203:
2087:
1938:
2006:
1843:
623:
28:
235:
comprises two signals in 1:2 ratio, as expected from the solid state structure. At 20 °C, only one signal is observed because exchange of terminal and bridging methyl groups is too fast to be resolved by NMR. The high Lewis acidity of the monomeric species is related to the size of the Al(III)
583:
Organoaluminum compounds can react with alkenes and alkynes, resulting in the net addition of one organyl group and the metal fragment across the multiple bond (carboalumination). This process can proceed in a purely thermal manner or in the presence of a transition metal catalyst. For the
480:
Aluminium powder reacts directly with certain terminal alkenes in the presence of hydrogen. The process entails two steps, the first producing dialkylaluminium hydrides. Such reactions are typically conducted at elevated temperatures and require activation by trialkylaluminium reagents:
633:
selectivity, even in the presence of propargylic or homopropargylic heteroatom substituents. Unfortunately, extension of the zirconocene-catalyzed methylalumination to alkylalumination with higher alkyls results in lower yields and poor regioselectivities.
131:. In accord with the usual trends, four-coordinate Al prefers to be tetrahedral. In contrast to boron, aluminium is a larger atom and easily accommodates four carbon ligands. The triorganoaluminium compounds are thus usually dimeric with a pair of
312:. The cluster was obtained from related investigations on the reduction of organoaluminium compounds. This dianion adopts an icosahedral structure reminiscent of dodecaborate (). Its formal oxidation state is less than one.
1266:
Rand, Cynthia L.; Horn, David E. Van; Moore, Mark W.; Negishi, Eiichi (2002-05-01). "A versatile and selective route to difunctional trisubstituted (E)-alkene synthons via zirconium-catalyzed carboalumination of alkynes".
219:
The trialkylaluminium dimers often participate in dynamic equilibria, resulting in the interchange of bridging and terminal ligands as well as ligand exchange between dimers. Even in noncoordinating
1194:"Alkyne Elementometalation−Pd-Catalyzed Cross-Coupling. Toward Synthesis of All Conceivable Types of Acyclic Alkenes in High Yields, Efficiently, Selectively, Economically, and Safely: "Green" Way"
1470:
Schmidt, Roland; Griesbaum, Karl; Behr, Arno; Biedenkapp, Dieter; Voges, Heinz-Werner; Garbe, Dorothea; Paetz, Christian; Collin, Gerd; Mayer, Dieter; Höke, Hartmut (2014). "Hydrocarbons".
907:
for the production of alcohols from ethylene. Several technologies exist for the oligomerization of ethylene to give alpha-olefins. Organoaluminium compounds are used as catalysts for
1107:
Michael J. Krause, Frank
Orlandi, Alfred T. Saurage and Joseph R. Zietz "Aluminum Compounds, Organic" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim.
400:. The term sesquichloride refers to the fact that, on average, the Cl:Al ratio is 1.5. These sesquichlorides can be converted to the triorganoaluminium derivatives by reduction:
578:
500:
For nonbulky R groups, the organoaluminium hydrides are typically trimeric. In a subsequent step, these hydrides are treated with more alkene to effect hydroalumiunation:
615:
is employed for the synthesis of stereodefined trisubstituted olefin fragments, a common substructure in terpene and polyketide natural products. The synthesis of (
1521:
1415:
W. Uhl; B. Jana (2008). "A persistent alkylaluminum peroxide: Surprising stability of a molecule with strong reducing and oxidizing functions in close proximity".
642:
Although the simple members are commercially available at low cost, many methods have been developed for their synthesis in the laboratory, including
1059:
1034:
815:
Similarly, the reaction between trialkylaluminum compounds and carbon dioxide has been used to synthesise alcohols, olefins, or ketones.
