282:
1533:
1384:
1452:
1289:
298:
535:
427:
304:
The stability and diversity of platinum(II) alkene complexes contrasts with the rarity of alkene complexes of nickel(II). Platinum allyl complexes are also common. In contrast to nickel chemistry, where compounds such as CpNi(L)X are common,
769:
Ebert, K. H.; Massa, W.; Donath, H.; Lorberth, J.; Seo, B. S.; Herdtweck, E. (1998). "Organoplatinum
Compounds: VI. Trimethylplatinum Thiomethylate and Trimethylplatinum Iodide. The Crystal Structures of
485:, and it is assumed that these useful reactions proceed via surface-bound organoplatinum intermediates. Better defined but less commercially significant are homogeneous catalysts based on platinum.
914:
Brian G. Hashiguchi; Steven M. Bischof; Michael M. Konnick; Roy A. Periana (2012). "Designing
Catalysts for Functionalization of Unactivated C–H Bonds Based on the CH Activation Reaction".
438:
Organoplatinum(IV) hydrides are rare. The first isolated representatives were prepared from organotin halides or acids with orthometalated arylplatinum(II) compounds. The compound Me(PEt
559:. Strenuous efforts have been made, thus far unsuccessfully, to extend this reactivity to practical methods for functionalizing methane. For example, platinum complexes of
967:
415:
and
Peachey in 1907. The compound adopts a cubane-like structure with four triply bridging iodide ligands. "Tetramethylplatinum" was claimed in 1952 by
156:-dichlorobis(triphenylphosphine)platinum(II). Nitrogen-based ligands do not often support the formation of platinum complexes of alkenes and alkynes.
50:
0 to IV, with oxidation state II most abundant. The general order in bond strength is Pt-C (sp) > Pt-O > Pt-N > Pt-C (sp). Organoplatinum and
289:
325:
892:
840:
678:
645:
879:
Stahl, Shannon S.; Labinger, Jay A.; Bercaw, John E. (1998). "Homogeneous
Oxidation of Alkanes by Electrophilic Late Transition Metals".
960:
1665:
756:
611:
Jwu-Ting Chen "Platinum: Organometallic
Chemistry" in Encyclopedia of Inorganic Chemistry 2006, John Wiley & Sons, New York.
508:
499:("Speier's catalyst") is an important catalyst. Mechanisms for this catalytic system usually assume intermediates that contain
256:
Platinum(I) compounds are uncommon but generally are diamagnetic because they have Pt-Pt bonds. An example is the dication .
953:
273:
137:
62:
Most organoplatinum(0) compounds contain alkene and alkyne ligands. Carbonyl complexes are rare, and the analogue of Ni(CO)
297:
419:
as a derivative of this tetramer, but this claim was later shown to be incorrect ("Tetramethylplatinum" proved to be
1522:
1517:
1512:
1507:
1502:
1497:
1492:
1487:
1482:
1477:
1472:
1467:
1457:
1400:
1294:
1215:
1210:
1067:
751:
Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the
Elements (2nd Edn.), Oxford: Butterworth-Heinemann.
516:
391:
Many organoplatinum(II) complexes arise via ortho-metalation and related intramolecular C-H activation processes.
1558:
1260:
1230:
1220:
1200:
1188:
1156:
1121:
1089:
1057:
1052:
1012:
1027:
991:
945:
1603:
1598:
1593:
1588:
1583:
1578:
1573:
1568:
1563:
1548:
1538:
1389:
1364:
1359:
1344:
1309:
1304:
1255:
1183:
1166:
1116:
1111:
1106:
1101:
1077:
1037:
852:
Kettler, P. B. (2003). "Platinum Group Metals in
Catalysis: Fabrication of Catalysts and Catalyst Precursors".
400:
24:
661:
Hill, Geoffrey S.; Irwin, Michael J.; Levy, Christopher J.; Rendina, Louis M.; Puddephatt, Richard J. (1998).
