579:
717:
613:
680:
503:
150:
633:
2377:
2228:
2296:
2133:
363:
279:
421:
85:
20:
485:
prompted the search for other catalysts capable of this reaction, with results in the finding of new catalysts that technically produced high molar mass polymers, like the modern
Ziegler–Natta catalysts.
161:
also exist with just 12 valence electrons. In solution however solvent always interact with the metal atom increasing the electron count. One 12 VE compound is di(mesityl)nickel prepared from (allyl)
612:
607:
The formation of organonickel compounds in this type of reaction is not always obvious but in a carefully designed experiment two such intermediates are formed quantitatively:
1148:
Jen-Chieh Hsieh and Chien-Hong Cheng (2005). "Nickel-catalyzed cocyclotrimerization of arynes with diynes; a novel method for synthesis of naphthalene derivatives".
1811:
679:
716:
100:
627:
7 isomers are possibly differing in the position of the substituents or the double bond positions. One strategy to remedy this problem employs certain diynes:
1284:
578:
1185:
Formation of an Aza-nickelacycle by
Reaction of an Imine and an Alkyne with Nickel(0): Oxidative Cyclization, Insertion, and Reductive Elimination
639:
The selected reaction conditions also minimize the amount formed of competing cycloaddition product to the corresponding substituted arene.
266:
with 10 electrons provided by nickel itself and 4x2 electrons more by the double bonds. This solid, which melts at 60 °C, is used as a
247:
1804:
502:
1105:
Danopoulos, Andreas A.; Simler, Thomas; Braunstein, Pierre (2019). "N-Heterocyclic
Carbene Complexes of Copper, Nickel, and Cobalt".
1761:
820:
801:
769:
729:
567:
412:
2509:
1696:
1797:
1345:
1277:
1022:
1449:
1318:
251:
552:
239:
71:
50:. Organonickel compounds are also short-lived intermediates in organic reactions. The first organonickel compound was
632:
450:
2366:
2361:
2356:
2351:
2346:
2341:
2336:
2331:
2326:
2321:
2316:
2311:
2301:
2244:
2138:
2059:
2054:
1911:
2402:
2104:
2074:
2064:
2044:
2032:
2000:
1965:
1933:
1901:
1896:
1856:
1270:
619:
It is noted in one study that this reaction only works with acetylene itself or with simple alkynes due to poor
47:
1871:
1835:
1789:
2447:
2442:
2437:
2432:
2427:
2422:
2417:
2412:
2407:
2392:
2382:
2233:
2208:
2203:
2188:
2173:
2153:
2148:
2099:
2027:
2010:
1960:
1955:
1950:
1945:
1921:
1881:
31:
2397:
2387:
2198:
2183:
2168:
2158:
2143:
2084:
2069:
2049:
2039:
2020:
2015:
2005:
1995:
1938:
1906:
1150:
734:
345:
1876:
1866:
2306:
2221:
2163:
2126:
2121:
2109:
2089:
2079:
1985:
1975:
1916:
1891:
1735:
1550:
1463:
1239:
Nickel(0)-Catalyzed
Cycloadditions of Terminal Diynes for the Synthesis of Substituted Cyclooctatetraenes
1223:
588:
elementary zinc serves to reduce nickel(II) to nickel(0) to which can then coordinate two alkyne bonds. A
62:
for nickel purification. Organonickel complexes are prominent in numerous industrial processes including
2114:
1926:
1861:
1851:
1188:
601:
362:
238:. Practical applications of this theme include polymerization or oligomerization of alkenes, as in the
1226:
first to nickelapyrroline and with a second insertion a nickeldihydroazepine and finally on heating a
2193:
2178:
1990:
1970:
1751:
1715:
1500:
1484:
1476:
1380:
1310:
878:
525:
466:
458:
51:
2094:
1663:
1532:
1524:
1516:
1403:
1372:
1337:
1630:
1574:
1562:
1492:
1426:
1395:
1130:
1054:
955:
708:
556:
420:
19:
1723:
1418:
1242:
1215:
1167:
1122:
1078:
1046:
1004:
922:
Göttker-Schnetmann, Inigo; Mecking, Stefan (2020). "A Practical
Synthesis of [(tmeda)Ni(CH
904:
816:
797:
765:
648:
494:
1026:
1025:; Breuil, P. A. R.; Magna, L.; Michel, T.; Espada Pastor, M. Fernandez; Delcroix, D. (2020).