1514:
962:
2219:
1250:
945:
903:
Organoaluminium compounds are widely used in the production of alkenes, alcohols, and polymers. Some relevant processes include the
1487:
1133:
1092:
1507:
584:
uncatalyzed process, monoaddition is only possible when the alkene is substituted. For ethylene, carboalumination leads to a
734:
The Al–C bond is polarized such that the carbon is highly basic. Acids react to give alkanes. For example, alcohols give
397:
106:
and colleagues discovered the direct synthesis of trialkylaluminium compounds and applied these compounds to catalytic
762:, trialkylaluminium compounds give the dialkylaluminium carboxylate, and subsequently alkyl aluminium dicarboxylates:
527:
32:
694:
The high reactivity of organoaluminium compounds toward electrophiles is attributed to the charge separation between
66:. The behavior of organoaluminium compounds can be understood in terms of the polarity of the C−Al bond and the high
2076:
2071:
2066:
2061:
2056:
2051:
2046:
2041:
2036:
2031:
2026:
2021:
2011:
1954:
1848:
1769:
1764:
1621:
475:
293:
2112:
1814:
1784:
1774:
1754:
1742:
1710:
1675:
1643:
1606:
1566:
1581:
1545:
1499:
2157:
2152:
2147:
2142:
2137:
2132:
2127:
2122:
2117:
2102:
2092:
1943:
1918:
1913:
1898:
1883:
1863:
1858:
1809:
1737:
1720:
1670:
1665:
1660:
1655:
1631:
1591:
892:
758:
A wide variety of acids can be employed beyond the simple mineral acids. Amines give amido derivatives. With
643:
51:
629:
For terminal alkynes, the reaction generally proceeds with good regioselectivity (>90:10 rr) and complete
2107:
2097:
1908:
1893:
1878:
1868:
1853:
1794:
1779:
1759:
1749:
1730:
1725:
1715:
1705:
1648:
1616:
264:(a dialane). They are typically prepared reduction of the dialkylaluminium chlorides by metallic potassium:
163:. Thus, despite its common name of triethylaluminium, this compound contains two aluminium centres, and six
1586:
1576:
202:
2016:
1931:
1873:
1836:
1831:
1819:
1799:
1789:
1695:
1690:
1685:
1626:
1601:
1297:
Yur'ev, V.P.; Kuchin, A.V.; Tolstikov, G.A. (1974). "Reaction of aluminum trialkyls with carbon dioxide".
1824:
1636:
1571:
1561:
1372:
908:
107:
604:
is an example of an asymmetric carboalumination of alkenes catalyzed by a chiral zirconocene catalyst.
288:
Another notable group of alanes are tetraalanes containing four Al(I) centres. These compounds adopt a
70:
of the three-coordinated species. Industrially, these compounds are mainly used for the production of
1903:
1888:
1700:
1680:
1333:
585:
451:
The overall reaction for the production of these simple alkylaluminium compounds is thus as follows:
59:
102:
was discovered in 1859. Organoaluminium compounds were, however, little known until the 1950s when
1804:
864:
124:
123:
Organoaluminium compounds generally feature three- and four-coordinate Al centers, although higher
207:
55:
63:
175:, these smaller ligands tend to occupy the bridging sites. Three coordination occurs when the
1483:
1432:
1246:
1223:
1129:
1088:
1055:
1030:
941:
916:
445:
977:
1475:
1424:
1341:
1306:
1276:
1213:
1205:
1172:
1162:
1108:
1080:
1007:
809:
373:
1403:
1385:
904:
719:
647:
601:
132:
1337:
17:
1218:
1193:
1151:"Discovery of ZACA reaction : Zr-catalyzed asymmetric carboalumination of alkenes"
884:
876:
759:
723:
589:
1084:
1077:
Organoelement
Compounds Possessing Al---Al, Ga---Ga, In---In, and Tl---Tl Single Bonds
619:)-4-iodo-3-methylbut-3-en-1-ol shown below is a typical application of this reaction:
2213:
2176:
1177:
597:
67:
880:
289:
248:
The first organoaluminium compound with an Al-Al bond was reported in 1988 as (((Me
103:
1479:
822:
one obtains the corresponding alkoxides, which can be hydrolysed to the alcohols:
530:, which is dimeric, is prepared by hydride elimination from triisobutylaluminium:
1167:
1449:
912:
711:
164:
622:
1399:
334:
237:
224:
176:
71:
1345:
1112:
1011:
1126:
Comprehensive
Organic Synthesis: Additions to and substitutions at C-C-Bonds
695:
555:
47:
27:
1436:
1428:
1227:
1192:
Negishi, Ei-ichi; Wang, Guangwei; Rao, Honghua; Xu, Zhaoqing (2010-05-14).
1150:
110:. This line of research ultimately resulted in the Nobel Prize to Ziegler.
