88:
96:
1418:
986:
1477:
827:
743:
3118:
2969:
3037:
2874:
1361:
36:
342:. Grignard reagents can be used in place of organolithium compounds. Gilman also investigated the dialkylcuprates. These are obtained by combining two equivalent of RLi with Cu(I) salts. Alternatively, these cuprates are prepared from oligomeric neutral organocopper compounds by treatment with one equivalent of organolithium reagent.
283:
423:
1086:
1726:
Yamamoto, Y.; Yamammoto, S.; Yatagai, H.; Maruyama, K (1980). "Lewis acid mediated reactions of organocopper reagent. A remarkably enhanced regioselective gamma- attack of allylic halides and direct alkylation of allylic alcohols via
738:{\displaystyle {\begin{aligned}{\ce {{ArX}+ (Ar')2CuLi}}\ &{\ce {<=> {ArAr'CuLi}+ Ar'X}}\\{\ce {2ArAr'CuLi}}\ &{\ce {<=> {(Ar)2CuLi}+ (Ar')2CuLi}}\\{\ce {{ArAr'CuLi}+ O2}}\ &{\ce {-> Ar-Ar'}}\end{aligned}}}
1673:
Muller and collaborators reported a vicinal functionalization of α,β-acetylenic esters using a carbocupration/Mukaiyama aldol reaction sequence (as shown in the figure above) carbocupration favors the formation of the Z-aldol.
1650:
attack occurs better in cyclohexyl carbamate due to sterics. The reaction is reported to be favorable in ethereal solvents. This method was proved to be very effective for the oxidative coupling of amines and alkyl, including
1696:
Yao, B.; Liu, Y.; Zhao, L.; Wang, D.; Wang, M. (2014). "Designing a Cu(II)−ArCu(II)−ArCu(III)−Cu(I) Catalytic Cycle: Cu(II)-Catalyzed
Oxidative Arene C−H Bond Azidation with Air as an Oxidant under Ambient Conditions".
2007:
Cairncross, Allan; Sheppard, William A; Wonchoba, Edward; Guilford, William J; House, Cynthia B; Coates, Robert M (1979). "Pentafluorophenylcopper tetramer, a reagent for synthesis of fluorinated aromatic compounds".
278:
Copper(I) salts have long been known to bind CO, albeit weakly. A representative complex is CuCl(CO), which is polymeric. In contrast to classical metal carbonyls, pi-backbonding is not strong in these compounds.
1356:{\displaystyle ^{-}{\ce {Li+}}\ {\xrightarrow {\color {Red}{\ce {R'-X}}}}\ \left^{-}{\ce {Li+}}{\ce {->R}}{-}{\color {Blue}{\ce {Cu}}}+{\ce {R}}{-}{\color {Red}{\ce {R'}}}+{\ce {Li}}{-}{\color {Red}{\ce {X}}}}
405:
Alkyl halides react with organocopper compounds with inversion of configuration. On the other hand, reactions of organocopper compound with alkenyl halides proceed with retention of subtrate’s configuration.
1412:
Generally the OA-RE mechanism is analogous to that of palladium-catalyzed cross coupling reactions. One difference between copper and palladium is that copper can undergo single-electron transfer processes.
2066:
Bertz, Steven H.; Cope, Stephen; Murphy, Michael; Ogle, Craig A.; Taylor, Brad J. (2007). "Rapid
Injection NMR in Mechanistic Organocopper Chemistry. Preparation of the Elusive Copper(III) Intermediate1".
1627:
359:. They also tend to be thermally unstable, which can be useful in certain coupling reactions. Despite or because of these difficulties, organocopper reagents are frequently generated and consumed
1024:
for almost a century. Palladium offers a faster, more selective reaction. Copper reagents and catalysts continue to be the subject of innovation. Relative to palladium, copper is cheaper but the
428:
294:
Alkenes bind to copper(I), although again generally weakly. The binding of ethylene to Cu in proteins is of broad significance in plant biology so much so that ethylene is classified as a
2165:
Posner, Gary H.; Whitten, Charles E. (2003). "Secondary and
Tertiary Alkyl Ketones from Carboxylic Acid Chlorides and Lithium Phenylthio(Alkyl)Cuprate Reagents:tert-Butyl Phenyl Ketone".
753:
Alkyl and aryl copper complexes aggregate both in crystalline form and in solution. Aggregation is especially evident for charge-neutral organocopper compounds, i.e. species with the
2525:
Muller, A.J.; Jennings, M.P. Vicinal
Functionalization of propionilate Esters via Tandem Catalytic Carbocupration-Mukaiyama Aldol Reaction sequence. Org. Lett. 2008, 10, 1649-1652
2437:
Cox, N.; Dang, H.; Whittaker, A.M.; Lalic, G. (2014). "NHC- copper hydrides as chemoselective reducing agents: catalytic reduction of alkynes, alkyl triflates and alkyl halides".
1463:
forming the arylcopper (ArCu) intermediate. Simultaneously, a palladium catalyst reacts with an aryl bromide to give an organopalladium intermediate (Ar'PdB), which undergoes
54:
2552:
1417:
1905:
Light, K. M.; Wisniewski, J. A.; Vinyard, W. A.; Kieber-Emmons, M. T. (2016). "Perception of the plant hormone ethylene: known-knowns and known-unknowns".
1668:
985:
1626:
2464:
Jurkauskas, V.; Sadighi, J. P.; Buchwald, S. L. (2003). "Conjugate addition of a,b- unsaturated compounds catalyzad by a copper carbene complex".
1610:
1540:
1476:
87:
95:
2545:
1366:
Many electrophiles participate in this reaction. The approximate order of reactivity, beginning with the most reactive, is as follows:
1491:
998:
263:
1948:
Delbaere, L. T. J.; McBride, D. W.; Ferguson, R. B. (1970). "Crystal structure of π-cyclopentadienyl(triethylphosphine)copper(I), π-C
1831:
1806:
1781:
945:
is formed (indefinitely stable at that temperature) and on increasing the temperature to −80 °C the conjugate addition product
72:
1849:"Ueber die Einwirkung des Leuchtgases auf verschiedene Salzsolutionen, insbesondere auf eine ammoniakalische Kupferchlorürlösung"
954:
3250:
2538:
2421:
2182:
1456:
802:
1585:
reaction, which utilizes both copper and palladium, entails the coupling of aryl and/or vinyl halides with terminal alkynes.