1471:
1441:
1296:
1250:
1195:
1159:
1114:
1038:
994:
986:
947:
894:
886:
845:
757:
739:
671:
620:
341:
337:
282:
174:
35:
391:
are unknown. From nickelocene, many derivatives are generated, e.g. CpNiLCl, CpNiNO, and Cp
1545:
1357:
1227:
704:
692:
667:
663:
624:
589:
585:
490:
470:
438:
349:
882:
1618:
1586:
1540:
999:
974:
899:
866:
761:
545:
259:
67:
2503:
2466:
1058:
959:
597:
510:
289:
263:
63:
1134:
592:
step follows to the nickelcyclopentadiene intermediate and then coordination of the
46:
bonds. They are used as a catalyst, as a building block in organic chemistry and in
696:
59:
951:
1118:
1073:
1042:
990:
571:
548:
380:
372:
313:
243:
149:
850:
833:
1773:
700:
514:
454:
453:
in the 1950s. That discovery shown by nickel impurities originating from an
1199:
1171:
1126:
1050:
1008:
908:
541:
518:
478:
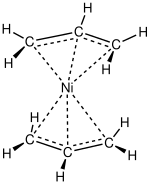
474:
267:
890:
1074:"Reaction of Aryl Halides with π-Allylnickel Halides: Methallylbenzene"
593:
537:
533:
529:
278:
1254:
555:
positions and reacts with a di-yne such as 1,7-octadiyne along with a
1821:
1293:
1163:
659:
446:
442:
235:
43:
39:
84:
1262:
1211:
973:
Shields, Jason D.; Gray, Erin E.; Doyle, Abigail G. (2015-05-01).
652:
293:
277:
148:
83:
865:
Tasker, Sarah Z.; Standley, Eric A.; Jamison, Timothy F. (2014).
383:
of nickel. It can be oxidized by one electron. The corresponding
754:
The
Organic Chemistry of Nickel Volume I: Organonickel Complexes
656:
560:
379:
with +2 Ni oxidation state and 20 valence electrons is the main
114:
Many alkyl and aryl complexes are known with the formula NiR(X)L
1793:
1266:
521:
to trans-1,4-hexadiene was an industrial process at one time.
489:
One practical implementation of alkyne oligomerization is the
834:"Nickel: The "Spirited Horse" of Transition Metal Catalysis"
361:
18:
1027:"Nickel Catalyzed Olefin Oligomerization and Dimerization"
449:. This property validated the research and development of
340:+2, and the electron counts are 16 and 18, respectively.
1214:
N-(benzenesulfonyl)benzaldimine with two equivalents of
938:], and Neutral Chelated-Nickel Methyl Complexes".
528:. This extensible trimerisation can generally include
695:
to alkenes and alkynes. The industrial production of
270:
and as a precursor for many other nickel compounds.
2458:
1744:
1708:
1330:
1303:
1072:Martin F. Semmelhack and Paul M. Helquist (1988).
867:"Recent Advances in Homogeneous Nickel Catalysis"
415:complexes, formally featuring C=Ni double bonds.
234:Many complexes exist of nickel coordinated to an
707:and water at 40-55 atm and 160-200 °C with
1187:Sensuke Ogoshi Haruo Ikeda, and Hideo Kurosawa
662:. Other coupling reactions involving nickel in
312:. These compounds in turn are sources of allyl
58:, reported in 1890 and quickly applied in the
1805:
1278:
930:], Isotopically Labeled [(tmeda)Ni(CH
604:liberates the tetrahydroanthracene compound.
8:
1249:; 129(44) pp 13402 - 13403; (Communication)
1812:
1798:
1790:
1285:
1271:
1263:
975:"A Modular, Air-Stable Nickel Precatalyst"
501:
1824:with other elements in the periodic table
998:
898:
849:
524:Formal cycloadditions also take place in
246:Ni and the bonding is described with the
242:. In these compounds nickel is formally
1754:
600:to the nickelcycloheptatriene compound.