1324:
Ziegler, K. (1956). "Neue
Entwicklungen der metallorganischen Synthese".
845:
842:
735:
715:
593:
128:
1280:
654:
Metathesis of aluminium trichloride with RLi or RMgX gives the trialkyl:
1310:
1079:. Advances in Organometallic Chemistry. Vol. 51. pp. 53–108.
963:"Organoaluminum chemistry at the forefront of research and development"
888:
220:
196:
168:
1209:
621:
1531:
868:
819:
699:
172:
43:
995:
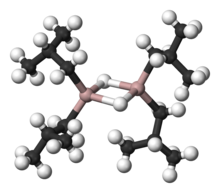
35:, showing aluminium as pink, carbon as black, and hydrogen as white.
333:(R = Me, Et) are prepared in a two-step process beginning with the
872:
860:
227:
spectroscopy. For example, at −25 °C the H NMR spectrum of Me
201:
26:
996:"Ueber die Verbindungen der Erdmetalle mit organischen Radicalen"
1503:
444:
This method is used for production of trimethylaluminium and
859:
The reaction between pure trialalkylaluminum compounds and
1124:
Barry M. Trost; Martin F. Semmelhack; Ian
Fleming (1992).
1243:
Organometallics In
Synthesis: A Manual (Ed. M. Schlosser)
1359:
Zakharkin, L.I.; Gavrilenko, V.V.; Ivanov, L.L. (1967).
1245:. Chichester, West Sussex, UK: Wiley. pp. 963–975.
54:. Illustrative organoaluminium compounds are the dimer
607:
The methylalumination of alkynes in the presence of Cp
464:+ 6MX (where M is an alkali metal and X is a halogen)
808:
The conversion is reminiscent of the carbonation of
579:
Reactions of alkenyl- and alkynylaluminium compounds
325:
Industrially, simple aluminium alkyls of the type Al
2168:
42:is the study of compounds containing bonds between
1050:Cotton, Frank Albert; Wilkinson, Geoffrey (1980).
210:, a compound that features five-coordinate carbon.
588:of higher alkylaluminum species. The reaction is
1472:Ullmann's Encyclopedia of Industrial Chemistry
215:Ligand exchange in trialkylaluminium compounds
1515:
244:Low oxidation state organoaluminium compounds
167:. When the organoaluminium compound contain
62:, and the titanium-aluminium compound called
8:
714:and readily form adducts with bases such as
127:are observed with inorganic ligands such as
1522:
1508:
1500:
223:, Al-Me exchange is fast, as confirmed by
1534:with other elements in the periodic table
1292:
1290:
1217:
1176:
1166:
726:. These adducts are tetrahedral at Al.
461:
457:
50:. It is one of the major themes within
928:
1551:
1460:Handling Chemicals Safely 1980. p. 929
1381:
1370:
236:center and its tendency to achieve an
1029:(3rd ed.). Weinheim: Wiley-VCH.
994:Hallwachs, W.; Schafarik, A. (1859).
372:The reaction resembles the synthesis
82:The first organoaluminium compound (C
7:
2193:Academic research, no widespread use
936:D. F. Shriver; P. W. Atkins (2006).
841:A structurally characterized organo
25:
2085:
2004:
1936:
1841:
1541:
1299:Organic and Biological Chemistry
1269:The Journal of Organic Chemistry
1198:The Journal of Organic Chemistry
321:From alkyl halides and aluminium
961:M. Witt; H. W. Roesky (2000).
710:Organoaluminium compounds are
1:
1480:10.1002/14356007.a13_227.pub3
1398:David W. Marshall, US patent
1085:10.1016/S0065-3055(03)51002-4
398:ethylaluminium sesquichloride
1168:10.3998/ark.5550190.0012.803
1052:Advanced Inorganic Chemistry
31:The ball-and-stick model of
940:. Oxford University Press.
915:, for example the catalyst
528:Diisobutylaluminium hydride
33:diisobutylaluminium hydride
2236:
1025:Elschenbroich, C. (2006).
576:
476:alkenylaluminium compounds
473:
2220:Organoaluminium compounds
2082:
2001:
1553:
1549:
1539:
1241:Negishi, Ei-ichi (2002).
1178:2027/spo.5550190.0012.803
1149:Negishi, Ei-ichi (2011).