761:. This effect is illustrated by the structure of mesitylcopper, which is a pentamer. A cyclic structure is also seen for
826:
1484:
757:(RCu), which adopt cyclic structures. Since each copper center requires at least two ligands, the organic group is a
151:
3107:
3102:
3097:
3092:
3087:
3082:
3077:
3072:
3067:
3062:
3057:
3052:
3042:
2985:
2879:
2800:
2795:
2652:
2378:
2102:
Hu, Haipeng; Snyder, James P. (2007). "Organocuprate
Conjugate Addition: The Square-Planar "CuIII" Intermediate".
305:, half-sandwich complexes can be produced. One such derivative is π-cyclopentadienyl(triethylphosphine)copper(I).
3143:
2845:
2815:
2805:
2785:
2773:
2741:
2706:
2674:
2642:
2637:
2597:
2249:
205:, the bonding behavior of Cu(I) is similar to Ni(0), but owing to its higher oxidation state, it engages in less
2612:
2576:
2530:
2380:
NOVEL METHODOLOGIES VIA THE CATALYTIC CARBOCUPRATION OF ALKYNOATES AND THE TOTAL SYNTHESIS OF (+)-ASPERGILLIDE B
3188:
3183:
3178:
3173:
3168:
3163:
3158:
3153:
3148:
3133:
3123:
2974:
2949:
2944:
2929:
2914:
2894:
2889:
2840:
2768:
2751:
2701:
2696:
2691:
2686:
2662:
2622:
1441:
111:
3138:
3128:
2939:
2924:
2909:
2899:
2884:
2825:
2810:
2790:
2780:
2761:
2756:
2746:
2736:
2679:
2647:
1013:
2617:
2607:
193:
Organocopper compounds are diverse in structure and reactivity, but almost all are based on copper with an
3047:
2962:
2904:
2867:
2862:
2850:
2830:
2820:
2721:
2716:
2657:
2632:
2301:
Normant, J; Bourgain, M. (1971). "Synthese stereospecifique and reactivite d' organocuivreux vinyliques".
2137:
Beletkaya, I.P.; Cheprakov, A.V. (2004). "Copper in Cross
Coupling Reactions: The Post Ullman Chemistry".
1483:
Redox neutral coupling is the coupling of terminal alkynes with halo-alkynes with a copper(I) salt in the
938:
794:, forming an 8-membered ring with alternating Cu-C bonds. In addition the four copper atoms form a planar
2198:
Goossen, L. J.; Deng, G; Levy, LM (2006). "Synthesis of
Biaryls via Catalytic Decarboxylative Coupling".
2855:
2667:
2602:
2592:
1505:
1073:
319:
210:
1997:
Posner, G. H. 2011. Substitution
Reactions Using Organocopper Reagents. Organic Reactions. 22:2:253–400
2934:
2919:
2731:
2711:
2207:
1602:
1582:
1437:
410:
287:
2835:
2404:
Daeuble, John F.; Stryker, Jeffrey M. (2001). "Hexa-μ-hydrohexakis(triphenylphosphine)hexacopper".
2241:
2035:
N. P. Lorenzen, E. Weiss (1990). "Synthesis and
Structure of a Dimeric Lithium Diphenylcuprate:2".
133:
1571:
involves copper-mediated reactions of aryl halides. Two types of
Ullmann reaction are recognized:
1930:
1667:
1065:
1061:
891:
368:
209:. Organic derivatives of copper's higher oxidation states +2 and +3 are sometimes encountered as
159:
2481:
2417:
2359:
2245:
2223:
2178:
2119:
2084:
2037:
1922:
1827:
1802:
1777:
1714:
1554:
1448:
834:
754:
376:
364:
335:
126:
2499:
Yamamoto, H.; Marouka, K. (1980). "Novel N-alkylation of amines with organocopper reagents".
1878:
Strauss, S. H. (2000). "Copper(I) and Silver(I) Carbonyls. To be or not to be Nonclassical".
2508:
2473:
2446:
2409:
2349:
2341:
2310:
2283:
2253:
2215:
2170:
2146:
2111:
2076:
2045:
2017:
1977:
1914:
1887:
1860:
1740:
1706:
1568:
1464:
1452:
977:
903:
414:
380:
372:
224:
202:
1543:
Catalytic cycle for carbocupration for the synthesis of aldol, Baylis-Hillman type products
890:
The involvement of the otherwise rare Cu(III) oxidation state has been demonstrated in the
2257:
1539:
1529:
1433:
1429:
1069:
1025:
783:
758:
388:
356:
298:. Its presence, detected by the Cu-protein, affects ripening and many other developments.
194:
2211:
2354:
2329:
2261:
1765:
1598:
919:
895:
787:
206:
2314:
3244:
3207:
1770:
1558:
1367:
923:
838:
339:
331:
295:
122:
1934:
1801:. Vol. 5, Copper, Silver, Gold, Zinc, Cadmium, and Mercury. Stuttgart: Thieme.
1663:
Vicinal functionalization using a carbocupration/Mukaiyama aldol reaction sequence:
1451:
of aryl halides with a stoichiometric equivalent of copper metal that occurs in the
1683:
1391:
1387:
1383:
1076:(RE). The nucleophilic attack is the rate-determining step. In the substitution of
1040:
773:
327:
323:
2174:
1618:
973:
817:
a 5-membered copper ring is formed, similar to (2,4,6-trimethylphenyl)gold, and
806:
302:
267:
217:
2274:"Addition of an Ethylcopper Complex to 1-Octyne: (E)-5-Ethyl-1,4-Undecadiene".
1557:
enables the formation of aryl carbon-hetoroatom bonds. It involves coupling of
841:, forming an 8-membered ring with two lithium atoms linking two methyl groups,
2450:
2413:
2150:
1981:
1918:
962:
931:
236:
2287:
2021:
1864:
2219:
1562:
1513:
1009:
950:
810:
399:
290:, the Cu centers are tetrahedral linked by triply bridging chloride ligands.
