
978:Structure and reactivity
533:Precautionary statements
400:3.54*10^5 mg/L at 25 °C
1277:changes were observed.
1237:Acute/subacute toxicity
1156:
1028:
715:20 ppm (34 mg/m) Skin
652:
1839:10.1089/jir.2008.0027
1755:10.1089/jir.2008.0027
1652:10.3390/toxins3070802
1154:
1033:trichodiene synthases
1026:
709:Threshold limit value
651:
1106:transcription factor
1104:pathway. NF-κB is a
634:(fire diamond)
1268:Subchronic toxicity
1096:Mechanism of action
1081:F. sporotrichiodies
395:Solubility in water
350: g·mol
21:
2040:10.1007/BF03543746
1202:growth retardation
1157:
1029:
791:Infobox references
653:
19:
1430:Safety Data Sheet
1352:phytoheamagglutin
1275:histopathological
1222:subcutaneous (SC)
1072:acetyltransferase
799:Chemical compound
797:
796:
777:20 ppm (34 mg/m)
764:40 ppm (70 mg/m)
491:Hazard statements
224:CompTox Dashboard
88:Interactive image
16:Type of mycotoxin
2155:
2143:Tetrahydropyrans
2086:
2085:
2079:
2071:
2044:
2043:
2023:
2017:
2016:
1988:
1982:
1981:
1945:
1939:
1938:
1915:Toxicol in Vitro
1910:
1904:
1903:
1867:
1861:
1860:
1850:
1818:
1812:
1811:
1783:
1777:
1776:
1766:
1734:
1728:
1727:
1725:
1724:
1713:
1707:
1706:
1688:
1675:
1674:
1664:
1654:
1630:
1619:
1618:
1602:
1596:
1595:
1577:
1571:
1570:
1568:
1544:
1538:
1537:
1509:
1498:
1497:
1485:
1474:
1473:
1471:
1469:
1455:
1449:
1448:
1446:
1444:
1438:
1432:. Archived from
1427:
1419:
1402:
1401:
1399:
1397:
1383:
1324:was given. "The
1216:of intravenous,
673:
666:
659:
644:
624:
620:
616:
612:
608:
604:
600:
596:
592:
588:
584:
580:
576:
572:
568:
564:
560:
556:
552:
548:
544:
540:
526:
522:
518:
514:
510:
506:
502:
498:
470:
465:
460:
349:
334:
328:
322:
315:Chemical formula
248:
247:
232:
230:
214:
194:
183:
169:
152:
141:
130:
110:
90:
66:
29:
22:
20:Nivalenol (NIV)
2163:
2162:
2158:
2157:
2156:
2154:
2153:
2152:
2113:
2112:
2094:
2089:
2077:
2073:
2072:
2047:
2025:
2024:
2020:
1990:
1989:
1985:
1947:
1946:
1942:
1912:
1911:
1907:
1869:
1868:
1864:
1820:
1819:
1815:
1785:
1784:
1780:
1736:
1735:
1731:
1722:
1720:
1715:
1714:
1710:
1703:
1690:
1689:
1678:
1632:
1631:
1622:
1604:
1603:
1599:
1592:
1579:
1578:
1574:
1559:(6): 3262–119.
1546:
1545:
1541:
1511:
1510:
1501:
1496:(6): 1–5. 2013.
1487:
1486:
1477:
1467:
1465:
1457:
1456:
1452:
1442:
1440:
1436:
1425:
1421:
1420:
1405:
1395:
1393:
1391:Cayman Chemical
1385:
1384:
1377:
1373:
1365:
1344:IgA-nephropathy
1335:
1317:
1300:
1283:
1270:
1250:
1244:
1239:
1230:
1218:intraperitoneal
1215:
1210:
1187:
1178:
1176:Adverse effects
1149:
1098:
1068:tetrahydropyran
1045:cytochrome P450
1021:
1009:
1007:Available forms
988:tetrahydropyran
980:
945:
910:
885:contamination.
855:
847:haematotoxicity
800:
793:
788:
774:
761:
738:
732:
712:
699:
696:
678:
677:
676:
675:
668:
661:
654:
650:
642:
535:
493:
479:
453:
424:
397:
347:
337:
331:
325:
317:
303:
300:
295:
294:
283:
280:
279:
273:
272:
269:
263:
262:
251:
233:
226:
217:
197:
184:
172:
133:
113:
93:
80:
69:
56:
42:
17:
12:
11:
5:
2161:
2159:
2151:
2150:
2145:
2140:
2135:
2130:
2125:
2115:
2114:
2111:
2110:
2105:
2100:
2093:
2092:External links
2090:
2088:
2087:
2045:
2018:
1999:(4): 619–624.