292:core, as illustrated by (
40:Organoaluminium chemistry
1346:10.1002/ange.19560682302
1113:10.1002/14356007.a01_543
1012:10.1002/jlac.18591090214
976:(4): 410. Archived from
893:halogenated hydrocarbons
119:Aluminium(III) compounds
52:organometallic chemistry
18:Organoaluminium compound
638:Laboratory preparations
135:alkyl ligands, e.g., Al
2188:Many uses in chemistry
2183:Core organic chemistry
1429:10.1002/chem.200701916
626:
211:
179:is bulky, e.g. Al(Mes)
36:
909:alkene polymerization
625:
456:2Al + 6RX + 6M → Al
337:of aluminium powder:
205:
114:Structure and bonding
108:olefin polymerization
30:
746:+ ROH → 1/n (R'
586:Poisson distribution
125:coordination numbers
60:triisobutylaluminium
1450:Cameo Chemicals SDS
1338:1956AngCh..68..721Z
1281:10.1021/jo00333a041
938:Inorganic Chemistry
238:octet configuration
1311:10.1007/BF00923507
627:
600:first reported by
376:. The product, (CH
212:
208:trimethylaluminium
56:trimethylaluminium
37:
2207:
2206:
2163:
2162:
1474:. pp. 1–74.
1380:Missing or empty
1275:(20): 4093–4096.
1210:10.1021/jo1003218
1204:(10): 3151–3182.
1061:978-0-471-02775-1
1036:978-3-527-29390-2
1000:Liebigs Ann. Chem
917:methylaluminoxane
810:Grignard reagents
663:+ 3 BuLi → Bu
446:triethylaluminium
374:Grignard reagents
16:(Redirected from
2227:
2199:
2194:
2189:
2184:
2089:
2088:
2008:
2007:
1940:
1939:
1845:
1844:
1542:
1524:
1517:
1510:
1501:
1494:
1493:
1467:
1461:
1458:
1452:
1447:
1441:
1440:
1412:
1406:
1396:
1390:
1389:
1383:
1378:
1376:
1368:
1361:Zh. Obshch. Khim
1356:
1350:
1349:
1321:
1315:
1314:
1294:
1285:
1284:
1263:
1257:
1256:
1238:
1232:
1231:
1221:
1189:
1183:
1182:
1180:
1170:
1146:
1140:
1139:
1121:
1115:
1105:
1099:
1098:
1075:Uhl, W. (2004).
1072:
1066:
1065:
1047:
1041:
1040:
1022:
1016:
1015:
991:
985:
984:
982:
967:
958:
952:
951:
933:
895:can be violent.
672:Transmetalation:
596:. The so-called
573:Carboalumination
470:Hydroalumination
465:
21:
2235:
2234:
2230:
2229:
2228:
2226:
2225:
2224:
2210:
2209:
2208:
2203:
2202:
2197:
2192:
2187:
2182:
2164:
2086:
2005:
1937:
1842:
1535:
1528:
1498:
1497:
1490:
1469:
1468:
1464:
1459:
1455:
1448:
1444:
1423:(10): 3067–71.
1414:
1413:
1409:
1404:Continental Oil
1397:
1393:
1379:
1369:
1358:
1357:
1353:
1332:(23): 721–729.
1323:
1322:
1318:
1296:
1295:
1288:
1265:
1264:
1260:
1253:
1240:
1239:
1235:
1191:
1190:
1186:
1161:(viii): 34–53.
1148:
1147:
1143:
1136:
1123:
1122:
1118:
1106:
1102:
1095:
1074:
1073:
1069:
1062:
1054:. p. 343.
1049:
1048:
1044:
1037:
1027:Organometallics
1024:
1023:
1019:
993:
992:
988:
980:
965:
960:
959:
955:
948:
935:
934:
930:
925:
905:Ziegler Process
901:
885:nitrogen oxides
855:
851:
837:
833:
829:
804:
800:
796:
792:
788:
781:
777:
773:
769:
753:
749:
745:
732:
724:tertiary amines
708:
692:
684:
680:
666:
662:
648:transmetalation
640:
614:
610:
602:Ei-ichi Negishi
581:
575:
567:
563:
559:
553:
549:
541:
523:
519:
515:
511:
507:
496:
492:
488:
478:
472:
463:
459:
455:
440:+ 2 Al + 6 NaCl
439:
435:
431:
427:
423:
419:
415:
411:
407:
395:
391:
387:
383:
379:
368:
364:
360:
356:
352:
348:
344:
332:
328:
323:
318:
311:
307:
303:
299:
283:
279:
275:
271:
263:
259:
255:
251:
246:
234:
230:
217:
199:) or isobutyl.
