307:
212:
53:
44:
35:
641:
733:
443:
610:
Apocynin was used to determine whether ionic activation due to proton flux across the membrane of renal medulla cells was coupled to NADPH oxidase production of superoxide. Apocynin was introduced to the cells and completely blocked the production of superoxide, and was a key component in determining
761:
Bowel disease: Apocynin treatment in rats has been proven to lessen damage in the colon as well as the enzymatic activity of myeloperoxidase which is associated with inflammation. In addition, apocynin also decreased the number of macrophages and polymorphonuclear leukocytes in the
1154:
Luchtefeld, Ron; Dasari, Mina S.; Richards, Kristy M.; Alt, Mikaela L.; Crawford, Clark F. P.; Schleiden, Amanda; Ingram, Jai; Hamidou, Abdel Aziz Amadou; Williams, Angela; Chernovitz, Patricia A.; Sun, Grace Y.; Luo, Rensheng; Smith, Robert E. (2008). "Synthesis of
Diapocynin".
772:
Atherosclerosis: Apocynin is used in the treatment of atherosclerosis in order to prevent the activity of NADPH oxidase activity, halting the production of reactive oxygen species. In effect, this inhibition stops initiation of disease in the endothelial
622:
and anti-inflammatory properties, it is still yet to be shown as biologically relevant molecule. Biotransformation of apocynin predominantly leads to glycosylated form of apocynin. Another molecule that is shown to form under experimental conditions is
532:. In 1990, Simons et al. isolated apocynin to a pharmacologically useful level using an actively guided isolation procedure. Apocynin's observed anti-inflammatory capabilities proved to be a result of its ability to selectively prevent the formation of
769:, is being investigated for the treatment of asthma for has been shown to prevent bronchial obstruction in guinea pigs. It is believed that the anti-asthmatic quality of apocynin comes from its interference with certain inflammatory processes.
1505:
Liu N, Matsumura H, Kato T, Ichinose S, Takada A, Namiki T, Asakawa K, Morinaga H, Mohri Y, De
Arcangelis A, Geroges-Labouesse E, Daisuke Nanba D, Nishimura EK (2019). "Stem cell competition orchestrates skin homeostasis and ageing".
1420:
Van den Worm E, Beukelman CJ, Van den Berg AJ, Kroes BH, Labadie RP, Van Dijk H (2001). "Effects of methoxylation of apocynin and analogs on the inhibition of reactive oxygen species production by stimulated human neutrophils".
1190:
Chandasana H, Chhonker YS, Bala V, Prasad YD, Chaitanya TK, Sharma VL, Bhatta RS (2015). "Pharmacokinetic, bioavailability, metabolism and plasma protein binding evaluation of NADPH-oxidase inhibitor apocynin using LC-MS/MS".
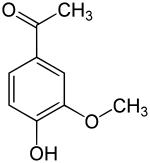
757:
and in the mechanisms that lead to the start of inflammation of the joints. The action of apocynin reduces the presence of such cells before the inflammation has begun but it is unable to reverse inflammation that is already
1301:
Stefanska J, Sarniak A, Wlodarczyk A, Sokolowska M, Pniewska E, Doniec Z, Nowak D, Pawliczak R (2012). "Apocynin reduces reactive oxygen species concentrations in exhaled breath condensate in asthmatics".
595:), which can be used by the immune system to kill bacteria and fungi. Apocynin is an inhibitor of NADPH oxidase activity and thus is effective in preventing the production of the superoxide in human
1390:
Palmen, M.J.H.J.; Beukelman, C.J.; Mooij, R.G.M.; Pena A.S.; van Rees, E.P. (1995). "Anti-inflammatory effect of apocynin, a plant-derived NADPH oxidase antagonist, in acute experimental colitis".
1370:
456:
651:
603:. It does not however obstruct the phagocytic or other defense roles of granulocytes. Due to the selectivity of its inhibition, apocynin can be widely used as an inhibitor of
1345:'T Hart BA, Simons JM, Knaan-Shanzer S, Bakker NP, Labadie RP (1990). "Antiarthritic activity of the newly developed neutrophil oxidative burst antagonist apocynin".
1111:
Li N, Zhang G, Yi FX, Zou AP, Li PL (2005). "Activation of NAD(P)H oxidase by outward movements of H+ ions in renal medullary thick ascending limb of Henle".