1983:
1950:Arch Anim Nutr
1940:
1905:
1862:
1833:(6): 313–326.
1813:
1778:
1749:(6): 313–326.
1729:
1708:
1702:978-9997320919
1701:
1676:
1645:(7): 802–814.
1620:
1597:
1590:
1572:
1539:
1499:
1475:
1450:
1403:
1374:
1372:
1369:
1364:
1361:
1334:
1331:
1322:teratogenicity
1316:
1313:
1299:
1296:
1282:
1279:
1269:
1266:
1248:
1242:
1238:
1235:
1229:
1226:
1213:
1208:
1186:
1183:
1177:
1174:
1148:
1145:
1097:
1094:
1020:
1017:
1008:
1005:
979:
976:
961:immunotoxicity
951:. A temporary
944:
941:
909:
906:
898:abdominal pain
896:symptoms were
883:deoxynivalenol
878:F. graminaerum
854:
851:
798:
795:
794:
789:
785:standard state
782:
779:
778:
775:
769:
766:
765:
762:
756:
753:
752:
743:
742:
739:
730:
728:
725:
724:
717:
716:
713:
707:
704:
703:
700:
693:
690:
689:
686:
680:
679:
669:
662:
655:
640:
639:
638:
637:
635:
626:
625:
536:
531:
528:
527:
494:
489:
486:
485:
480:
475:
472:
471:
454:
449:
446:
445:
435:
434:
430:
429:
426:
422:
412:
411:
408:
402:
401:
398:
393:
390:
389:
386:
380:
379:
376:
370:
369:
366:
360:
359:
356:
352:
351:
345:
339:
338:
335:
329:
323:
318:
313:
310:
309:
305:
304:
302:
301:
298:
290:
289:
288:
285:
284:
282:
281:
277:
276:
274:
270:
267:
266:
258:
257:
256:
253:
252:
250:
249:
236:
234:
222:
219:
218:
216:
215:
207:
205:
199:
198:
196:
195:
187:
185:
177:
174:
173:
171:
170:
162:
160:
154:
153:
143:
135:
134:
132:
131:
123:
121:
115:
114:
112:
111:
103:
101:
95:
94:
92:
91:
83:
81:
74:
71:
70:
68:
67:
59:
57:
52:
49:
48:
44:
43:
40:
36:
35:
31:
30:
15:
13:
10:
9:
6:
4:
3:
2:
2160:
2149:
2146:
2144:
2141:
2139:
2136:
2134:
2131:
2129:
2128:Cyclohexenols
2126:
2124:
2121:
2120:
2118:
2109:
2106:
2104:
2101:
2099:
2096:
2095:
2091:
2083:
2076:
2070:
2068:
2066:
2064:
2062:
2060:
2058:
2056:
2054:
2052:
2050:
2046:
2041:
2037:
2033:
2029:
2022:
2019:
2014:
2010:
2006:
2002:
1998:
1994:
1987:
1984:
1979:
1975:
1971:
1967:
1963:
1959:
1956:(5): 383–93.
1955:
1951:
1944:
1941:
1936:
1932:
1928:
1924:
1920:
1916:
1909:
1906:
1901:
1897:
1893:
1889:
1885:
1881:
1878:(8): 619–36.
1877:
1873:
1866:
1863:
1858:
1854:
1849:
1844:
1840:
1836:
1832:
1828:
1824:
1817:
1814:
1809:
1805:
1801:
1797:
1794:(3): 1014–7.