815:III Robert Crabtree, Mike Mingos 2006
785:
1841:
813:Comprehensive organometallic chemistry
570:/ Zn) to synthesize the corresponding
1241:Paul A. Wender and Justin P. Christy
7:
2483:Academic research, no widespread use
699:at one time consisted of combining
300:to form pi-allyl complexes, (allyl)
762:10.1016/B978-0-12-388401-5.X5001-5
752:P.W. Jolly, G. Wilke, ed. (1974).
493:; for example in the synthesis of
205:MgBr → 2 (allyl)MgBr + 2 MgBr
16:Branch of organometallic chemistry
14:
730:Nickel(IV) organometallic complex
2375:
2294:
2226:
2131:
1831:
715:
678:
631:
611:
577:
419:
250:. One common representative is
157:Nickel compounds of the type NiR
88:Bis(1,5-cyclooctadiene)nickel(0)
832:Ananikov, Valentine P. (2015).
437:Nickel compounds catalyze the
433:Alkene/alkyne oligomerizations
1:
952:10.1021/acs.organomet.0c00500
691:Ni catalyzes the addition of
568:bis(diphenylphosphino) ethane
288:Nickel forms several simple
252:Bis(cyclooctadiene)nickel(0)
109:(tetramethylethylenediamine)
1119:10.1021/acs.chemrev.8b00505
1043:10.1021/acs.chemrev.0c00076
991:10.1021/acs.orglett.5b00766
647:Nickel compounds cause the
248:Dewar–Chatt–Duncanson model
240:Shell Higher Olefin Process
72:Shell higher olefin process
2526:
1093:, vol. 6, p. 161
794:Advanced Organic Chemistry
356:Cyclopentadienyl complexes
142:-tolyl)Cl, and (TMEDA)NiMe
2372:
2291:
1843:
1839:
1829:
792:F.A. Carey R.J. Sundberg
513:. The oligomerization of
336:), nickel is assigned to
296:halides react with Ni(CO)
48:chemical vapor deposition
851:10.1021/acscatal.5b00072
473:: the polymerization of
95:Alkyl and aryl complexes
32:organometallic chemistry
1151:Chemical Communications
735:Nickel(II) precatalysts
540:compound attached to a
532:. Benzyne is generated
451:Ziegler–Natta catalysts
346:allyl magnesium bromide
138:-tolyl)Cl]], (TMEDA)Ni(
2510:Organonickel compounds
2478:Many uses in chemistry
2473:Core organic chemistry
1224:tricyclohexylphosphine
1200:10.1002/anie.200700688
598:C-H insertion reaction
367:
285:
258:), which contains two
173:and the corresponding
154:
134:-tolyl)Cl]], (dppf)Ni(
118:. Examples include ,
89:
28:Organonickel chemistry
24:
1189:Angew. Chem. Int. Ed.
1023:Olivier-Bourbigou, H.
711:and a copper halide.
602:Reductive elimination
563:catalyst system (NiBr
365:
281:
152:
99:A popular reagent is
87:
22:
526:alkyne trimerisation
477:suddenly stopped at
467:termination reaction
459:propagation reaction
78:Classes of compounds
52:nickel tetracarbonyl
891:10.1038/nature13274
883:2014Natur.509..299T
756:. Academic Press.
709:nickel(II) bromide
643:Coupling reactions
557:nickel(II) bromide
368:
286:
155:
90:
25:
2497:
2496:
2453:
2452:
1787:
1786:
1255:10.1021/ja0763044
1243:J. Am. Chem. Soc.
1216:diphenylacetylene
1194:, 46, 4930 –4932
1158:(19): 2459–2461.
1091:Collected Volumes
1079:Organic Syntheses
1037:(15): 7919–7983.
946:(18): 3433–3440.
877:(7500): 299–309.
664:catalytic amounts
649:coupling reaction
509:This is a formal
495:cyclooctatetraene
481:. This so-called
457:which killed the
407:Carbene complexes
344:is prepared from
262:ligands. It is a
36:organic compounds
2517:
2489:
2484:
2479:
2474:
2379:
2378:
2298:
2297:
2230:
2229:
2135:
2134:
1832:
1814:
1807:
1800:
1791:
1778:
1757:
1692:
1691:
1690:
1682:
1681:
1673:
1672:
1659:
1658:
1657:
1649:
1648:
1640:
1639:
1612:
1611:
1599:
1598:
1435:
1434:
1412:
1411:
1389:
1388:
1366:
1365:
1287:
1280:
1273:
1264:
1257:
1236:
1230:
1210:Reaction of the
1208:
1202:
1182:
1176:
1175:
1164:10.1039/b415691a
1145:
1139:
1138:
1113:(6): 3730–3961.