356:
797:
Skin stem cells: Apocynin promotes the synthesis of collagen 17 and by doing this it increases the survival of the mother cells derived from stem cells.
788:(ALS, or Lou Gehrig's disease). Researchers believe the benefit derives from a newly discovered role for SOD1 as a self-regulating redox sensor for
1563:
856:
743:
826:
321:
1019:"Apocynin prevents cyclooxygenase 2 expression in human monocytes through NADPH oxidase and glutathione redox-dependent mechanisms"
1017:
Barbieri, Silvia S; Cavalca, Viviana; Eligini, Sonia; Brambilla, Marta; Caiani, Alessia; Tremoli, Elena; Colli, Susanna (2004).
540:
in the body. Apocynin has since been extensively studied to help determine its disease-fighting capabilities and applications.
1456:
Harraz MM, Marden JJ, Zhou W, Zhang Y, Williams A, Sharov VS, Nelson K, Luo M, Paulson H, Schöneich C, Engelhardt JF (2008).
490:. It has been isolated from a variety of plant sources and is being studied for its variety of pharmacological properties.
614:
The mechanism of action of apocynin is not understood. In the experimental studies, apocynin is shown to dimerize and form
1376:
1066:"Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol"
785:
463:
502:, a German pharmacologist, in 1883 and was first isolated by Horace Finnemore, in 1908, from the root of Canadian hemp (
264:
712:
659:
285:
684:
754:
969:
670:
655:
1573:
691:
129:
619:
207:
781:
698:
169:
1515:
1239:
1164:
680:
65:
52:
43:
1568:
533:
504:
499:
302:
95:
1578:
1539:
1327:
1289:
1136:
1458:"SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model"
1288:
for "Hydrogen
Peroxide and Nitrite Reduction in Exhaled Breath Condensate of COPD Patients" at
873:
792:-derived O2• production. The findings in mice may point to new drug targets for hereditary ALS.
1531:
1487:
1438:
1362:
1319:
1265:
1208:
1128:
1093:
1085:
1046:
1038:
951:
933:
852:
836:
822:
816:
766:
569:
189:
1523:
1477:
1469:
1430:
1399:
1354:
1311:
1255:
1247:
1200:
1172:
1120:
1077:
1030:
941:
923:
885:
844:
483:
379:
273:
1018:
105:
1519:
1243:
1168:
1034:
306:
211:
1482:
1457:
1260:
1227:
946:
911:
434:
149:
1434:
705:
1557:
1403:
1358:
1331:
789:
624:
604:
580:
422:
412:
200:
1543:
1140:
596:
1284:
1204:
1315:
1065:
848:
253:
1124:
1081:
600:
508:). At the time, this plant was already used for its known effectiveness against
993:
1527:
1251:
777:
615:
588:
561:
548:
Apocynin is a solid with a melting point of 115 °C and the faint odor of
402:
180:
1089:
1042:
937:
611:
that the proton outflow was responsible for the activation of NADPH oxidase.
17:
517:
34:
1535:
1491:
1442:
1323:
1269:
1212:
1132:
1050:
955:
1366:
1097:
928:
889:
746:(COPD) in 2011 and asthma in 2012 but they did not progress any further.
537:
525:
487:
742:
Small scale early stage clinical trials for apocynin were conducted for
841:
Moderne
Methoden der Pflanzenanalyse / Modern Methods of Plant Analysis
753:
Anti-arthritic: Neutrophils are a key component of the pathogenesis of
669:
if you can. Unsourced or poorly sourced material may be challenged and
557:
553:
549:
240:
1176:
1473:
776:
Familial ALS: Apocynin extended the lives of mutant mice and reduced
529:
160:
433:
Except where otherwise noted, data are given for materials in their
618:. Although, diapocynin seems to have beneficial effect in reducing
228:
784:(SOD1) gene—a genetic defect found in some people with hereditary
509:
140:
128:
118:
1228:"4-Hydr-oxy-3-meth-oxy-5-nitro-aceto-phenone (5-nitro-apocynin)"
1064:
Stolk, J; Hiltermann, T J; Dijkman, J H; Verhoeven, A J (1994).