155:
17:
2485:
2363:
2227:
2123:
2088:
2049:
1926:
1718:
972:
group and anti-parallel to the methine proton. With other ligands than the
1460:
1375:
1371:
1021:
791:
2512:
1744:
1638:
Generally, the alkylation reaction of organocopper reagents proceed via
786:(1972 by Lappert). This compound is relatively stable because the bulky
2345:
1517:
1403:
1379:
1028:
are often lower with copper and the reaction conditions more vigorous.
969:
360:
248:
2477:
2115:
2080:
1710:
375:
tolerance than corresponding Grignard and organolithium reagents. The
2562:
1891:
1468:
1407:
1395:
1077:
1017:
871:
395:
220:
119:
115:
106:
is the study of the physical properties, reactions, and synthesis of
1848:
1152:
1487:. Thermal coupling of two organocopper compounds is also possible.
262:, copper(I) complexes have symmetrical structures - either linear,
1614:
1538:
1399:
958:
899:
856:. Similarly, lithium diphenylcuprate(I) forms a dimeric etherate,
282:
281:
1080:, a single-electron transfer mechanism is proposed (see figure).
1574:
Classic copper-promoted synthesis of symmetric biaryl compounds)
384:
2534:
782:, the first 1:1 organocopper compound to be analyzed by X-ray
29:
91:
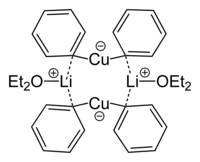
Lithium diphenylcuprate etherate dimer from crystal structure
379:
of copper is much higher than its next-door neighbor in the
1799:
Synthetic Methods of Organometallic and Inorganic Chemistry
1459:, one coupling partner is a carboxylate. Cu(I) displaces a
1467:
to give ArPdAr', which in turn reductively eliminates the
322:
to give organocopper compounds. The area was pioneered by
99:
Skeletal formula of lithium diphenylcuprate etherate dimer
701:
663:
630:
463:
1772:
An introduction to synthesis using organocopper reagents
216:
Organocopper compounds form complexes with a variety of
50:
592:
497:
1226:
1212:
1089:
930:) enabling the detection of the copper — alkene
426:
409:
Organocopper compounds couple with aryl halides (see
1880:
Journal of the Chemical Society, Dalton Transactions
1565:, or siloxanes with NH- or OH-containing substrates.
3199:
1577:
Copper-promoted nucleophilic aromatic substitution.
45:
may be too technical for most readers to understand
1769:
1613:. A related but catalytic reaction uses copper(I)
1355:
737:
363:with no attempt to isolate them. They are used in
600:
599:
582:
581:
505:
504:
487:
486:
274:Simple complexes with CO, alkene, and Cp ligands
1617:complex with hydride equivalents provided by a
906:experiment at −100 °C, the Gilman reagent
355:are reactive towards oxygen and water, forming
132:The first organocopper compound, the explosive
2406:Encyclopedia of Reagents for Organic Synthesis
2546:
1428:Oxidative coupling is the coupling of copper
1068:(OA) of the alkyl halide to Cu(I), forming a
8:
2330:"Copper mediated carbometalation reactions"
953:experiments the Cu(III) intermediate has a
326:, who reported methylcopper in 1936. Thus,
286:Part of the framework of CuCl(CO). In this
2553:
2539:
2531:
790:groups provide steric protection. It is a
2565:with other elements in the periodic table
2353:
1346:
1344:
1339:
1334:
1319:
1317:
1312:
1307:
1297:
1295:
1290:
1282:
1275:
1270:
1264:
1250:
1249:
1241:
1229:
1225:
1214:
1211:
1206:
1199:
1195:
1190:
1185:
1163:
1154:
1147:
1137:
1132:
1126:
1113:
1112:
1105:
1103:
1098:
1093:
1088:
717:
710:
700:
695:
675:
674:
662:
657:
641:
629:
624:
613:
611:
601:
594:
593:
591:
583:
576:
574:
573:
571:
570:
552:
548:
516:
506:
499:
498:
496:
488:
481:
479:
478:
476:
475:
462:
457:
441:
432:
431:
427:
425:
258:Due to the spherical electronic shell of
73:Learn how and when to remove this message
57:, without removing the technical details.
2104:Journal of the American Chemical Society
2069:Journal of the American Chemical Society
2061:
2059:
1733:Journal of the American Chemical Society
1523:
1520:resulting in an alkenylcopper compound (
1052:
1034:
913:
909:
878:
874:
867:
863:
859:
852:
848:
844:
797:
778:
768:
764:
348:
242:
230:
213:, but rarely isolated or even observed.
180:
176:
172:
168:
142:
138:
94:
86:
1757:
575:
480:
2582:
813:compared to 256 pm in bulk copper. In
270:, depending on the number of ligands.
1993:
1991:
1345:
1318:
1296:
1227:
1213:
1198:
1153:
1104:
55:make it understandable to non-experts
7:
3224:Academic research, no widespread use
886:Alkyl and aryl copper(III) compounds
27:Compound with carbon to copper bonds
1826:(3 ed.). Weinheim: Wiley-VCH.
1659:Vicinal functionalization reactions
1609:. It reduces the alkene portion of
1601:. The well-known copper hydride is
1611:α,β-Unsaturated carbonyl compounds
1492:Reactions of organocopper reagents
1224:
1205:
1072:Cu(III) intermediate, followed by
999:Reactions of organocopper reagents
314:Alkyl and aryl copper(I) compounds
197:of +1, sometimes denoted Cu(I) or
25:
2328:Müller, D. S.; Marek, I. (2016).
1436:(for example in the synthesis of
968:with respect to the cyclohexenyl
902:: In a so-called rapid-injection
394:Copper salts react with terminal
3116:
3035:
2967:
2872:
2572:
1853:Annalen der Chemie und Pharmacie
1822:Christoph Elschenbroich (2006).
1666:
1625:
1597:Copper hydrides are specialized
1475:
1416:
984:
955:square planar molecular geometry
833:Lithium dimethylcuprate(I) is a
825:
301:Although copper does not form a
34:
949:. According to an accompanying
803:three-center two-electron bonds
309:Alkyl and aryl copper compounds
1457:decarboxylative cross-coupling
1283:
1242:
1207:
1123:
1090:
711:
653:
642:
620:
614:
602:
577:
507:
482:
453:
442:
1:
2377:HENDRIX, AMANDA JOY MUELLER.