1793:
1789:
1782:
1779:
1774:
1770:
1765:
1760:
1756:
1752:
1748:
1744:
1740:
1733:
1730:
1719:
1712:
1709:
1704:
1698:
1694:
1687:
1685:
1683:
1681:
1677:
1672:
1668:
1663:
1658:
1653:
1648:
1644:
1640:
1636:
1629:
1627:
1625:
1621:
1616:
1612:
1608:
1601:
1598:
1593:
1591:9780128001592
1587:
1583:
1576:
1573:
1567:
1562:
1558:
1554:
1550:
1543:
1540:
1535:
1531:
1527:
1523:
1519:
1515:
1508:
1506:
1504:
1500:
1495:
1491:
1484:
1482:
1480:
1476:
1464:
1460:
1454:
1451:
1439:on 2021-08-20
1435:
1431:
1424:
1418:
1416:
1414:
1412:
1410:
1408:
1404:
1392:
1388:
1382:
1380:
1376:
1370:
1368:
1362:
1360:
1357:
1353:
1349:
1348:blastogenesis
1345:
1341:
1332:
1330:
1327:
1323:
1314:
1312:
1310:
1306:
1297:
1295:
1293:
1289:
1288:Peyer's patch
1280:
1278:
1276:
1267:
1265:
1263:
1259:
1255:
1251:
1236:
1234:
1227:
1225:
1223:
1219:
1211:
1203:
1198:
1194:
1192:
1184:
1182:
1175:
1173:
1169:
1165:
1163:
1153:
1146:
1144:
1142:
1136:
1134:
1129:
1127:
1123:
1119:
1115:
1111:
1107:
1103:
1095:
1093:
1090:
1089:deacetylation
1085:
1083:
1082:
1075:
1073:
1069:
1065:
1061:
1057:
1051:
1049:
1048:monooxygenase
1046:
1042:
1038:
1037:pyrophosphate
1034:
1025:
1018:
1016:
1014:
1006:
1004:
1002:
997:
993:
989:
985:
977:
975:
972:
967:
964:
962:
958:
957:haematoxicity
954:
950:
942:
940:
937:
934:
931:
930:trichothecene
927:
922:
919:
915:
907:
905:
903:
899:
894:
889:
886:
884:
880:
879:
874:
870:
866:
862:
861:
852:
850:
848:
844:
840:
839:gastroenteric
835:
833:
828:
826:
823:species. The
822:
821:
816:
815:trichothecene
812:
808:
804:
792:
786:
780:
776:
773:(Recommended)
772:
768:
767:
763:
760:(Permissible)
759:
755:
754:
750:
749:
744:
740:
736:
727:
726:
722:
718:
714:
710:
706:
705:
701:
698:
692:
691:
687:
685:
682:
681:
674:
667:
660:
636:
633:
632:
628:
627:
537:
534:
530:
529:
495:
492:
488:
487:
484:
481:
478:
474:
473:
469:
464:
459:
455:
452:
448:
447:
443:
441:
436:
431:
427:
421:
417:
414:
413:
409:
407:
404:
403:
399:
396:
392:
391:
387:
385:
384:Boiling point
382:
381:
377:
375:
374:Melting point
372:
371:
368:1.6±0.1 g/cm
367:
365:
362:
361:
357:
354:
353:
346:
344:
341:
340:
319:
316:
312:
311:
306:
297:
293:
286:
275:
265:
261:
254:
246:
242:
241:DTXSID3021067
238:
237:
235:
225:
221:
220:
213:
209:
208:
206:
204:
201:
200:
193:
189:
188:
186:
180:
176:
175:
168:
164:
163:
161:
159:
156:
155:
151:
147:
144:
142:
140:ECHA InfoCard
137:
136:
129:
125:
124:
122:
120:
117:
116:
109:
105:
104:
102:
100:
97:
96:
89:
85:
84:
82:
78:
73:
72:
65:
61:
60:
58:
55:
51:
50:
45:
37:
32:
28:
23:
2084:: 2–6. 2000.
2081:
2031:
2027:
2021:
1996:
1992:
1986:
1953:
1949:
1943:
1918:
1914:
1908:
1875:
1871:
1865:
1830:
1826:
1816:
1791:
1787:
1781:
1746:
1742:
1732:
1721:. Retrieved
1711:
1692:
1642:
1638:
1606:
1600:
1581:
1575:
1556:
1553:EFSA Journal
1552:
1542:
1520:(1): 13–24.
1517:
1513:
1493:
1489:
1466:. Retrieved
1462:
1453:
1441:. Retrieved
1434:the original
1429:
1394:. Retrieved
1390:
1366:
1336:
1318:
1301:
1298:Genotoxicity
1284:
1271:
1258:erythropenia
1240:
1231:
1199:
1195:
1190:
1188:
1179:
1170:
1166:
1158:
1137:
1130:
1118:inflammation
1109:
1099:
1086:
1080:
1076:
1060:cyclopentane
1052:
1030:
1012:
1010:
981:
968:
965:
946:
938:
935:
923:
911:
901:
892:
890:
887:
877:
867:, vomiting,
858:
856:
836:
831:
829:
824:
818:
806:
802:
801:
747:
720:
695:Autoignition
630:
482:
439:
419:
388:585.1±50 °C
47:Identifiers
39:Other names
1921:(1): 21–8.