565:
219:
524:
was used for ages as a treatment for liver and heart problems,
634:
512:
and heart problems. In 1971, apocynin was also isolated from
330:
InChI=1S/C9H10O3/c1-6(10)7-3-4-8(11)9(5-7)12-2/h3-5,11H,1-2H3
516:, a small plant that grows at high altitudes in the western
340:
InChI=1/C9H10O3/c1-6(10)7-3-4-8(11)9(5-7)12-2/h3-5,11H,1-2H3
290:
607:
without interfering in other aspects of the immune system.
1070:
American
Journal of Respiratory Cell and Molecular Biology
843:. Springer-Verlag Berlin Heidelberg. pp. 392–427.
666:
451:
1226:
Babu S, Raghavamenon AC, Fronczek FR, Uppu RM (2009).
874:"The Constituents of Canadian Hemp. Part I. Apocynin"
780:toxicity of cultured cells lines with a defective
749:Other preliminary pre-clinical research includes:
427:295–300 °C (563–572 °F; 568–573 K)
1113:American Journal of Physiology. Renal Physiology
252:
1415:
1413:
104:
665:Please review the contents of the section and
8:
765:Anti-asthmatic: The glucoside of apocynin,
305:
210:
188:
26:
1481:
1259:
945:
927:
272:
835:de Stevens, George; Nord, F. F. (1955).
70:1-(4-Hydroxy-3-methoxyphenyl)ethan-1-one
815:Paech, K.; Tracey, M. V. (2012-12-06).
807:
583:is an enzyme that effectively reduces O
361:
326:
301:
994:"498-02-2 Acetovanillone AKSci J20139"
970:"Apocynin [498-02-2] Biotrend"
839:. In Paech, K.; Tracey, M. V. (eds.).
417:115 °C (239 °F; 388 K)
201:
1462:The Journal of Clinical Investigation
910:Stefanska, J.; Pawliczak, R. (2008).
744:chronic obstructive pulmonary disease
333:Key: DFYRUELUNQRZTB-UHFFFAOYSA-N
168:
148:
79:1-(4-Hydroxy-3-methoxyphenyl)ethanone
7:
1392:The Netherlands Journal of Medicine
1347:Free Radical Biology & Medicine
1035:10.1016/j.freeradbiomed.2004.04.020
837:"Natural Phenylpropane Derivatives"
343:Key: DFYRUELUNQRZTB-UHFFFAOYAW
243:
227:
25:
1023:Free Radical Biology and Medicine
1423:European Journal of Pharmacology
731:
639:
498:Apocynin was first described by
441:
51:
42:
33:
912:"Apocynin: Molecular Aptitudes"
878:Journal of the Chemical Society
437:(at 25 °C , 100 kPa).
81:4-Hydroxy-3-methoxyacetophenone
667:add the appropriate references
552:. It is soluble in hot water,
1:
1435:10.1016/S0014-2999(01)01516-3
1205:10.1016/j.jchromb.2015.01.025
1157:Journal of Chemical Education
786:amyotrophic lateral sclerosis
1564:O-methylated natural phenols
1404:10.1016/0300-2977(95)97051-P
1359:10.1016/0891-5849(90)90115-Y
1316:10.3109/01902148.2011.649823
849:10.1007/978-3-642-64958-5_10
821:. Springer. pp. 410–1.
1193:Journal of Chromatography B
1125:10.1152/ajprenal.00416.2004
1082:10.1165/ajrcmb.11.1.8018341
652:reliable medical references
1595:
1304:Experimental Lung Research
872:Horace, Finnemore (1908).
755:collagen-induced arthritis
407:166.17 g/mol
1528:10.1038/s41586-019-1085-7
1252:10.1107/S160053680903390X
916:Mediators of Inflammation
658:or relies too heavily on
431:
372:
352:
317:
88:
76:
64:
59:
50:
41:
32:
1232:Acta Crystallographica E
486:structurally related to
620:reactive oxygen species
1282:Clinical trial number
782:superoxide dismutase 1
890:10.1039/ct9089301513
66:Preferred IUPAC name
1520:2019Natur.568..344L
1244:2009AcCrE..65o2292B
1169:2008JChEd..85..411D
929:10.1155/2008/106507
544:Physical properties
536:, oxygen ions, and
505:Apocynum cannabinum
500:Oswald Schmiedeberg
364:Oc1ccc(cc1OC)C(C)=O
29:
1290:ClinicalTrials.gov
464:Infobox references
27:
1514:(7752): 344–350.