194:
190:
186:
183:(Mes = 2,4,6-Me
182:
162:
158:
154:
150:
146:
142:
138:
121:
116:
101:
97:
93:
89:
85:
80:
64:Tebbe's reagent
23:
22:
15:
12:
11:
5:
2233:
2231:
2223:
2222:
2212:
2211:
2205:
2204:
2201:
2200:
2195:
2190:
2185:
2180:
2177:Chemical bonds
2173:
2172:
2170:
2166:
2165:
2161:
2160:
2155:
2150:
2145:
2140:
2135:
2130:
2125:
2120:
2115:
2110:
2105:
2100:
2095:
2090:
2083:
2080:
2079:
2074:
2069:
2064:
2059:
2054:
2049:
2044:
2039:
2034:
2029:
2024:
2019:
2014:
2009:
2002:
1999:
1998:
1994:
1993:
1990:
1987:
1984:
1981:
1978:
1975:
1972:
1969:
1966:
1963:
1960:
1957:
1952:
1949:
1946:
1941:
1934:
1929:
1925:
1924:
1921:
1916:
1911:
1906:
1901:
1896:
1891:
1886:
1881:
1876:
1871:
1866:
1861:
1856:
1851:
1846:
1839:
1834:
1828:
1827:
1822:
1817:
1812:
1807:
1802:
1797:
1792:
1787:
1782:
1777:
1772:
1767:
1762:
1757:
1752:
1747:
1745:
1740:
1734:
1733:
1728:
1723:
1718:
1713:
1708:
1703:
1698:
1693:
1688:
1683:
1678:
1673:
1668:
1663:
1658:
1653:
1651:
1646:
1640:
1639:
1634:
1629:
1624:
1619:
1614:
1609:
1604:
1598:
1597:
1594:
1589:
1584:
1579:
1574:
1569:
1564:
1558:
1557:
1554:
1552:
1550:
1548:
1540:
1537:
1536:
1529:
1527:
1526:
1519:
1512:
1504:
1496:
1495:
1488:
1462:
1453:
1442:
1407:
1402:, assigned to
1391:
1351:
1316:
1305:(4): 817–819.
1286:
1258:
1252:978-0471984160
1251:
1233:
1184:
1141:
1134:
1116:
1100:
1093:
1067:
1060:
1042:
1035:
1017:
1006:(2): 206–209.
986:
983:on 2014-10-06.
953:
947:978-0199264636
946:
927:
926:
924:
921:
900:
897:
877:carbon dioxide
853:
852:}Al(R)-O-O-CMe
849:
839:
838:
835:
831:
827:
806:
805:
802:
798:
794:
790:
786:
783:
779:
775:
771:
767:
760:carbon dioxide
756:
755:
751:
747:
743:
731:
728:
707:
704:
691:
688:
687:
686:
682:
678:
674:
673:
669:
668:
664:
660:
656:
655:
639:
636:
612:
608:
590:regioselective
577:Main article:
574:
571:
570:
569:
565:
561:
557:
551:
547:
539:
525:
524:
521:
517:
513:
509:
505:
498:
497:
494:
490:
486:
471:
468:
467:
466:
442:
441:
437:
433:
429:
425:
424:+ 6 Na → (CH
421:
417:
413:
409:
405:
393:
389:
385:
381:
377:
370:
369:
366:
362:
358:
354:
350:
346:
342:
330:
326:
322:
319:
317:
314:
309:
305:
301:
297:
286:
285:
281:
277:
273:
269:
261:
257:
253:
249:
245:
242:
232:
228:
216:
213:
192:
188:
184:
180:
160:
156:
152:
148:
144:
140:
136:
120:
117:
115:
112:
99:
95:
91:
87:
83:
79:
76:
58:, the monomer
24:
14:
13:
10:
9:
6:
4:
3:
2:
2232:
2221:
2218:
2217:
2215:
2196:
2191:
2186:
2181:
2178:
2175:
2174:
2171:
2167:
2159:
2156:
2154:
2151:
2149:
2146:
2144:
2141:
2139:
2136:
2134:
2131:
2129:
2126:
2124:
2121:
2119:
2116:
2114:
2111:
2109:
2106:
2104:
2101:
2099:
2096:
2094:
2091:
2084:
2081:
2078:
2075:
2073:
2070:
2068:
2065:
2063:
2060:
2058:
2055:
2053:
2050:
2048:
2045:
2043:
2040:
2038:
2035:
2033:
2030:
2028:
2025:
2023:
2020:
2018:
2015:
2013:
2010:
2003:
2000:
1996:
1995:
1991:
1988:
1985:
1982:
1979:
1976:
1973:
1970:
1967:
1964:
1961:
1958:
1956:
1953:
1950:
1947:
1945:
1942:
1935:
1933:
1930:
1927:
1926:
1922:
1920:
1917:
1915:
1912:
1910:
1907:
1905:
1902:
1900:
1897:
1895:
1892:
1890:
1887:
1885:
1882:
1880:
1877:
1875:
1872:
1870:
1867:
1865:
1862:
1860:
1857:
1855:
1852:
1850:
1847:
1840:
1838:
1835:
1833:
1830:
1829:
1826:
1823:
1821:
1818:
1816:
1813:
1811:
1808:
1806:
1803:
1801:
1798:
1796:
1793:
1791:
1788:
1786:
1783:
1781:
1778:
1776:
1773:
1771:
1768:
1766:
1763:
1761:
1758:
1756:
1753:
1751:
1748:
1746:
1744:
1741:
1739:
1736:
1735:
1732:
1729:
1727:
1724:
1722:
1719:
1717:
1714:
1712:
1709:
1707:
1704:
1702:
1699:
1697:
1694:
1692:
1689:
1687:
1684:
1682:
1679:
1677:
1674:
1672:
1669:
1667:
1664:
1662:
1659:
1657:
1654:
1652:
1650:
1647:
1645:
1642:
1641:
1638:
1635:
1633:
1630:
1628:
1625:
1623:
1620:
1618:
1615:
1613:
1610:
1608:
1605:
1603:
1600:
1599:
1595:
1593:
1590:
1588:
1585:
1583:
1580:
1578:
1575:
1573:
1570:
1568:
1565:
1563:
1560:
1559:
1555:
1547:
1544:
1543:
1538:
1533:
1530:Compounds of
1525:
1520:
1518:
1513:
1511:
1506:
1505:
1502:
1491:
1489:9783527306732
1485:
1481:
1477:
1473:
1466:
1463:
1457:
1454:
1451:
1446:
1443:
1438:
1434:
1430:
1426:
1422:
1418:
1411:
1408:
1405:
1401:
1395:
1392:
1387:
1374:
1366:
1362:
1355:
1352:
1347:
1343:
1339:
1335:
1331:
1327:
1320:
1317:
1312:
1308:
1304:
1300:
1293:
1291:
1287:
1282:
1278:
1274:
1270:
1262:
1259:
1254:
1248:
1244:
1237:
1234:
1229:
1225:
1220:
1215:
1211:
1207:
1203:
1199:
1195:
1188:
1185:
1179:
1174:
1169:
1164:
1160:
1156:
1152:
1145:
1142:
1137:
1135:9780080405957
1131:
1127:
1120:
1117:
1114:
1110:
1104:
1101:
1096:
1094:9780120311514
1090:
1086:
1082:
1078:
1071:
1068:
1063:
1057:
1053:
1046:
1043:
1038:
1032:
1028:
1021:
1018:
1013:
1009:
1005:
1001:
997:
990:
987:
979:
975:
971:
964:
957:
954:
949:
943:
939:
932:
929:
922:
920:
918:
914:
910:
906:
898:
896:
894:
890:
886:
882:
881:sulfur oxides
878:
874:
870:
866:
862:
857:
847:
844:
825:
824:
823:
821:
816:
813:
811:
784:
765:
764:
763:
761:
741:
740:
739:
737:
730:Electrophiles
729:
727:
725:
721:
717:
713:
706:Lewis acidity
705:
703:
701:
697:
689:
677:2 Al + 3 HgPh
676:
675:
671:
670:
658:
657:
653:
652:
651:
649:
645:
637:
635:
632:
624:
620:
618:
605:
603:
599:
598:ZACA reaction
595:
591:
587:
580:
572:
568:
545:
537:
533:
532:
531:
529:
503:
502:
501:
484:
483:
482:
477:
469:
454:
453:
452:
449:
447:
403:
402:
401:
399:
375:
340:
339:
338:
336:
320:
315:
313:
295:
291:
267:
266:
265:
243:
241:
239:
226:
222:
214:
209:
206:Structure of
204:
200:
198:
178:
174:
170:
166:
134:
130:
126:
118:
113:
111:
109:
105:
77:
75:
73:
69:
68:Lewis acidity
65:
61:
57:
53:
49:
45:
41:
34:
29:
19:
2198:Bond unknown
1611:
1471:
1465:
1456:
1445:
1420:
1417:Chem. Eur. J
1416:
1410:
1394:
1382:|title=
1373:cite journal
1364:
1360:
1354:
1329:
1325:
1319:
1302:
1298:
1272:
1268:
1261:
1242:
1236:
1201:
1197:
1187:
1158:
1154:
1144:
1128:. Pergamon.