1553:
1543:
1354:
1339:
1324:
1314:
1299:
1240:
1225:
1205:
1195:
1176:
1171:
1161:
1151:
1094:
1062:
54:
chemistry are similar, but organoplatinum compounds are more stable and therefore less useful as catalysts.
1032:
1022:
1462:
1377:
1319:
1282:
1277:
1265:
1245:
1235:
1141:
1136:
1131:
1072:
1047:
512:
482:
478:
408:
281:
66:
is elusive. The alkene and alkyne ligands serve as two-electron donors, for example in the complexes (PPh
1270:
1082:
1017:
1007:
404:
1349:
1334:
1146:
1126:
412:
309:
derivatives of Pt(II) are rare, consistent with the reluctance of Pt(II) to become pentacoordinate.
1250:
520:
489:
467:
141:
313:
149:
931:
896:
836:
752:
674:
641:
122:
91:
923:
888:
861:
814:
791:
734:
703:
666:
633:
612:
568:
528:
306:
43:
564:
556:
524:
51:
47:
265:
159:
Zerovalent organoplatinum complexes lacking phosphine ligands are often prepared via PtCl
818:
795:
1659:
1622:
548:
35:
560:
447:
416:
317:
893:
10.1002/(SICI)1521-3773(19980904)37:16<2180::AID-ANIE2180>3.0.CO;2-A
321:
670:
637:
245:
616:
145:
20:
935:
900:
588:
Nickel, Palladium and
Platinum (Comprehensive Organometallic Chemistry II)
738:
707:
269:
152:
and the alkene or alkyne. Such reactions proceed via the intermediacy of
39:
32:
344:. Alternatively, platinum(II) chlorides are susceptible to alkylation:
117:) is labile and exchanges with alkynes and electrophilic alkenes, even C
552:
500:
466:
at -20 °C. Weak acids often suffice even water and alcohol and in
451:
927:
865:
977:
538:
Idealized mechanism for metal-catalysed hydrosilylation of an alkene.
28:
722:
534:
533:
426:
425:
949:
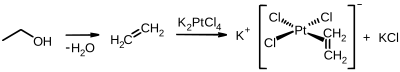
312:
Alkyl and aryl platinum(II) complexes are often prepared by
264:
A historically significant organoplatinum(II) compound is
809:
Puddephatt, R. (2001). "Platinum(IV) hydride chemistry".
694:"Proceedings of the Chemical Society, Vol. 23, No. 323".
600:
531:
have repeating units based on organoplatinum compounds.
399:
The first organoplatinum compound ever synthesised was
292:
is a more modern relative, and is more widely used.
1614:
665:. Inorganic Syntheses. Vol. 32. p. 149.
632:. Inorganic Syntheses. Vol. 31. p. 284.
563:catalyze the conversion of methane, oxygen, and
547:Organoplatinum compounds are implicated in the
628:Costa, E.; Pringle, P. G.; Ravetz, M. (1997).
961:
727:Journal of the Chemical Society, Transactions
602:Sanshiro Komiya Ed. S. Komiya, M. Hurano 1997
136:Pt(un) (un = alkene, alkyne) is reduction of
8:
696:Proceedings of the Chemical Society (London)
509:Cis-dichlorobis(diethyl sulfide)platinum(II)
423:). Salts of and have been characterized.
481:based on platinum play a major role in the
968:
954:
946:
854:Organic Process Research & Development
663:Platinum(II) Complexes of Dimethyl Sulfide
148:in presence of a phosphine ligand such as
980:with other elements in the periodic table
723:"LXXIII.?The alkyl compounds of platinum"
290:dichloro(cycloocta-1,5-diene)platinum(II)
590:R.J. Puddephatt (Editor) 2002 0080423167
881:Angewandte Chemie International Edition
580:
326:tetrakis(triphenylphosphine)platinum(0)
997:
835:(2006) Wiley and Sons-VCH: Weinheim.
450:between -60 and -80 °C, forming
7:
1639:Academic research, no widespread use
721:Pope, W. J.; Peachey, S. J. (1909).