1238:(Pt 9): o2292–3.
1177:10.1021/ed085p411
858:978-3-642-64958-5
740:
739:
716:
514:Picrorhiza kurroa
472:Chemical compound
470:
469:
286:CompTox Dashboard
130:Interactive image
16:(Redirected from
1586:
1574:Aromatic ketones
1548:
1547:
1502:
1496:
1495:
1485:
1474:10.1172/JCI34060
1453:
1447:
1446:
1417:
1408:
1407:
1387:
1381:
1380:
1375:
1342:
1336:
1335:
1298:
1292:
1280:
1274:
1273:
1263:
1223:
1217:
1216:
1187:
1181:
1180:
1151:
1145:
1144:
1108:
1102:
1101:
1061:
1055:
1054:
1014:
1008:
1007:
1005:
1004:
990:
984:
983:
981:
980:
974:www.biotrend.com
966:
960:
959:
949:
931:
907:
901:
900:
898:
896:
869:
863:
862:
832:
812:
735:
734:
726:
723:
717:
715:
674:
643:
642:
635:
599:or neutrophilic
484:organic compound
478:, also known as
454:
448:
445:
444:
380:Chemical formula
310:
309:
294:
292:
276:
256:
245:
231:
214:
203:
192:
172:
152:
132:
108:
55:
46:
37:
30:
21:
1594:
1593:
1589:
1588:
1587:
1585:
1584:
1583:
1554:
1553:
1552:
1551:
1504:
1503:
1499:
1455:
1454:
1450:
1429:(2–3): 225–30.
1419:
1418:
1411:
1389:
1388:
1384:
1373:
1344:
1343:
1339:
1300:
1299:
1295:
1281:
1277:
1225:
1224:
1220:
1189:
1188:
1184:
1153:
1152:
1148:
1119:(5): F1048–56.
1110:
1109:
1105:
1063:
1062:
1058:
1016:
1015:
1011:
1002:
1000:
992:
991:
987:
978:
976:
968:
967:
963:
909:
908:
904:
894:
892:
871:
870:
866:
859:
834:
829:
814:
813:
809:
804:
736:
732:
727:
721:
718:
675:
664:
660:primary sources
644:
640:
633:
594:
586:
578:
546:
496:
482:, is a natural
473:
466:
461:
460:
459: ?)
450:
446:
442:
438:
396:
392:
388:
382:
368:
365:
360:
359:
348:
345:
344:
341:
335:
334:
331:
325:
324:
313:
295:
288:
279:
259:
246:
234:
195:
175:
155:
135:
122:
111:
98:
84:
82:
80:
72:
71:
23:
22:
15:
12:
11:
5:
1592:
1590:
1582:
1581:
1576:
1571:
1566:
1556:
1555:
1550:
1549:
1497:
1448:
1409:
1382:
1337:
1293:
1275:
1218:
1182:
1146:
1103:
1056:
1029:(2): 156–165.
1009:
985:
961:
902:
864:
857:
827:
818:Acetovanillone
806:
805:
803:
800:
799:
798:
794:
793:
774:
770:
763:
759:
738:
737:
730:
728:
647:
645:
638:
632:
629:
592:
584:
577:
576:Mode of action
574:
545:
542:
495:
492:
480:acetovanillone
471:
468:
467:
462:
440:
439:
435:standard state
432:
429:
428:
425:
419:
418:
415:
409:
408:
405:
399:
398:
394:
390:
386:
383:
378:
375:
374:
370:
369:
367:
366:
363:
355:
354:
353:
350:
349:
347:
346:
342:
339:
338:
336:
332:
329:
328:
320:
319:
318:
315:
314:
312:
311:
298:
296:
284:
281:
280:
278:
277:
269:
267:
261:
260:
258:
257:
249:
247:
239:
236:
235:
233:
232:
224:
222:
216:
215:
205:
197:
196:
194:
193:
185:
183:
177:
176:
174:
173:
165:
163:
157:
156:
154:
153:
145:
143:
137:
136:
134:
133:
125:
123:
116:
113:
112:
110:
109:
101:
99:
94:
91:
90:
86:
85:
83:Acetovanillone
78:
74:
73:
69:
68:
62:
61:
57:
56:
48:
47:
39:
38:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1591:
1580:
1577:
1575:
1572:
1570:
1567:
1565:
1562:
1561:
1559:
1545:
1541:
1537:
1533:
1529:
1525:
1521:
1517:
1513:
1509:
1501:
1498:
1493:
1489:
1484:
1479:
1475:
1471:
1468:(2): 659–70.