2315:10.1016/S0040-4039(01)96925-4
1432:to conjugated alkynes in the
371:because they exhibit greater
1455:. A related reaction called
1440:) or to aryl halides in the
1008:Prior to the development of
937:. On subsequent addition of
2175:10.1002/0471264180.os055.28
1797:W.A. Herrmann, ed. (1999).
1528:). It is a special case of
1485:Cadiot-Chodkiewicz coupling
1055:] + R'−X → R−R' + CuR + LiX
1046:give the coupling product:
993:Reactions of organocuprates
330:is prepared by reaction of
201:. With 10 electrons in its
3267:
1776:. New York: Wiley: Wiley.
1655:-butyl, and aryl halides.
1634:Copper alkylation reaction
1508:of organocopper reagents (
1489:
996:
980:stable Cu(III) compounds.
318:Copper halides react with
158:gas through a solution of
3113:
3032:
2584:
2580:
2570:
2451:10.1016/j.tet.2014.04.004
2414:10.1002/047084289X.rh011m
2250:palladium acetylacetonate
2151:10.1016/j.ccr.2004.09.014
1982:10.1107/S056774087000273X
1919:10.1007/s00775-016-1378-3
2334:Chemical Society Reviews
2288:10.15227/orgsyn.064.0001
2022:10.15227/orgsyn.059.0122
1970:Acta Crystallographica B
1865:10.1002/jlac.18591090318
1447:Reductive coupling is a
1442:Castro-Stephens Coupling
1014:cross coupling reactions
1004:Cross-coupling reactions
391:for its carbon ligands.
387:, suggesting diminished
152:Rudolf Christian Böttger
112:organometallic compounds
2220:10.1126/science.1128684
819:pentafluorophenylcopper
815:pentamesitylpentacopper
805:. The copper to copper
125:. They are reagents in
3251:Organocopper compounds
3219:Many uses in chemistry
3214:Core organic chemistry
2050:10.1002/anie.199003001
1847:R. C. Böttger (1859).
1548:Synthetic applications
1544:
1357:
939:trimethylsilyl cyanide
882:, in the solid state.
772:, where Me stands for
739:
345:Compounds of the type
320:organolithium reagents
291:
211:reaction intermediates
150:), was synthesized by
108:organocopper compounds
104:Organocopper chemistry
100:
92:
2038:Angew. Chem. Int. Ed.
1542:
1506:nucleophilic addition
1358:
1074:reductive elimination
740:
285:
189:Structure and bonding
98:
90:
2445:(27–28): 4219–4231.
2163:For an example see:
1907:J. Biol. Inorg. Chem
1583:Sonogashira coupling
1532:and also called the
1438:cyclooctadecanonaene
1087:
976:this study predicts
941:the Cu(III) species
922:) was introduced to
424:
411:Ullmann condensation
288:coordination polymer
2513:10.1021/jo01301a048
2303:Tetrahedron Letters
2242:potassium carbonate
2212:2006Sci...313..662G
1745:10.1021/ja00527a032
1705:(22): 11139–11145.
1605:, with the formula
1173:
703:
665:
632:
588:
493:
465:
369:alkylating reagents
154:in 1859 by passing
134:copper(I) acetylide
2346:10.1039/C5CS00897B
1545:
1424:Coupling reactions
1353:
1351:
1329:
1302:
1239:
1238:
1223:
1220:
1219:
1204:
1172:
1110:
1066:oxidative addition
1062:reaction mechanism
1020:was the preferred
892:conjugate addition
735:
733:
691:
640:
612:
607:
512:
440:
292:
160:copper(I) chloride
101:
93:
3238:
3237:
3194:
3193:
2507:(13): 2739–2740.
2478:10.1021/ol034560p
2472:(14): 2417–2420.
2340:(16): 4552–4566.
2276:Organic Syntheses
2167:Organic Syntheses
2116:10.1021/ja0675346
2081:10.1021/ja067533d
2010:Organic Syntheses
1711:10.1021/jo502115a
1603:Stryker's reagent
1555:Chan-Lam coupling
1449:coupling reaction
1349:
1337:
1323:
1310:
1300:
1288:
1274:
1257:
1247:
1246:
1232:
1222:
1217:
1202:
1197:
1188:
1178:
1174:
1170:
1158:
1146:
1136:
1120:
1108:
1096:
755:empirical formula
725:
716:
707:
694:
686:
679:
668:
648:
635:
619:
609:
567:
563:
556:
542:
535:
527:
520:
514:
472:
468:
448:
435:
381:group 12 elements
377:electronegativity
365:organic synthesis
336:copper(I) bromide
127:organic chemistry
83:
82:
75:
16:(Redirected from
3258:
3230:
3225:
3220:
3215:
3120:
3119:
3039:
3038:
2971:
2970:
2876:
2875:
2573:
2555:
2548:
2541:
2532:
2526:
2523:
2517:
2516:
2496:
2490:
2489:
2461:
2455:
2454:
2434:
2428:
2427:
2401:
2395:
2394:
2392:
2390:
2385:
2374:
2368:
2367:
2357:
2325:
2319:
2318:
2298:
2292:
2291:
2272:For an example:
2270:
2264:
2258:molecular sieves
2256:, MS stands for
2254:Copper(I) iodide
2238:
2232:
2231:
2195:
2189:
2188:
2161:
2155:
2154:
2139:Coord. Chem. Rev
2134:
2128:
2127:
2099:
2093:
2092:
2063:
2054:
2053:
2032:
2026:
2025:
2004:
1998:
1995:
1986:
1985:
1945:
1939:
1938:
1913:(5–6): 715–728.
1902:
1896:
1895:
1892:10.1039/A908459B
1875:
1869:
1868:
1844:
1838:
1837:
1819:
1813:
1812:
1794:
1788:
1787:
1775:
1762:
1748:
1739:(7): 2318–2325.