46:. Organoplatinum compounds exist in
388:can be displaced by other ligands.
376:The dimethylsulfide ligands in PtMe
14:
128:A general synthetic route to (PPh
38:, and the study of platinum as a
1531:
1450:
1382:
1287:
987:
470:the proton source is an alkane.
296:
288:The colourless diolefin complex
280:
101:). The ethylene ligand in (PPh
811:Coordination Chemistry Reviews
274:potassium tetrachloroplatinate
138:potassium tetrachloroplatinate
1:
819:10.1016/S0010-8545(01)00325-3
796:10.1016/S0022-328X(98)00414-8
446:PtOTf reacts reversibly with
324:to a Pt(0) precursor such as
517:divinyltetramethyldisiloxane
1682:
671:10.1002/9780470132630.ch25
638:10.1002/9780470132623.ch49
1528:
1447:
999:
995:
985:
507:Si), and alkene ligands.
268:, which is obtained from
1666:Organoplatinum compounds
617:10.1002/0470862106.ia195
401:trimethylplatinum iodide
25:organometallic compounds
17:Organoplatinum chemistry
479:Heterogeneous catalysts
1634:Many uses in chemistry
1629:Core organic chemistry
551:for the conversion of
539:
488:For hydrosilylation,
483:petrochemical industry
435:
409:methylmagnesium iodide
221:+ 2 COD → Pt(COD)
630:Dimethyl-Platinum(II)
537:
429:
405:platinum(IV) chloride
813:. 219–221: 157–185.
739:10.1039/CT9099500571
708:10.1039/PL9072300081
702:(323): 81–94. 1907.
360:+ 2 MeLi → PtMe
521:chloroplatinic acid
513:Karstedt's catalyst
468:C-H bond activation
142:potassium hydroxide
831:C. Elschenbroich,
784:J. Organomet. Chem
540:
436:
395:Organoplatinum(IV)
314:oxidative addition
260:Organoplatinum(II)
191:→ + 2 LiCl + C
150:triphenylphosphine
1653:
1652:
1609:
1608:
928:10.1021/ar200250r
887:(16): 2180–2192.
866:10.1021/op034017o
841:978-3-527-29390-2
680:978-0-470-13263-0
647:978-0-470-13262-3
529:metallodendrimers
503:, silyl ligand (R
252:Organoplatinum(I)
58:Organoplatinum(0)
44:organic reactions
1673:
1645:
1640:
1635:
1630:
1535:
1534:
1454:
1453:
1386:
1385:
1291:
1290:
988:
970:
963:
956:
947:
940:
939:
911:
905:
904:
876:
870:
869:
849:
843:
829:
823:
822:
806:
800:
799:
790:(1–2): 203–207.
766:
760:
749:
743:
742:
718:
712:
711:
691:
685:
684:
658:
652:
651:
625:
619:
609:
603:
597:
591:
585:
569:methyl bisulfate
523:) also catalyse
307:cyclopentadienyl
300:
284:
1681:
1680:
1676:
1675:
1674:
1672:
1671:
1670:
1656:
1655:
1654:
1649:
1648:
1643:
1638:
1633:
1628:
1610:
1532:
1451:
1383:
1288:
981:
974:
944:
943:
913:
912:
908:
878:
877:
873:
851:
850:
846:
833:Organometallics
830:
826:
808:
807:
803:
781:
777:
773:
768:
767:
763:
750:
746:
720:
719:
715:
693:
692:
688:
681:
660:
659:
655:
648:
627:
626:
622:
610:
606:
598:
594:
586:
582:
577:
565:sulfur trioxide