1467:
1463:
1459:
1452:
1449:
1444:
1440:
1436:
1432:
1428:
1424:
1416:
1414:
1410:
1405:
1401:
1397:
1393:
1386:
1383:
1378:
1372:
1368:
1364:
1360:
1356:
1353:(2): 127–31.
1352:
1348:
1341:
1338:
1333:
1329:
1325:
1321:
1317:
1313:
1309:
1305:
1297:
1294:
1291:
1287:
1286:
1279:
1276:
1271:
1267:
1262:
1257:
1253:
1249:
1245:
1241:
1237:
1233:
1229:
1222:
1219:
1214:
1210:
1206:
1202:
1198:
1194:
1186:
1183:
1178:
1174:
1170:
1166:
1162:
1158:
1150:
1147:
1142:
1138:
1134:
1130:
1126:
1122:
1118:
1114:
1107:
1104:
1099:
1095:
1091:
1087:
1083:
1079:
1076:(1): 95–102.
1075:
1071:
1067:
1060:
1057:
1052:
1048:
1044:
1040:
1036:
1032:
1028:
1024:
1020:
1013:
1010:
999:
995:
989:
986:
975:
971:
965:
962:
957:
953:
948:
943:
939:
935:
930:
925:
921:
917:
913:
906:
903:
891:
887:
884:(2): 1513–9.
883:
879:
875:
868:
865:
860:
854:
850:
846:
842:
838:
830:
828:9783642649585
824:
820:
819:
811:
808:
801:
796:
795:
791:
790:NADPH oxidase
787:
783:
779:
775:
771:
768:
764:
760:
756:
752:
751:
750:
747:
745:
729:
725:
714:
711:
707:
704:
700:
697:
693:
690:
686:
683: –
682:
678:
677:Find sources:
672:
668:
662:
661:
657:
653:
648:This section
646:
637:
636:
630:
628:
626:
625:nitroapocynin
621:
617:
612:
608:
606:
605:NADPH oxidase
602:
598:
597:agranulocytes
590:
582:
581:NADPH oxidase
575:
573:
571:
567:
563:
559:
555:
551:
543:
541:
539:
535:
534:free radicals
531:
527:
523:
519:
515:
511:
507:
506:
501:
493:
491:
489:
485:
481:
477:
465:
458:
453:
436:
430:
426:
424:
423:Boiling point
421:
420:
416:
414:
413:Melting point
411:
410:
406:
404:
401:
400:
384:
381:
377:
376:
371:
362:
358:
351:
337:
327:
323:
316:
308:
304:
303:DTXSID7060097
300:
299:
297:
287:
283:
282:
275:
271:
270:
268:
266:
263:
262:
255:
251:
250:
248:
242:
238:
237:
230:
226:
225:
223:
221:
218:
217:
213:
209:
206:
204:
202:ECHA InfoCard
199:
198:
191:
187:
186:
184:
182:
179:
178:
171:
167:
166:
164:
162:
159:
158:
151:
147:
146:
144:
142:
139:
138:
131:
127:
126:
124:
120:
115:
114:
107:
103:
102:
100:
97:
93:
92:
87:
75:
67:
63:
58:
54:
49:
45:
40:
36:
31:
19:
18:Acetovanillon
1511:
1507:
1500:
1465:
1461:
1451:
1426:
1422:
1395:
1391:
1385:
1350:
1346:
1340:
1307:
1303:
1296:
1283:
1278:
1235:
1231:
1221:
1196:
1192:
1185:
1160:
1156:
1149:
1116:
1112:
1106:
1073:
1069:
1059:
1026:
1022:
1012:
1001:. Retrieved
997:
988:
977:. Retrieved
973:
964:
919:
915:
905:
893:. Retrieved
881:
877:
867:
840:
817:
810:
748:
741:
722:January 2017
719:
709:
702:
695:
688:
676:
656:verification
649:
613:
609:
601:granulocytes
579:
547:
521:
513:
503:
497:
479:
475:
474:
170:ChEMBL346919
89:Identifiers
77:Other names
1310:(2): 90–9.