1125:
1119:
1103:
1076:
1070:
1051:
1045:
1026:
1020:
1003:
999:
989:
978:the original
973:
969:
956:
937:
931:
902:
899:Applications
858:
840:
817:
814:
807:
757:
733:
709:
693:
641:
630:
628:
616:
606:
582:
543:
535:
526:
512:=CHR → 3 [Al
499:
479:
450:
443:
396:, is called
371:
324:
290:tetrahedrane
287:
247:
218:
165:ethyl groups
122:
104:Karl Ziegler
81:
39:
38:
1326:Angew. Chem
913:polyolefins
793:CR + CO
667:Al + 3 LiCl
341:2 Al + 3 CH
316:Preparation
72:polyolefins
923:References
834:→ Al(OR)
712:hard acids
681:→ 2 AlPh
644:metathesis
485:6 Al + 3 H
474:See also:
335:alkylation
225:proton NMR
2179:to carbon
1400:US3168570
970:Curr. Sci
797:→ RAl(O
736:alkoxides
696:aluminium
690:Reactions
594:1-alkenes
493:=CHR → 2
276:+ 2 K → R
48:aluminium
2214:Category
1437:18283706
1228:20465291
889:halogens
865:alcohols
846:peroxide
843:aluminum
830:+ 3/2 O
716:pyridine
349:Cl → (CH
300:and ((Me
221:solvents
177:R groups
133:bridging
129:fluoride
1997:
1334:Bibcode
1219:2933819
1155:Arkivoc
869:phenols
489:+ 12 CH
284:+ 2 KCl
197:mesityl
169:hydride
78:History
2169:Legend
1532:carbon
1486:
1435:
1367:: 992.
1249:
1226:
1216:
1132:
1091:
1058:
1033:
944:
891:, and
873:amines
820:oxygen
754:+ R'H
750:Al−OR)
702:atom.
700:carbon
685:+ 3 Hg
542:Al → (
508:+ 3 CH
280:Al-AlR
173:halide
44:carbon
981:(PDF)
966:(PDF)
861:water
818:With
774:→ R
770:+ CO
404:2 (CH
308:C)Al)
272:AlCl)
1484:ISBN
1433:PMID
1386:help
1247:ISBN
1224:PMID
1159:2011
1130:ISBN
1089:ISBN
1056:ISBN
1031:ISBN
942:ISBN
856:] .