557:methyl chloride
545:
543:Research themes
525:hydrosilylation
506:
497:
493:
476:
465:
461:
457:
445:
441:
433:
422:
397:
387:
383:
379:
371:
367:
363:
359:
355:
351:
343:
339:
335:
331:
262:
254:
243:
239:
232:
228:
224:
220:
216:
212:
206:
202:
198:
194:
190:
186:
182:
178:
174:
170:
162:
140:with ethanolic
135:
131:
120:
116:
112:
108:
104:
99:
95:
89:
85:
81:
77:
73:
69:
65:
60:
52:organopalladium
48:oxidation state
12:
11:
5:
1679:
1677:
1669:
1668:
1658:
1657:
1651:
1650:
1647:
1646:
1641:
1636:
1631:
1626:
1623:Chemical bonds
1619:
1618:
1616:
1612:
1611:
1607:
1606:
1601:
1596:
1591:
1586:
1581:
1576:
1571:
1566:
1561:
1556:
1551:
1546:
1541:
1536:
1529:
1526:
1525:
1520:
1515:
1510:
1505:
1500:
1495:
1490:
1485:
1480:
1475:
1470:
1465:
1460:
1455:
1448:
1445:
1444:
1440:
1439:
1436:
1433:
1430:
1427:
1424:
1421:
1418:
1415:
1412:
1409:
1406:
1403:
1398:
1395:
1392:
1387:
1380:
1375:
1371:
1370:
1367:
1362:
1357:
1352:
1347:
1342:
1337:
1332:
1327:
1322:
1317:
1312:
1307:
1302:
1297:
1292:
1285:
1280:
1274:
1273:
1268:
1263:
1258:
1253:
1248:
1243:
1238:
1233:
1228:
1223:
1218:
1213:
1208:
1203:
1198:
1193:
1191:
1186:
1180:
1179:
1174:
1169:
1164:
1159:
1154:
1149:
1144:
1139:
1134:
1129:
1124:
1119:
1114:
1109:
1104:
1099:
1097:
1092:
1086:
1085:
1080:
1075:
1070:
1065:
1060:
1055:
1050:
1044:
1043:
1040:
1035:
1030:
1025:
1020:
1015:
1010:
1004:
1003:
1000:
998:
996:
994:
986:
983:
982:
975:
973:
972:
965:
958:
950:
942:
941:
922:(6): 885–898.
916:Acc. Chem. Res
906:
871:
860:(3): 342–354.
844:
824:
801:
779:
775:
771:
761:
744:
713:
686:
679:
653:
646:
620:
604:
592:
579:
578:
576:
573:
544:
541:
504:
495:
491:
475:
472:
463:
459:
455:
443:
439:
431:
420:
411:, reported by
396:
393:
385:
381:
377:
374:
373:
369:
365:
361:
357:
353:
349:
341:
337:
333:
329:
302:
301:
286:
285:
261:
258:
253:
250:
241:
237:
234:
233:
230:
226:
222:
218:
214:
210:
207:
204:
200:
196:
192:
188:
184:
180:
176:
172:
168:
160:
133:
129:
118:
114:
110:
106:
102:
97:
93:
87:
83:
79:
75:
71:
67:
63:
59:
56:
13:
10:
9:
6:
4:
3:
2:
1678:
1667:
1664:
1663:
1661:
1642:
1637:
1632:
1627:
1624:
1621:
1620:
1617:
1613:
1605:
1602:
1600:
1597:
1595:
1592:
1590:
1587:
1585:
1582:
1580:
1577:
1575:
1572:
1570:
1567:
1565:
1562:
1560:
1557:
1555:
1552:
1550:
1547:
1545:
1542:
1540:
1537:
1530:
1527:
1524:
1521:
1519:
1516:
1514:
1511:
1509:
1506:
1504:
1501:
1499:
1496:
1494:
1491:
1489:
1486:
1484:
1481:
1479:
1476:
1474:
1471:
1469:
1466:
1464:
1461:
1459:
1456:
1449:
1446:
1442:
1441:
1437:
1434:
1431:
1428:
1425:
1422:
1419:
1416:
1413:
1410:
1407:
1404:
1402:
1399:
1396:
1393:
1391:
1388:
1381:
1379:
1376:
1373:
1372:
1368:
1366:
1363:
1361:
1358:
1356:
1353:
1351:
1348:
1346:
1343:
1341:
1338:
1336:
1333:
1331:
1328:
1326:
1323:
1321:
1318:
1316:
1313:
1311:
1308:
1306:
1303:
1301:
1298:
1296:
1293:
1286:
1284:
1281:
1279:
1276:
1275:
1272:
1269:
1267:
1264:
1262:
1259:
1257:
1254:
1252:
1249:
1247:
1244:
1242:
1239:
1237:
1234:
1232:
1229:
1227:
1224:
1222:
1219:
1217:
1214:
1212:
1209:
1207:
1204:
1202:
1199:
1197:
1194:
1192:
1190:
1187:
1185:
1182:
1181:
1178:
1175:
1173:
1170:
1168:
1165:
1163:
1160:
1158:
1155:
1153:
1150:
1148:
1145:
1143:
1140:
1138:
1135:
1133:
1130:
1128:
1125:
1123:
1120:
1118:
1115:
1113:
1110:
1108:
1105:
1103:
1100:
1098:
1096:
1093:
1091:
1088:
1087:
1084:
1081:
1079:
1076:
1074:
1071:
1069:
1066:
1064:
1061:
1059:
1056:
1054:
1051:
1049:
1046:
1045:
1041:
1039:
1036:
1034:
1031:
1029:
1026:
1024:
1021:
1019:
1016:
1014:
1011:
1009:
1006:
1005:
1001:
993:
990:
989:
984:
979:
976:Compounds of
971:
966:
964:
959:
957:
952:
951:
948:
937:
933:
929:
925:
921:
917:
910:
907:
902:
898:
894:
890:
886:
882:
875:
872:
867:
863:
859:
855:
848:
845:
842:
838:
834:
828:
825:
820:
816:
812:
805:
802:
797:
793:
789:
785:
765:
762:
758:
757:0-7506-3365-4
754:
748:
745:
740:
736:
732:
728:
724:
717:
714:
709:
705:
701:
697:
690:
687:
682:
676:
672:
668:
664:
657:
654:
649:
643:
639:
635:
631:
624:
621:
618:
614:
608:
605:
601:
596:
593:
589:
584:
581:
574:
572:
570:
566:
562:
558:
554:
550:
549:Shilov system
542:
536:
532:
530:
526:
522:
518:
514:
510:
502:
498:
486:
484:
480:
473:
471:
469:
453:
449:
430:Structure of
428:
424:
418:
414:
410:
406:
402:
394:
392:
389:
347:
346:
345:
327:
323:
319:
315:
310:
308:
299:
295:
294:
293:
291:
283:
279:
278:
277:
275:
271:
267:
259:
257:
251:
249:
247:
208:
183:(COD) + 3 C
166:
165:
164:
157:
155:
151:
147:
143:
139:
126:
124:
100:
57:
55:
53:
49:
45:
41:
37:
36:chemical bond
34:
30:
27:containing a
26:
22:
18:
1644:Bond unknown
1329:
919:
915:
909:
884:
880:
874:
857:
853:
847:
832:
827:
810:
804:
787:
783:
764:
747:
730:
726:
716:
699:
695:
689:
662:
656:
629:
623:
607:
599:
595:
587:
583:
561:bipyrimidine
546:
487:
477:
448:triflic acid
437:
417:Henry Gilman
398:
390:
375:
318:alkyl halide
311:
303:
287:
266:Zeise's salt
263:
255:
235:
158:
153:
127:
61:
16:
15:
733:: 571–576.
515:(adduct of
322:aryl halide
575:References
246:norbornene
82:) and (PPh
1625:to carbon
474:Catalysis
372:+ 2 LiCl
146:hydrazine
123:fullerene
21:chemistry
1660:Category
936:22482496
901:29711451
454:and (PEt
270:ethylene
40:catalyst
33:platinum
1443:
553:methane
527:. Many
501:hydride
462:Pt(OTf)
452:methane
328:or Pt(C
236:where C
179:+ PtCl
163:(COD).