1107:Chemical Reviews
1102:
1096:
1094:
1087:
1069:
1063:
1062:
1031:Chemical Reviews
1019:
1013:
1012:
1002:
985:(9): 2166–2169.
970:
964:
963:
919:
913:
912:
902:
862:
856:
855:
853:
844:(3): 1964–1971.
829:
823:
810:
804:
790:
775:
740:Lactate racemase
719:
687:Ni carbonylation
682:
672:Negishi coupling
635:
621:regioselectivity
615:
581:
505:
423:
342:Bis(allyl)nickel
338:oxidation number
283:Bis(allyl)nickel
230:Alkene complexes
175:Grignard reagent
34:that deals with
2525:
2524:
2520:
2519:
2518:
2516:
2515:
2514:
2500:
2499:
2498:
2493:
2492:
2487:
2482:
2477:
2472:
2454:
2376:
2295:
2227:
2132:
1825:
1818:
1788:
1783:
1779:
1776:
1769:
1765:
1756:
1752:
1740:
1731:
1727:
1719:
1704:
1700:
1689:
1686:
1685:
1684:
1680:
1677:
1676:
1675:
1671:
1668:
1667:
1666:
1664:
1656:
1653:
1652:
1651:
1647:
1644:
1643:
1642:
1638:
1635:
1634:
1633:
1631:
1626:
1622:
1610:
1607:
1606:
1605:
1603:
1597:
1594:
1593:
1592:
1590:
1582:
1578:
1570:
1566:
1558:
1554:
1536:
1528:
1520:
1512:
1508:
1504:
1496:
1488:
1480:
1467:
1457:
1453:
1445:
1433:
1430:
1429:
1428:
1422:
1410:
1407:
1406:
1405:
1399:
1387:
1384:
1383:
1382:
1376:
1364:
1361:
1360:
1359:
1353:
1349:
1341:
1326:
1322:
1314:
1299:
1291:
1261:
1260:
1237:
1233:
1228:dihydropyridine
1221:
1209:
1205:
1183:
1179:
1147:
1146:
1142:
1104:
1103:
1099:
1089:
1071:
1070:
1066:
1021:
1020:
1016:
979:Organic Letters
972:
971:
967:
940:Organometallics
937:
933:
929:
925:
921:
920:
916:
864:
863:
859:
831:
830:
826:
811:
807:
791:
787:
782:
772:
751:
748:
746:Further reading
726:
705:carbon monoxide
693:carbon monoxide
689:
668:Kumada coupling
645:
625:terminal alkyne
590:cyclometalation
586:catalytic cycle
566:
491:Reppe synthesis
471:terminal alkene
439:oligomerization
435:
430:
409:
402:
398:
394:
378:
358:
350:nickel chloride
335:
331:
328:and (allyl)Ni(C
327:
323:
319:
311:
307:
303:
299:
290:allyl complexes
276:
274:Allyl complexes
257:
232:
224:
220:
216:
212:
208:
204:
200:
196:
192:
188:
184:
172:
168:
164:
160:
145:
129:
125:
117:
108:
104:
97:
80:
57:
30:is a branch of
17:
12:
11:
5:
2523:
2521:
2513:
2512:
2502:
2501:
2495:
2494:
2491:
2490:
2485:
2480:
2475:
2470:
2467:Chemical bonds
2463:
2462:
2460:
2456:
2455:
2451:
2450:
2445:
2440:
2435:
2430:
2425:
2420:
2415:
2410:
2405:
2400:
2395:
2390:
2385:
2380:
2373:
2370:
2369:
2364:
2359:
2354:
2349:
2344:
2339:
2334:
2329:
2324:
2319:
2314:
2309:
2304:
2299:
2292:
2289:
2288:
2284:
2283:
2280:
2277:
2274:
2271:
2268:
2265:
2262:
2259:
2256:
2253:
2250:
2247:
2242:
2239:
2236:
2231:
2224:
2219:
2215:
2214:
2211:
2206:
2201:
2196:
2191:
2186:
2181:
2176:
2171:
2166:
2161:
2156:
2151:
2146:
2141:
2136:
2129:
2124:
2118:
2117:
2112:
2107:
2102:
2097:
2092:
2087:
2082:
2077:
2072:
2067:
2062:
2057:
2052:
2047:
2042:
2037:
2035:
2030:
2024:
2023:
2018:
2013:
2008:
2003:
1998:
1993:
1988:
1983:
1978:
1973:
1968:
1963:
1958:
1953:
1948:
1943:
1941:
1936:
1930:
1929:
1924:
1919:
1914:
1909:
1904:
1899:
1894:
1888:
1887:
1884:
1879:
1874:
1869:
1864:
1859:
1854:
1848:
1847:
1844:
1842:
1840:
1838:
1830:
1827:
1826:
1819:
1817:
1816:
1809:
1802:
1794:
1785:
1784:
1782:
1781:
1775:
1771:
1767:
1763:
1759:
1748:
1746:
1742:
1741:
1739:
1738:
1733:
1729:
1725:
1721:
1717:
1712:
1710:
1706:
1705:
1703:
1702:
1698:
1694:
1687:
1678:
1669:
1661:
1654:
1645:
1636:
1628:
1624:
1620:
1616:
1615:
1614:
1608:
1601:
1595:
1588:
1580:
1576:
1572:
1568:
1564:
1560:
1556:
1552:
1548:
1543:
1538:
1534:
1530:
1526:
1522:
1518:
1514:
1510:
1506:
1502:
1498:
1494:
1490:
1486:
1482:
1478:
1474:
1469:
1465:
1461:
1460:
1459:
1455:
1451:
1443:
1439:
1438:
1437:
1431:
1420:
1416:
1415:
1414:
1408:
1397:
1393:
1392:
1391:
1385:
1374:
1370:
1369:
1368:
1362:
1355:
1351:
1347:
1339:
1334:
1332:
1328:
1327:
1325:
1324:
1320:
1316:
1312:
1307:
1305:
1301:
1300:
1292:
1290:
1289:
1282:
1275:
1267:
1259:
1258:
1231:
1219:
1203:
1177:
1140:
1097:
1064:
1014:
965:
935:
931:
927:
923:
914:
857:
824:
805:
784:
783:
781:
778:
777:
776:
770:
747:
744:
743:
742:
737:
732:
725:
722:
721:
720:
688:
685:
684:
683:
644:
641:
637:
636:
617:
616:
596:which gives a
564:
546:trimethylsilyl
507:
506:
465:) in favor of
434:
431:
429:
426:
425:
424:
408:
405:
400:
396:
392:
376:
370:
369:
357:
354:
333:
329:
325:
321:
317:
309:
305:
301:
297:
275:
272:
260:cyclooctadiene
255:
231:
228:
227:
226:
222:
218:
214:
210:
206:
202:
198:
194:
190:
186:
182:
170:
166:
162:
158:
153:Synthesis of .
143:
127:
123:
115:
106:
102:
96:
93:
92:
91:
79:
76:
68:hydrocyanation
64:carbonylations
55:
15:
13:
10:
9:
6:
4:
3:
2:
2522:
2511:
2508:
2507:
2505:
2486:
2481:
2476:
2471:
2468:
2465:
2464:
2461:
2457:
2449:
2446:
2444:
2441:
2439:
2436:
2434:
2431:
2429:
2426:
2424:
2421:
2419:
2416:
2414:
2411:
2409:
2406:
2404:
2401:
2399:
2396:
2394:
2391:
2389:
2386:
2384:
2381:
2374:
2371:
2368:
2365:
2363:
2360:
2358:
2355:
2353:
2350:
2348:
2345:
2343:
2340:
2338:
2335:
2333:
2330:
2328:
2325:
2323:
2320:
2318:
2315:
2313:
2310:
2308:
2305:
2303:
2300:
2293:
2290:
2286:
2285:
2281:
2278:
2275:
2272:
2269:
2266:
2263:
2260:
2257:
2254:
2251:
2248:
2246:
2243:
2240:
2237:
2235:
2232:
2225:
2223:
2220:
2217:
2216:
2212:
2210:
2207:
2205:
2202:
2200:
2197:
2195:
2192:
2190:
2187:
2185:
2182:
2180:
2177:
2175:
2172:
2170:
2167:
2165:
2162:
2160:
2157:
2155:
2152:
2150:
2147:
2145:
2142:
2140:
2137:
2130:
2128:
2125:
2123:
2120:
2119:
2116:
2113:
2111:
2108:
2106:
2103:
2101:
2098:
2096:
2093:
2091:
2088:
2086:
2083:
2081:
2078:
2076:
2073:
2071:
2068:
2066:
2063:
2061:
2058:
2056:
2053:
2051:
2048:
2046:
2043:
2041:
2038:
2036:
2034:
2031:
2029:
2026:
2025:
2022:
2019:
2017:
2014:
2012:
2009:
2007:
2004:
2002:
1999:
1997:
1994:
1992:
1989:
1987:
1984:
1982:
1979:
1977:
1974:
1972:
1969:
1967:
1964:
1962:
1959:
1957:
1954:
1952:
1949:
1947:
1944:
1942:
1940:
1937:
1935:
1932:
1931:
1928:
1925:
1923:
1920:
1918:
1915:
1913:
1910:
1908:
1905:
1903:
1900:
1898:
1895:
1893:
1890:
1889:
1885:
1883:
1880:
1878:
1875:
1873:
1870:
1868:
1865:
1863:
1860:
1858:
1855:
1853:
1850:
1849:
1845:
1837:
1834:
1833:
1828:
1823:
1820:Compounds of
1815:
1810:
1808:
1803:
1801:
1796:
1795:
1792:
1780:
1772:
1770:
1760:
1758:
1750:
1749:
1747:
1743:
1737:
1734:
1732:
1722:
1720:
1714:
1713:
1711:
1707:
1701:
1695:
1693:
1662:
1660:
1629:
1627:
1617:
1613:
1585:
1584:
1583:
1573:
1571:
1561:
1559:
1549:
1547:
1544:
1542:
1539:
1537:
1531:
1529:
1523:
1521:
1515:
1513:
1499:
1497:
1491:
1489:
1483:
1481:
1475:
1473:
1470:
1468:
1462:
1458:
1448:
1447:
1446:
1440:
1436:
1425:
1424:
1423:
1417:
1413:
1402:
1401:
1400:
1394:
1390:
1379:
1378:
1377:
1371:
1367:
1356:
1354:
1344:
1343:
1342:
1336:
1335:
1333:
1329:
1323:
1317:
1315:
1309:
1308:
1306:
1302:
1298:
1295:
1288:
1283:
1281:
1276:
1274:
1269:
1268:
1265:
1256:
1252:
1248:
1244:
1240:
1235:
1232:
1229:
1225:
1217:
1213:
1207:
1204:
1201:
1197:
1193:
1190:
1186:
1181:
1178:
1173:
1169:
1165:
1161:
1157:
1153:
1152:
1144:
1141:
1136:
1132:
1128:
1124:
1120:
1116:
1112:
1108:
1101:
1098:
1092:
1085:
1081:
1080:
1075:
1068:
1065:
1060:
1056:
1052:
1048:
1044:
1040:
1036:
1032:
1028:
1024:
1018:
1015:
1010:
1006:
1001:
996:
992:
988:
984:
980:
976:
969:
966:
961:
957:
953:
949:
945:
941:
918:
915:
910:
906:
901:
896:
892:
888:
884:
880:
876:
872:
868:
861:
858:
852:
847:
843:
839:
838:ACS Catalysis
835:
828:
825:
822:
821:0-08-044590-X
818:
814:
809:
806:
803:
802:0-306-41199-7
799:
795:
789:
786:
779:
773:
771:9780123884015
767:
763:
759:
755:
750:
749:
745:
741:
738:
736:
733:
731:
728:
727:
723:
718:
714:
713:
712:
710:
706:
702:
698:
694:
686:
681:
677:
676:
675:
673:
669:
665:
661:
658:
654:
650:
642:
640:
634:
630:
629:
628:
626:
622:
614:
610:
609:
608:
605:
603:
599:
595:
591:
587:
582:
580:
575:
573:
569:
562:
558:
554:
550:
547:
543:
539:
535:
531:
527:
522:
520:
516:
512:
511:cycloaddition
504:
500:
499:
498:
496:
492:
487:
484:
483:nickel effect
480:
476:
472:
468:
464:
460:
456:
452:
448:
444:
440:
432:
427:
422:
418:
417:
416:
414:
411:Nickel forms
406:
404:
390:
386:
382:
374:
364:
360:
359:
355:
353:
351:
347:
343:
339:
315:
295:
291:
284:
280:
273:
271:
269:
265:
264:18VE compound
261:
253:
249:
245:
241:
237:
229:
180:
179:
178:
176:
151:
147:
141:
137:
133:
121:
112:
110:
94:
86:
82:
81:
77:
75:
73:
69:
65:
61:
53:
49:
45:
41:
37:
33:
29:
21:
2488:Bond unknown
1980:
1246:
1238:
1234:
1206:
1191:
1184:
1180:
1155:
1149:
1143:
1110:
1106:
1100:
1090:
1083:
1077:
1067:
1034:
1030:
1017:
982:
978:
968:
943:
939:
917:
874:
870:
860:
841:
837:
827:
812:
808:
793:
788:
753:
697:acrylic acid
690:
646:
638:
618:
606:
583:
576:
574:derivative.