1722:
1670:
1629:
1569:Ullmann reaction
1534:Normant reaction
1527:
1511:
1479:
1465:transmetallation
1453:Ullmann reaction
1420:
1362:
1360:
1359:
1354:
1352:
1350:
1347:
1343:
1338:
1335:
1330:
1328:
1327:
1321:
1316:
1311:
1308:
1303:
1301:
1298:
1294:
1289:
1286:
1281:
1280:
1279:
1272:
1269:
1268:
1263:
1259:
1258:
1255:
1254:
1248:
1245:
1240:
1237:
1233:
1230:
1221:
1218:
1215:
1210:
1203:
1200:
1196:
1194:
1189:
1186:
1176:
1175:
1171:
1168:
1167:
1162:
1156:
1148:
1144:
1143:
1142:
1141:
1134:
1131:
1130:
1121:
1118:
1117:
1111:
1109:
1106:
1102:
1097:
1094:
1056:
1045:
1038:
1026:turnover numbers
988:
978:room temperature
917:
881:
855:
829:
800:
781:
771:
744:
742:
741:
736:
734:
730:
729:
723:
721:
714:
705:
704:
702:
699:
692:
687:
684:
683:
677:
669:
666:
664:
661:
656:
652:
646:
636:
633:
631:
628:
623:
617:
610:
608:
606:
605:
598:
590:
589:
587:
580:
572:
565:
564:
561:
560:
554:
543:
540:
539:
533:
528:
525:
524:
518:
515:
513:
511:
510:
503:
495:
494:
492:
485:
477:
470:
469:
466:
464:
461:
456:
452:
446:
436:
433:
415:Ullmann reaction
373:functional group
354:
261:
254:
246:
234:
200:
184:
149:
145:
78:
71:
67:
64:
58:
38:
37:
30:
21:
3266:
3265:
3261:
3260:
3259:
3257:
3256:
3255:
3241:
3240:
3239:
3234:
3233:
3228:
3223:
3218:
3213:
3195:
3117:
3036:
2968:
2873:
2566:
2559:
2529:
2524:
2520:
2498:
2497:
2493:
2463:
2462:
2458:
2436:
2435:
2431:
2424:
2403:
2402:
2398:
2388:
2386:
2383:
2376:
2375:
2371:
2327:
2326:
2322:
2300:
2299:
2295:
2273:
2271:
2267:
2240:Reagents: base
2239:
2235:
2206:(5787): 662–4.
2197:
2196:
2192:
2185:
2164:
2162:
2158:
2136:
2135:
2131:
2101:
2100:
2096:
2065:
2064:
2057:
2034:
2033:
2029:
2006:
2005:
2001:
1996:
1989:
1967:
1963:
1959:
1955:
1951:
1947:
1946:
1942:
1904:
1903:
1899:
1877:
1876:
1872:
1846:
1845:
1841:
1834:
1824:Organometallics
1821:
1820:
1816:
1809:
1796:
1795:
1791:
1784:
1764:
1763:
1759:
1755:
1730:
1725:
1695:
1692:
1690:Further reading
1680:
1661:
1636:
1608:
1599:reducing agents
1595:
1593:Reducing agents
1589:
1550:
1530:carbometalation
1525:
1521:
1509:
1499:
1494:
1434:Glaser coupling
1426:
1320:
1271:
1228:
1184:
1180:
1179:
1155:
1133:
1122:
1085:
1084:
1054:
1050:
1043:
1036:
1032:
1006:
1001:
995:
918:(stabilized by
915:
911:
907:
888:
880:
876:
869:
865:
861:
857:
854:
850:
846:
842:
821:is a tetramer.
799:
795:
784:crystallography
780:
776:
770:
766:
762:
759:bridging ligand
751:
732:
731:
722:
708:
676:
671:
670:
645:
568:
553:
545:
544:
532:
517:
473:
445:
422:
421:
389:nucleophilicity
357:copper(I) oxide
352:
346:
316:
311:
276:
264:trigonal planar
259:
252:
244:
240:
232:
228:
225:alkylphosphines
198:
195:oxidation state
191:
182:
178:
174:
170:
166:
147:
144:
140:
136:
79:
68:
62:
59:
51:help improve it
48:
39:
35:
28:
23:
22:
15:
12:
11:
5:
3264:
3262:
3254:
3253:
3243:
3242:
3236:
3235:
3232:
3231:
3226:
3221:
3216:
3211:
3208:Chemical bonds
3204:
3203:
3201:
3197:
3196:
3192:
3191:
3186:
3181:
3176:
3171:
3166:
3161:
3156:
3151:
3146:
3141:
3136:
3131:
3126:
3121:
3114:
3111:
3110:
3105:
3100:
3095:
3090:
3085:
3080:
3075:
3070:
3065:
3060:
3055:
3050:
3045:
3040:
3033:
3030:
3029:
3025:
3024:
3021:
3018:
3015:
3012:
3009:
3006:
3003:
3000:
2997:
2994:
2991:
2988:
2983:
2980:
2977:
2972:
2965:
2960:
2956:
2955:
2952:
2947:
2942:
2937:
2932:
2927:
2922:
2917:
2912:
2907:
2902:
2897:
2892:
2887:
2882:
2877:
2870:
2865:
2859:
2858:
2853:
2848:
2843:
2838:
2833:
2828:
2823:
2818:
2813:
2808:
2803:
2798:
2793:
2788:
2783:
2778:
2776:
2771:
2765:
2764:
2759:
2754:
2749:
2744:
2739:
2734:
2729:
2724:
2719:
2714:
2709:
2704:
2699:
2694:
2689:
2684:
2682:
2677:
2671:
2670:
2665:
2660:
2655:
2650:
2645:
2640:
2635:
2629:
2628:
2625:
2620:
2615:
2610:
2605:
2600:
2595:
2589:
2588:
2585:
2583:
2581:
2579:
2571:
2568:
2567:
2560:
2558:
2557:
2550:
2543:
2535:
2528:
2527:
2518:
2491:
2456:
2429:
2422:
2396:
2369:
2320:
2293:
2265:
2262:phenanthroline
2233:
2190:
2183:
2156:
2129:
2110:(23): 7210–1.
2094:
2075:(23): 7208–9.
2055:
2044:(3): 300–302.
2027:
1999:
1987:
1965:
1961:
1957:
1953:
1949:
1940:
1897:
1870:
1859:(3): 351–362.