19:is the
1615:Legend
978:carbon
934:
899:
839:
778:·0.5CH
755:
677:
644:
316:of an
225:+ 3 C
29:carbon
567:into
555:into
403:from
336:)(PPh
932:PMID
897:PMID
837:ISBN
782:I".
774:and
753:ISBN
675:ISBN
642:ISBN
519:and
511:and
494:PtCl
413:Pope
407:and
380:(SMe
364:(SMe
352:(SMe
348:PtCl
272:and
209:Pt(C
199:+ C
109:Pt(C
74:Pt(C
1589:CEs
1584:CCf
1579:CBk
1574:CCm
1569:CAm
1564:CPu
1559:CNp
1549:CPa
1544:CTh
1523:CYb
1518:CTm
1513:CEr
1508:CHo
1503:CDy
1498:CTb
1493:CGd
1488:CEu
1483:CSm
1478:CPm
1473:CNd
1468:CPr
1463:CCe
1458:CLa
1438:Og
1435:Ts
1432:Lv
1429:Mc
1426:Fl
1423:Nh
1420:Cn
1417:Rg
1414:Ds
1411:Mt
1408:Hs
1405:Bh
1401:CSg
1397:Db
1394:Rf
1378:CRa
1374:Fr
1369:Rn
1365:CAt
1360:CPo
1355:CBi
1350:CPb
1345:CTl
1340:CHg
1335:CAu
1330:CPt
1325:CIr
1320:COs
1315:CRe
1305:CTa
1300:CHf
1295:CLu
1283:CBa
1278:CCs
1271:CXe
1261:CTe
1256:CSb
1251:CSn
1246:CIn
1241:CCd
1236:CAg
1231:CPd
1226:CRh
1221:CRu
1216:CTc
1211:CMo
1206:CNb
1201:CZr
1189:CSr
1184:CRb
1177:CKr
1172:CBr
1167:CSe
1162:CAs
1157:CGe
1152:CGa
1147:CZn
1142:CCu
1137:CNi
1132:CCo
1127:CFe
1122:CMn
1117:CCr
1107:CTi
1102:CSc
1095:CCa
1083:CAr
1078:CCl
1063:CSi
1058:CAl
1053:CMg
1048:CNa
1042:Ne
1013:CBe
1008:CLi
1002:He
924:doi
889:doi
862:doi
815:doi
792:doi
788:559
735:doi
704:doi
667:doi
634:doi
613:doi
320:or
244:is
154:cis
144:or
90:Pt(
42:in
31:to
23:of
1662::
1604:No
1599:Md
1594:Fm
1554:CU
1539:Ac
1390:Lr
1310:CW
1266:CI
1196:CY
1112:CV
1090:CK
1073:CS
1068:CP
1038:CF
1033:CO
1028:CN
1023:CC
1018:CB
992:CH
930:.
920:45
918:.
895:.
885:37
883:.
856:.
786:.
731:95
729:.
725:.
700:23
698:.
673:.
640:.
571:.
276::
248:.
242:10
231:10
215:10
205:12
189:10
167:Li
125:.
121:a
119:60
96:Ph
969:e
962:t
955:v
938:.
926::
903:.
891::
868:.
864::
858:7
821:.
817::
798:.
794::
780:3
776:4
772:4
759:.
741:.
737::
710:.
706::
683:.
669::
650:.
636::
615::
505:3
496:6
492:2
490:H
464:2
460:2
458:)
456:3
444:2
442:)
440:3
434:.
432:4
421:4
386:2
384:)
382:2
378:2
370:2
368:)
366:2
362:2
358:2
356:)
354:2
350:2
342:2
340:)
338:3
334:4
332:H
330:2
240:H
238:7
229:H
227:7
223:2
219:3
217:)
213:H
211:7
203:H
201:8
197:8
195:H
193:8
187:H
185:7
181:2
177:8
175:H
173:8
171:C
169:2
161:2
134:2
132:)
130:3
115:4
113:H
111:2
107:2
105:)
103:3
98:2
94:2
92:C
88:2
86:)
84:3
80:4
78:H
76:2
72:2
70:)
68:3
64:4
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.