742:AlR'
722:and
698:and
659:AlCl
611:ZrCl
592:for
564:C=CH
554:+ 2
550:AlH)
520:CHR)
151:(μ-C
46:and
2143:CEs
2138:CCf
2133:CBk
2128:CCm
2123:CAm
2118:CPu
2113:CNp
2103:CPa
2098:CTh
2077:CYb
2072:CTm
2067:CEr
2062:CHo
2057:CDy
2052:CTb
2047:CGd
2042:CEu
2037:CSm
2032:CPm
2027:CNd
2022:CPr
2017:CCe
2012:CLa
1992:Og
1989:Ts
1986:Lv
1983:Mc
1980:Fl
1977:Nh
1974:Cn
1971:Rg
1968:Ds
1965:Mt
1962:Hs
1959:Bh
1955:CSg
1951:Db
1948:Rf
1932:CRa
1928:Fr
1923:Rn
1919:CAt
1914:CPo
1909:CBi
1904:CPb
1899:CTl
1894:CHg
1889:CAu
1884:CPt
1879:CIr
1874:COs
1869:CRe
1859:CTa
1854:CHf
1849:CLu
1837:CBa
1832:CCs
1825:CXe
1815:CTe
1810:CSb
1805:CSn
1800:CIn
1795:CCd
1790:CAg
1785:CPd
1780:CRh
1775:CRu
1770:CTc
1765:CMo
1760:CNb
1755:CZr
1743:CSr
1738:CRb
1731:CKr
1726:CBr
1721:CSe
1716:CAs
1711:CGe
1706:CGa
1701:CZn
1696:CCu
1691:CNi
1686:CCo
1681:CFe
1676:CMn
1671:CCr
1661:CTi
1656:CSc
1649:CCa
1637:CAr
1632:CCl
1617:CSi
1612:CAl
1607:CMg
1602:CNa
1596:Ne
1567:CBe
1562:CLi
1556:He
1476:doi
1425:doi
1365:377
1342:doi
1307:doi
1277:doi
1214:PMC
1206:doi
1173:hdl
1163:doi
1109:doi
1081:doi
1008:doi
1004:109
911:to
848:is
826:AlR
801:CR)
789:AlO
778:AlO
766:AlR
720:THF
650:.
646:or
631:syn
556:(CH
546:-Bu
538:-Bu
516:(CH
296:Al)
294:Cp*
260:Al)
256:CH)
252:Si)
195:or
171:or
2216::
2158:No
2153:Md
2148:Fm
2108:CU
2093:Ac
1944:Lr
1864:CW
1820:CI
1750:CY
1666:CV
1644:CK
1627:CS
1622:CP
1592:CF
1587:CO
1582:CN
1577:CC
1572:CB
1546:CH
1482:.
1431:.
1421:14
1419:.
1377::
1375:}}
1371:{{
1363:.
1340:.
1330:68
1328:.
1303:23
1301:.
1289:^
1273:46
1271:.
1222:.
1212:.
1202:75
1200:.
1196:.
1171:.
1157:.
1153:.
1087:.
1002:.
998:.
974:78
972:.
968:.
919:.
887:,
883:,
879:,
875:,
871:,
867:,
863:,
812:.
782:CR
738::
718:,
534:2
504:2
448:.
436:Al
428:CH
420:Cl
416:Al
408:CH
392:Cl
388:Al
380:CH
365:Cl
361:Al
353:CH
345:CH
304:Si
268:(R
240:.
231:Al
139:(C
94:Al
74:.
1523:e
1516:t
1509:v
1492:.
1478::
1439:.
1427::
1388:)
1384:(
1348:.
1344::
1336::
1313:.
1309::
1283:.
1279::
1255:.
1230:.
1208::
1181:.
1175::
1165::
1138:.
1111::
1097:.
1083::
1064:.
1039:.
1014:.
1010::
950:.
854:3
850:2
836:3
832:2
828:3
803:2
799:2
795:2
791:2
787:2
785:R
780:2
776:2
772:2
768:3
752:n
748:2
744:3
683:3
679:2
665:3
661:3
617:E
613:2
609:2
566:2
562:2
560:)
558:3
552:2
548:2
544:i
540:3
536:i
522:3
518:2
514:2
510:2
506:3
495:3
491:2
487:2
462:6
460:R
458:2
438:2
434:6
432:)
430:2
426:3
422:3
418:2
414:3
412:)
410:2
406:3
394:3
390:2
386:3
384:)
382:2
378:3
367:3
363:2
359:3
357:)
355:2
351:3
347:2
343:3
331:6
329:R
327:2
310:4
306:3
302:3
298:4
282:2
278:2
274:2
270:2
262:2
258:2
254:2
250:3
233:2
229:6
193:2
191:H
189:6
187:C
185:3
181:3
161:2
159:)
157:5
155:H
153:2
149:4
147:)
145:5
143:H
141:2
137:2
100:3
98:I
96:2
92:3
90:)
88:5
86:H
84:2
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.