523:
508:
488:
482:
462:
436:
410:
388:
384:
371:
316:. In (allyl)
314:nucleophiles
287:
233:
156:
139:
135:
131:
119:
113:
98:
60:Mond process
27:
26:
23:organonickel
1709:Nickel(III)
572:naphthalene
549:substituent
389:platinocene
385:palladocene
381:metallocene
373:Nickelocene
366:Nickelocene
1745:Nickel(IV)
1331:Nickel(II)
1218:with NiCOD
780:References
244:zerovalent
70:, and the
38:featuring
2469:to carbon
1304:Nickel(0)
1297:compounds
1059:221124789
960:224930545
701:acetylene
623:. From a
515:butadiene
455:autoclave
428:Reactions
2504:Category
1697:Ni(acac)
1172:15886770
1135:73515728
1127:30843688
1051:32786672
1009:25886092
909:24828188
796:2nd Ed.
724:See also
670:and the
666:are the
651:between
542:triflate
519:ethylene
479:1-butene
475:ethylene
268:catalyst
254:(Ni(COD)
2287:
1600:/ Ni(NO
1464:Ni(SCN)
1319:Ni(COD)
1000:4719147
900:4344729
879:Bibcode
660:halides
594:benzyne
584:In the
551:in the
538:benzene
536:from a
534:in situ
530:benzyne
447:alkynes
443:alkenes
413:carbene
209:+ 2 (C
193:+ 4 C
181:(allyl)
2459:Legend
1822:carbon
1551:Ni(ClO
1477:Ni(OH)
1454:Ni(CN)
1442:Ni(CN)
1311:Ni(CO)
1294:Nickel
1170:
1133:
1125:
1057:
1049:
1007:
997:
958:
907:
897:
871:Nature
819:
800:
768:
553:ortho-
544:and a
463:Aufbau
236:alkene
54:Ni(CO)
44:carbon
40:nickel
1736:NiOOH
1619:Ni(CO
1587:Ni(NO
1575:Ni(NO
1563:Ni(NO
1533:NiSeO
1525:NiTiO
1517:NiCrO
1212:imine
1131:S2CID
1086:: 115
1055:S2CID
956:S2CID
653:allyl
517:with
469:to a
294:Allyl
122:-(PCy
120:trans
101:Ni(CH
1774:MNiO
1546:NiSe
1493:NiSO
1485:NiCO
1404:NiBr
1396:NiBr
1381:NiCl
1373:NiCl
1247:2007
1222:and
1192:2007
1168:PMID
1156:2005
1123:PMID
1047:PMID
1005:PMID
905:PMID
817:ISBN
798:ISBN
766:ISBN
657:aryl
655:and
561:zinc
445:and
399:(CO)
387:and
375:NiCp
348:and
2433:CEs
2428:CCf
2423:CBk
2418:CCm
2413:CAm
2408:CPu
2403:CNp
2393:CPa
2388:CTh
2367:CYb
2362:CTm
2357:CEr
2352:CHo
2347:CDy
2342:CTb
2337:CGd
2332:CEu
2327:CSm
2322:CPm
2317:CNd
2312:CPr
2307:CCe
2302:CLa
2282:Og
2279:Ts
2276:Lv
2273:Mc
2270:Fl
2267:Nh
2264:Cn
2261:Rg
2258:Ds
2255:Mt
2252:Hs
2249:Bh
2245:CSg
2241:Db
2238:Rf
2222:CRa
2218:Fr
2213:Rn
2209:CAt
2204:CPo
2199:CBi
2194:CPb
2189:CTl
2184:CHg
2179:CAu
2174:CPt
2169:CIr
2164:COs
2159:CRe
2149:CTa