1839:
1832:
1814:
1807:
1789:
1782:
1766:Gary H. Posner
1756:
1754:
1751:
1750:
1749:
1728:
1723:
1691:
1688:
1687:
1686:
1679:
1676:
1660:
1657:
1642:- alkylation.
1635:
1632:
1631:
1630:
1606:
1594:
1591:
1587:
1586:
1580:
1579:
1578:
1575:
1566:
1549:
1546:
1502:Carbocupration
1498:
1497:Carbocupration
1495:
1490:Main article:
1481:
1480:
1425:
1422:
1368:acid chlorides
1364:
1363:
1342:
1333:
1326:
1315:
1306:
1293:
1285:
1278:
1267:
1262:
1253:
1244:
1236:
1209:
1193:
1183:
1166:
1161:
1151:
1140:
1129:
1125:
1116:
1101:
1092:
1058:
1057:
1005:
1002:
994:
991:
990:
989:
920:lithium iodide
896:Gilman reagent
887:
884:
831:
830:
801:ring based on
788:trimethylsilyl
750:
747:
746:
745:
728:
720:
713:
709:
698:
690:
682:
673:
672:
660:
655:
651:
644:
639:
627:
622:
616:
604:
597:
586:
579:
569:
559:
551:
547:
546:
538:
531:
523:
509:
502:
491:
484:
474:
460:
455:
451:
444:
439:
430:
429:
315:
312:
310:
307:
275:
272:
207:pi-backbonding
190:
187:
186:
185:
81:
80:
42:
40:
33:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
3263:
3252:
3249:
3248:
3246:
3227:
3222:
3217:
3212:
3209:
3206:
3205:
3202:
3198:
3190:
3187:
3185:
3182:
3180:
3177:
3175:
3172:
3170:
3167:
3165:
3162:
3160:
3157:
3155:
3152:
3150:
3147:
3145:
3142:
3140:
3137:
3135:
3132:
3130:
3127:
3125:
3122:
3115:
3112:
3109:
3106:
3104:
3101:
3099:
3096:
3094:
3091:
3089:
3086:
3084:
3081:
3079:
3076:
3074:
3071:
3069:
3066:
3064:
3061:
3059:
3056:
3054:
3051:
3049:
3046:
3044:
3041:
3034:
3031:
3027:
3026:
3022:
3019:
3016:
3013:
3010:
3007:
3004:
3001:
2998:
2995:
2992:
2989:
2987:
2984:
2981:
2978:
2976:
2973:
2966:
2964:
2961:
2958:
2957:
2953:
2951:
2948:
2946:
2943:
2941:
2938:
2936:
2933:
2931:
2928:
2926:
2923:
2921:
2918:
2916:
2913:
2911:
2908:
2906:
2903:
2901:
2898:
2896:
2893:
2891:
2888:
2886:
2883:
2881:
2878:
2871:
2869:
2866:
2864:
2861:
2860:
2857:
2854:
2852:
2849:
2847:
2844:
2842:
2839:
2837:
2834:
2832:
2829:
2827:
2824:
2822:
2819:
2817:
2814:
2812:
2809:
2807:
2804:
2802:
2799:
2797:
2794:
2792:
2789:
2787:
2784:
2782:
2779:
2777:
2775:
2772:
2770:
2767:
2766:
2763:
2760:
2758:
2755:
2753:
2750:
2748:
2745:
2743:
2740:
2738:
2735:
2733:
2730:
2728:
2725:
2723:
2720:
2718:
2715:
2713:
2710:
2708:
2705:
2703:
2700:
2698:
2695:
2693:
2690:
2688:
2685:
2683:
2681:
2678:
2676:
2673:
2672:
2669:
2666:
2664:
2661:
2659:
2656:
2654:
2651:
2649:
2646:
2644:
2641:
2639:
2636:
2634:
2631:
2630:
2626:
2624:
2621:
2619:
2616:
2614:
2611:
2609:
2606:
2604:
2601:
2599:
2596:
2594:
2591:
2590:
2586:
2578:
2575:
2574:
2569:
2564:
2561:Compounds of
2556:
2551:
2549:
2544:
2542:
2537:
2536:
2533:
2522:
2519:
2514:
2510:
2506:
2502:
2495:
2492:
2487:
2483:
2479:
2475:
2471:
2467:
2460:
2457:
2452:
2448:
2444:
2440:
2433:
2430:
2425:
2419:
2415:
2411:
2407:
2400:
2397:
2382:
2381:
2373:
2370:
2365:
2361:
2356:
2351:
2347:
2343:
2339:
2335:
2331:
2324:
2321:
2316:
2312:
2308:
2304:
2297:
2294:
2289:
2285:
2281:
2277:
2269:
2266:
2263:
2259:
2255:
2251:
2247:
2243:
2237:
2234:
2229:
2225:
2221:
2217:
2213:
2209:
2205:
2201:
2194:
2191:
2186:
2180:
2176:
2172:
2168:
2160:
2157:
2152:
2148:
2145:: 2337–2364.
2144:
2140:
2133:
2130:
2125:
2121:
2117:
2113:
2109:
2105:
2098:
2095:
2090:
2086:
2082:
2078:
2074:
2070:
2062:
2060:
2056:
2051:
2047:
2043:
2040:
2039:
2031:
2028:
2023:
2019:
2015:
2011:
2003:
2000:
1994:
1992:
1988:
1983:
1979:
1976:(5): 515–21.