1459:"Nivalenol"
1423:"Nivalenol"
1387:"Nivalenol"
1260:and slight
1254:haemorrhage
1241:The oral LD
1162:trichodiene
1064:cyclohexene
984:cyclohexene
926:yellow rain
735:median dose
721:Lethal dose
697:temperature
684:Flash point
477:Signal word
355:Appearance
308:Properties
146:100.150.573
2133:Mycotoxins
2117:Categories
1723:2018-03-23
1371:References
1262:leukopenia
1147:Metabolism
1133:chemokines
1066:forming a
918:mycotoxins
451:Pictograms
406:Solubility
343:Molar mass
212:5WOP02RM1U
119:ChemSpider
108:CHEBI:7599
75:3D model (
64:23282-20-4
54:CAS Number
1141:apoptosis
1114:cytokines
1019:Synthesis
914:T-2 toxin
873:abdominal
811:mycotoxin
803:Nivalenol
615:P403+P233
583:P304+P340
579:P302+P350
575:P301+P310
442:labelling
2138:Epoxides
2123:Fusarium
2013:15019186
1978:20521758
1970:21114234
1935:14630058
1892:11766169
1857:19441883
1808:22964157
1773:19441883
1671:22069741
1468:28 March
1443:28 March
1396:28 March
1356:pokeweed
1309:necrotic
1191:Fusarium
1110:In vitro
1013:Fusarium
959:and the
902:Fusarium
893:Fusarium
869:diarrhea
860:Fusarium
843:necrosis
832:Fusarium
825:Fusarium
820:Fusarium
631:NFPA 704
433:Hazards
1848:2755091
1764:2755091
1662:3202860
1534:9205733
1463:PubChem
1346:". The
1305:jejunum
1056:isomere
1041:terpene
996:epoxide
853:History
813:of the
809:) is a
416:Acidity
364:Density
348:312.318
179:PubChem
2148:Enones
2011:
1976:
1968:
1933:
1900:104946
1898:
1890:
1855:
1845:
1806:
1771:
1761:
1699:
1669:
1659:
1639:Toxins
1609:: 22.
1588:
1532:
986:and a
865:nausea
483:Danger
428:11.78
358:solid
292:SMILES
167:C06080
34:Names
2078:(PDF)
1974:S2CID
1896:S2CID
1437:(PDF)
1426:(PDF)
1326:LOAEL
1292:NOAEL
1122:MCP-1
1102:NF-κB
748:NIOSH
711:(TLV)
260:InChI
192:31829
128:29515
99:ChEBI
77:JSmol
2032:25–3
2009:PMID
1966:PMID
1931:PMID
1888:PMID
1853:PMID
1804:PMID
1769:PMID
1697:ISBN
1667:PMID
1586:ISBN
1530:PMID
1518:50–1
1470:2018
1445:2018
1398:2018
1354:and
1220:and
1126:CCL2
1001:keto
992:keto
871:and
623:P501
619:P405
611:P363
607:P361
603:P330
599:P322
595:P321
591:P320
587:P310
571:P284
567:P280
563:P271
559:P270
555:P264
551:P262
547:P260
543:P241
539:P210
525:H332
521:H330
517:H319
513:H312
509:H310
505:H302
501:H300
497:H225
203:UNII
158:KEGG
2036:doi
2001:doi
1958:doi
1923:doi
1880:doi
1843:PMC
1835:doi
1796:doi
1759:PMC
1751:doi
1657:PMC
1647:doi
1611:doi
1561:doi
1522:doi
1340:IgA
1015:).
807:NIV
771:REL
758:PEL
440:GHS
229:EPA
182:CID
2119::
2080:.
2048:^
2030:.
2007:.
1997:42
1995:.
1972:.
1964:.
1954:64
1952:.
1929:.
1919:18
1917:.
1894:.
1886:.
1876:64
1874:.
1851:.
1841:.
1831:29
1829:.
1825:.
1802:.
1792:34
1790:.
1767:.
1757:.
1747:29
1745:.
1741:.
1679:^
1665:.
1655:.
1641:.
1637:.
1623:^
1557:11
1555:.
1551:.
1528:.
1516:.
1502:^
1494:11
1492:.
1478:^
1461:.
1428:.
1406:^
1389:.
1378:^
1264:.
1249:50
1247:LD
1243:50
1214:50
1209:50
1207:LD
731:50
729:LD
621:,
617:,
613:,
609:,
605:,
601:,
597:,
593:,
589:,
585:,
581:,
577:,
573:,
569:,
565:,
561:,
557:,
553:,
549:,
545:,
541:,
523:,
519:,
515:,
511:,
507:,
503:,
499:,
444::
425:)
418:(p
330:20
324:15
2042:.
2038::
2015:.
2003::
1980:.
1960::
1937:.
1925::
1902:.
1882::
1859:.
1837::
1810:.
1798::
1775:.
1753::
1726:.
1705:.
1673:.
1649::
1643:3
1617:.
1613::
1594:.
1569:.
1563::
1536:.
1524::
1472:.
1447:.
1400:.
1124:/
805:(
737:)
733:(
672:0
665:3
658:1
423:a
420:K
336:7
333:O
327:H
321:C
231:)
227:(
79:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.