1285:NCT01402297
650:needs more
373:Properties
208:100.007.141
1569:Vanilloids
1558:Categories
1163:(3): 411.
1003:2024-06-01
979:2024-06-01
922:: 106507.
802:References
778:glial cell
692:newspapers
681:"Apocynin"
616:diapocynin
589:superoxide
562:chloroform
403:Molar mass
274:B6J7B9UDTR
181:ChemSpider
150:CHEBI:2781
117:3D model (
96:CAS Number
1579:Catechols
1398:(2): 41.
1332:207441506
1199:: 180–8.
1090:1044-1549
1043:0891-5849
998:aksci.com
938:0962-9351
538:peroxides
522:P. kurroa
518:Himalayas
28:Apocynin
1544:92997308
1536:30944469
1492:18219391
1443:11755156
1377:19326251
1324:22296407
1270:21577684
1213:25682338
1141:25646988
1133:15972387
1051:15203187
956:19096513
895:10 April
767:androsin
758:present.
631:Research
526:jaundice
488:vanillin
476:Apocynin
190:21106900
106:498-02-2
1516:Bibcode
1483:2213375
1367:2172098
1261:2969931
1240:Bibcode
1165:Bibcode
1098:8018341
947:2593395
706:scholar
671:removed
558:benzene
554:alcohol
550:vanilla
494:History
457:what is
455: (
397:
241:PubChem
1542:
1534:
1508:Nature
1490:
1480:
1441:
1374:
1365:
1330:
1322:
1268:
1258:
1211:
1139:
1131:
1096:
1088:
1049:
1041:
954:
944:
936:
855:
825:
773:cells.
762:colon.
708:
701:
694:
687:
679:
530:asthma
528:, and
452:verify
449:
357:SMILES
229:C11380
161:ChEMBL
60:Names
1540:S2CID
1371:INIST
1328:S2CID
1137:S2CID
713:JSTOR
699:books
510:edema
322:InChI
141:ChEBI
119:JSmol
1532:PMID
1488:PMID
1439:PMID
1363:PMID
1320:PMID
1266:PMID
1209:PMID
1129:PMID
1094:PMID
1086:ISSN
1047:PMID
1039:ISSN
952:PMID
934:ISSN
920:2008
897:2014
853:ISBN
823:ISBN
685:news
654:for
568:and
566:DMSO
265:UNII
254:2214
220:KEGG
1524:doi
1512:568
1478:PMC
1470:doi
1466:118
1431:doi
1427:433
1400:doi
1355:doi
1312:doi
1256:PMC
1248:doi
1201:doi
1197:985
1173:doi
1121:doi
1117:289
1078:doi
1031:doi
942:PMC
924:doi
886:doi
845:doi
833:in
587:to
570:DMF
291:EPA
244:CID
1560::
1538:.
1530:.
1522:.
1510:.
1486:.
1476:.
1464:.
1460:.
1437:.
1425:.
1412:^
1396:47
1394:.
1369:.
1361:.
1349:.
1326:.
1318:.
1308:38
1306:.
1264:.
1254:.
1246:.
1236:65
1234:.
1230:.
1207:.
1195:.
1171:.
1161:85
1159:.
1135:.
1127:.
1115:.
1092:.
1084:.
1074:11
1072:.
1068:.
1045:.
1037:.
1027:37
1025:.
1021:.
996:.
972:.
950:.
940:.
932:.
918:.
914:.
882:93
880:.
876:.
851:.
673:.
627:.
591:(O
572:.
564:,
560:,
556:,
520:.
391:10
1546:.
1526::
1518::
1494:.
1472::
1445:.
1433::
1406:.
1402::
1379:.
1357::
1351:9
1334:.
1314::
1272:.
1250::
1242::
1215:.
1203::
1179:.
1175::
1167::
1143:.
1123::
1100:.
1080::
1053:.
1033::
1006:.
982:.
958:.
926::
899:.
888::
861:.
847::
831:.
724:)
720:(
710:·
703:·
696:·
689:·
663:.
593:2
585:2
447:Y
395:3
393:O
389:H
387:9
385:C
293:)
289:(
121:)
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.