1975:
1971:
1944:
1941:
1936:
1932:
1928:
1924:
1920:
1916:
1912:
1908:
1901:
1898:
1893:
1889:
1885:
1881:
1874:
1871:
1866:
1862:
1858:
1854:
1850:
1843:
1840:
1835:
1833:3-527-29390-6
1829:
1825:
1818:
1815:
1810:
1808:3-13-103061-5
1804:
1800:
1793:
1790:
1785:
1783:0-471-69538-6
1779:
1774:
1773:
1767:
1761:
1758:
1752:
1746:
1742:
1738:
1734:
1724:
1720:
1716:
1712:
1708:
1704:
1700:
1694:
1693:
1689:
1685:
1682:
1681:
1677:
1675:
1671:
1669:
1664:
1658:
1656:
1654:
1649:
1645:
1641:
1633:
1628:
1624:
1623:
1622:
1620:
1616:
1612:
1604:
1600:
1592:
1590:
1584:
1581:
1576:
1573:
1572:
1570:
1567:
1564:
1560:
1559:boronic acids
1556:
1552:
1551:
1547:
1541:
1537:
1535:
1531:
1519:
1515:
1507:
1503:
1496:
1493:
1488:
1486:
1478:
1474:
1473:
1472:
1470:
1466:
1462:
1458:
1454:
1450:
1445:
1443:
1439:
1435:
1431:
1423:
1421:
1419:
1414:
1410:
1409:
1405:
1401:
1397:
1393:
1389:
1385:
1381:
1377:
1373:
1369:
1340:
1331:
1324:
1313:
1304:
1291:
1276:
1265:
1260:
1251:
1234:
1191:
1181:
1164:
1159:
1149:
1138:
1127:
1114:
1099:
1083:
1082:
1081:
1079:
1075:
1071:
1067:
1063:
1049:
1048:
1047:
1042:
1041:alkyl halides
1031:Reactions of
1029:
1027:
1023:
1019:
1015:
1011:
1003:
1000:
992:
987:
983:
982:
981:
979:
975:
971:
967:
965:
960:
956:
952:
948:
944:
940:
936:
933:
929:
925:
924:cyclohexenone
921:
905:
901:
897:
893:
885:
883:
873:
858:([Li(O(CH
843:(Li[Cu(CH
840:
839:diethyl ether
836:
828:
824:
823:
822:
820:
816:
812:
808:
804:
793:
789:
785:
775:
760:
756:
748:
726:
718:
696:
688:
680:
658:
649:
637:
625:
595:
584:
557:
549:
536:
529:
521:
500:
489:
458:
449:
437:
420:
419:
418:
416:
412:
407:
403:
401:
397:
392:
390:
386:
382:
378:
374:
370:
366:
362:
358:
351:
343:
341:
340:diethyl ether
337:
333:
332:phenyllithium
329:
325:
321:
313:
308:
306:
304:
299:
297:
296:plant hormone
289:
284:
280:
273:
271:
269:
265:
256:
250:
238:
226:
222:
219:
214:
212:
208:
204:
203:valence shell
196:
188:
175:+ 2 CuCl → Cu
165:
164:
163:
161:
157:
153:
135:
130:
128:
124:
123:chemical bond
121:
117:
114:containing a
113:
109:
105:
97:
89:
85:
77:
74:
66:
56:
52:
46:
43:This article
41:
32:
31:
19:
3229:Bond unknown
2726:
2521:
2504:
2501:J. Org. Chem
2500:
2494:
2469:
2465:
2459:
2442:
2438:
2432:
2405:
2399:
2387:. Retrieved
2379:
2372:
2337:
2333:
2323:
2309:(27): 2583.
2306:
2302:
2296:
2279:
2275:
2268:
2248:, catalysts
2236:
2203:
2199:
2193:
2166:
2159:
2142:
2138:
2132:
2107:
2103:
2097:
2072:
2068:
2041:
2036:
2030:
2013:
2009:
2002:
1973:
1969:
1943:
1910:
1906:
1900:
1883:
1879:
1873:
1856:
1852:
1842:
1823:
1817:
1798:
1792:
1771:
1760:
1736:
1732:
1702:
1699:J. Org. Chem
1698:
1684:Ethyl copper
1672:
1665:
1662:
1652:
1647:
1643:
1639:
1637:
1596:
1588:
1533:
1516:or terminal
1501:
1500:
1482:
1446:
1427:
1415:
1411:
1365:
1059:
1030:
1007:
963:
946:
942:
934:
927:
908:Li[Cu(CH
889:
832:
818:
814:
774:methyl group
752:
408:
404:
398:to form the
393:
349:
344:
328:phenylcopper
324:Henry Gilman
317:
300:
293:
277:
257:
215:
192:
131:
110:, which are
107:
103:
102:
84:
69:
63:January 2023
60:
44:
18:Organocopper
2439:Tetrahedron
2389:January 17,
2282:: 1. 1986.
1619:hydrosilane
1012:-catalyzed
974:cyano group
966:orientation
807:bond length
303:metallocene
268:tetrahedral
2423:0471936235
2244:, solvent
2184:0471264229
1753:References
1430:acetylides
1051:Li[CuR
1033:Li[CuR
997:See also:
932:pi complex
749:Structures
400:acetylides
237:thioethers
3210:to carbon
2466:Org. Lett
2260:, ligand
1563:stannanes
1526:C=C(R)−Cu
1514:acetylene
1406:>>
1392:chlorides
1376:tosylates
1372:aldehydes
1341:−
1314:−
1292:−
1284:⟶
1266:−
1252:−
1192:−
1165:−
1128:−
1115:−
1100:−
1064:involves
1010:palladium
961:group in
957:with the
951:in silico
870:)][Cu
719:−
712:⟶
603:⇀
596:−
585:−
578:↽
508:⇀
501:−
490:−
483:↽
156:acetylene
148:Cu−C≡C−Cu
3245:Category
2486:12841744
2364:26808300
2228:16888137
2124:17506553
2089:17506552
1935:14399214
1927:27456611
1768:(1980).
1719:25350606
1678:See also
1461:carboxyl
1404:nitriles
1388:bromides
1380:epoxides
1325:′
1235:′
1160:′
1150:→
1022:catalyst
792:tetramer
727:′
681:′
650:′
558:′
537:′
522:′
450:′
347:[CuR
223:such as
3028:
2355:5166570
2208:Bibcode
2200:Science
2169:: 122.
2016:: 122.
1886:: 1–6.