2144:CHf
2139:CLu
2127:CBa
2122:CCs
2115:CXe
2105:CTe
2100:CSb
2095:CSn
2090:CIn
2085:CCd
2080:CAg
2075:CPd
2070:CRh
2065:CRu
2060:CTc
2055:CMo
2050:CNb
2045:CZr
2033:CSr
2028:CRb
2021:CKr
2016:CBr
2011:CSe
2006:CAs
2001:CGe
1996:CGa
1991:CZn
1986:CCu
1981:CNi
1976:CCo
1971:CFe
1966:CMn
1961:CCr
1951:CTi
1946:CSc
1939:CCa
1927:CAr
1922:CCl
1907:CSi
1902:CAl
1897:CMg
1892:CNa
1886:Ne
1857:CBe
1852:CLi
1846:He
1766:NiF
1753:NiF
1716:NiF
1683:NiO
1650:NiO
1541:NiS
1505:(PO
1472:NiO
1427:NiI
1419:NiI
1358:NiF
1350:NiF
1338:NiF
1251:doi
1196:doi
1160:doi
1115:doi
1111:119
1039:doi
1035:120
995:PMC
987:doi
948:doi
895:PMC
887:doi
875:509
846:doi
758:doi
441:of
130:Ni(
126:Ph)
2506::
2448:No
2443:Md
2438:Fm
2398:CU
2383:Ac
2234:Lr
2154:CW
2110:CI
2040:CY
1956:CV
1934:CK
1917:CS
1912:CP
1882:CF
1877:CO
1872:CN
1867:CC
1862:CB
1836:CH
1724:Ni
1679:70
1670:36
1646:46
1637:24
1623:H)
1501:Ni
1245:;
1166:.
1154:.
1129:.
1121:.
1109:.
1088:;
1084:52
1082:.
1076:.
1053:.
1045:.
1033:.
1029:.
1003:.
993:.
983:17
981:.
977:.
954:.
944:39
942:.
903:.
893:.
885:.
873:.
869:.
840:.
836:.
764:.
703:,
674:.
559:/
497::
403:.
395:Ni
352:.
324:Br
320:Ni
308:Cl
304:Ni
292:.
225:Ni
217:Me
201:Me
189:Br
185:Ni
177:.
169:Br
165:Ni
146:.
111:.
74:.
66:,
1813:e
1806:t
1799:v
1777:x
1768:6
1764:2
1762:K
1755:4
1730:3
1728:O
1726:2
1718:3
1699:2
1688:4
1674:H
1665:C
1655:4
1641:H
1632:C
1625:2
1621:2
1609:6
1604:)
1602:2
1596:5
1591:)
1589:2
1581:2
1579:)
1577:2
1569:2
1567:)
1565:3
1557:2
1555:)
1553:4
1535:4
1527:3
1519:4
1511:2
1509:)
1507:4
1503:3
1495:4
1487:3
1479:2
1466:2
1456:4
1452:2
1450:K
1444:2
1432:4
1421:2
1409:4
1398:2
1386:4
1375:2
1363:4
1352:4
1348:2
1346:K
1340:2
1321:2
1313:4
1286:e
1279:t
1272:v
1253::
1220:2
1198::
1174:.
1162::
1137:.
1117::
1095:.
1061:.
1041::
1011:.
989::
962:.
950::
936:2
934:)
932:3
928:2
926:)
924:3
911:.
889::
881::
854:.
848::
842:5
774:.
760::
565:2
461:(
401:3
397:2
393:2
377:2
334:5
332:H
330:5
326:2
322:2
318:2
310:2
306:2
302:2
298:4
256:2
223:2
221:)
219:3
215:2
213:H
211:6
207:2
203:3
199:2
197:H
195:6
191:2
187:2
183:2
171:2
167:2
163:2
159:2
144:2
140:o
136:o
132:o
128:2
124:2
116:2
107:2
105:)
103:3
56:4
42:-
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.