1518:alkynes
1408:alkenes
1396:ketones
1384:iodides
970:methine
894:of the
809:is 242
396:alkynes
361:in situ
249:cyanide
247:), and
221:ligands
183:+ 2 HCl
49:Please
3200:Legend
2563:carbon
2484:
2420:
2362:
2352:
2226:
2181:
2122:
2087:
1933:
1925:
1830:
1805:
1780:
1727:RCu.BF
1717:
1469:biaryl
1400:esters
1177:
1145:
1078:iodide
1070:planar
1018:copper
898:to an
706:
566:
471:
120:copper
116:carbon
2384:(PDF)
1956:CuP(C
1931:S2CID
1648:gamma
1640:gamma
1512:) to
1504:is a
1402:>
1398:>
1394:>
1390:>
1386:>
1382:>
1374:>
1370:>
1039:with
959:cyano
900:enone
835:dimer
334:with
2482:PMID
2418:ISBN
2391:2018
2360:PMID
2224:PMID
2179:ISBN
2120:PMID
2085:PMID
1923:PMID
1884:2000
1828:ISBN
1803:ISBN
1778:ISBN
1715:PMID
1653:tert
1553:The
1510:R−Cu
1060:The
1044:R'−X
767:SiMe
763:CuCH
685:CuLi
678:ArAr
667:CuLi
634:CuLi
562:CuLi
555:ArAr
526:CuLi
519:ArAr
467:CuLi
413:and
385:zinc
218:soft
3174:CEs
3169:CCf
3164:CBk
3159:CCm
3154:CAm
3149:CPu
3144:CNp
3134:CPa
3129:CTh
3108:CYb
3103:CTm
3098:CEr
3093:CHo
3088:CDy
3083:CTb
3078:CGd
3073:CEu
3068:CSm
3063:CPm
3058:CNd
3053:CPr
3048:CCe
3043:CLa
3023:Og
3020:Ts
3017:Lv
3014:Mc
3011:Fl
3008:Nh
3005:Cn
3002:Rg
2999:Ds
2996:Mt
2993:Hs
2990:Bh
2986:CSg
2982:Db
2979:Rf
2963:CRa
2959:Fr
2954:Rn
2950:CAt
2945:CPo
2940:CBi
2935:CPb
2930:CTl
2925:CHg
2920:CAu
2915:CPt
2910:CIr
2905:COs
2900:CRe
2890:CTa
2885:CHf
2880:CLu
2868:CBa
2863:CCs
2856:CXe
2846:CTe
2841:CSb
2836:CSn
2831:CIn
2826:CCd
2821:CAg
2816:CPd
2811:CRh
2806:CRu
2801:CTc
2796:CMo
2791:CNb
2786:CZr
2774:CSr
2769:CRb
2762:CKr
2757:CBr
2752:CSe
2747:CAs
2742:CGe
2737:CGa
2732:CZn
2727:CCu
2722:CNi
2717:CCo
2712:CFe
2707:CMn
2702:CCr
2692:CTi
2687:CSc
2680:CCa
2668:CAr
2663:CCl
2648:CSi
2643:CAl
2638:CMg
2633:CNa
2627:Ne
2598:CBe
2593:CLi
2587:He
2509:doi
2474:doi
2447:doi
2410:doi
2350:PMC
2342:doi
2311:doi
2284:doi
2246:NMP
2216:doi
2204:313
2171:doi
2147:doi
2143:248
2112:doi
2108:129
2077:doi
2073:129
2046:doi
2018:doi
1978:doi
1968:".
1915:doi
1888:doi
1861:doi
1857:109
1741:doi
1737:102
1731:".
1707:doi
1644:Cis
1615:NHC
964:cis
904:NMR
837:in
434:ArX
417:):
367:as
338:in
266:or
255:).
235:),
118:to
53:to
3247::
3189:No
3184:Md
3179:Fm
3139:CU
3124:Ac
2975:Lr
2895:CW
2851:CI
2781:CY
2697:CV
2675:CK
2658:CS
2653:CP
2623:CF
2618:CO
2613:CN
2608:CC
2603:CB
2577:CH
2505:45
2503:.
2480:.
2468:.
2443:70
2441:.
2416:.
2408:.
2358:.
2348:.
2338:45
2336:.
2332:.
2307:12
2305:.
2280:64
2278:.
2252:,
2222:.
2214:.
2202:.
2177:.
2141:.
2118:.
2106:.
2083:.
2071:.
2058:^
2042:29
2014:59
2012:.
1990:^
1974:26
1972:.
1929:.
1921:.
1911:21
1909:.
1882:.
1855:.
1851:.
1735:.
1713:.
1703:79
1701:.
1646:-
1621:.
1561:,
1536:.
1471:.
1444:.
1378:~
1336:Li
1299:Cu
1273:Li
1201:Cu
1135:Li
1107:Cu
1016:,
877:])
872:Ph
862:CH
851:])
811:pm
796:Cu
777:CH
724:Ar
715:Ar
647:Ar
618:Ar
534:Ar
447:Ar
402:.
383:,
260:Cu
253:CN
199:Cu
162::
137:Cu
129:.
2554:e
2547:t
2540:v
2515:.
2511::
2488:.
2476::
2470:5
2453:.
2449::
2426:.
2412::
2393:.
2366:.
2344::
2317:.
2313::
2290:.
2286::
2230:.
2218::
2210::
2187:.
2173::
2153:.
2149::
2126:.
2114::
2091:.
2079::
2052:.
2048::
2024:.
2020::
1984:.
1980::
1966:3
1964:)
1962:5
1960:H
1958:2
1954:5
1952:H
1950:5
1937:.
1917::
1894:.
1890::
1867:.
1863::
1836:.
1811:.
1786:.
1747:.
1743::
1729:3
1721:.
1709::
1607:6
1524:2
1522:R
1348:X
1332:+
1322:R
1309:R
1305:+
1287:R
1277:+
1261:]
1256:R
1243:|
1231:R
1216:X
1208:|
1187:R
1182:[
1169:X
1157:R
1139:+
1124:]
1119:R
1095:R
1091:[
1053:2
1037:]
1035:2
947:4
943:3
935:2
928:1
926:(
916:]
914:2
912:)
910:3
879:2
875:2
868:2
866:)
864:3
860:2
853:2
849:2
847:)
845:3
798:4
779:3
769:3
765:2
697:2
693:O
689:+
659:2
654:)
643:(
638:+
626:2
621:)
615:(
550:2
541:X
530:+
459:2
454:)
443:(
438:+
353:]
350:n
251:(
245:S
243:2
241:R
239:(
233:P
231:3
229:R
227:(
181:2
179:C
177:2
173:2
171:H
169:2
167:C
146:(
143:2
141:C
139:2
76:)
70:(
65:)
61:(
47:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.