127:
246:
285:
275:
325:
318:
101:
86:
135:
The metal center is electron withdrawing. This effect is enhanced if the metal is also attached to a carbonyl. Electron poor metals do not back bond well to the carbonyl. The more electron withdrawing the metal is, the more triple bond character the CO ligand has. This gives the ligand a higher force
254:
In this example the ring system can be thought of as analogous to 1,3-butadiene. Following the Green–Davies–Mingos rules, since butadiene is an open π-ligand of even hapticity, nucleophilic attack will occur at one of the terminal positions of the π-system. This occurs because the LUMO of butadiene
565:
Periana, Roy A (1986). "Carbon-carbon activation of organic small ring compounds by arrangement of cycloalkylhydridorhodium complexes to rhodacycloalkanes. Synthesis of metallacyclobutanes, including one with a tertiary metal-carbon bond, by nucleophilic addition to π-allyl complexes".
235:
338:
Periana Roy A.; Bergman Robert G. (1984). "Rapid intramolecular rearrangement of a hydrido(cyclopropyl)rhodium complex to a rhodacyclobutane. Independent synthesis of the metallacycle by addition of hydride to the central carbon atom of a cationic rhodium π-allyl complex".
112:
Nucleophiles preferentially add to even-hapticity polyene ligands at a terminus. Nucleophiles add to odd-hapticity acyclic polyene ligands at a terminal position if the metal is highly electrophilic, otherwise they add at an internal site.
154:
507:
Aranyos, Attila; Szabó, Kálmán J.; Castaño, Ana M.; Bäckvall, Jan-E. (1997). "Central versus
Terminal Attack in Nucleophilic Addition to (π-Allyl)palladium Complexes. Ligand Effects and Mechanism".
142:
The following figure shows a ligated metal attached to a carbonyl group. This group has a partial positive charge and therefore is susceptible to nucleophilic attack. If the ligand represented by L
314:
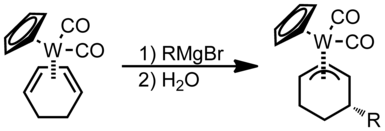
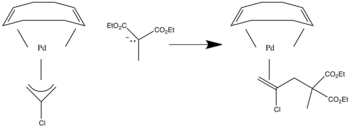
Nucleophilic addition to π ligands can be used in synthesis. One example of this is to make cyclic metal compounds. Nucleophiles add to the center of the π ligand and produces a metallobutane.
136:
constant. The resultant force constant found for a ligated carbonyl represents the same force constant for π ligands if they replaced the CO ligand in the same complex.
300:
568:
480:
341:
362:
Suzuki, Tomohiro; Okada, Goro; Hioki, Yasunori; Fujimoto, Hiroshi (2003). "Theoretical Study of the
Reactivity of (π-Allyl)molybdenum Complexes".
385:
Schörshusen, Sonja; Heck, Jürgen (2007). "Metal-Mediated
Transformations of Cyclooctatetraene to Novel Methylene-Bridged, Bicyclic Compounds".
441:(1978). "Nucleophilic Addition to Organotransition Metal Cations Containing Unsaturated Hydrocarbon Ligands: A Survey and Interpretation".
230:{\displaystyle {\ce {L_{\mathit {n}}-{}}}{\overset {\color {Red}\delta +}{\ce {M}}}{\ce {-C#}}{\overset {\color {Red}\delta +}{\ce {O}}}}
536:
Delbecq, F.; Lapouge, C. (2000). "Regioselectivity of the
Nucleophilic Addition to (η-allyl) Palladium Complexes. A Theoretical Study".
126:
306:
In this case the attack will occur on the carbon with both R groups attached to it since that is the more substituted position.
245:
139:
Nucleophilic addition does not occur if kCO* (the effective force constant for the CO ligand) is below a threshold value
600:
281:
If sigma donating ligands are present they pump electrons into the ligand and attack occurs at the internal position.
31:
434:
59:
121:
The following is a diagram showing the reactivity trends of even/odd hapticity and open/closed π-ligands.
443:
43:
471:
324:
67:
478:
force constants as predictors of π-ethylene and π-benzene complex reactivity with nucleophiles".
268:
317:
240:
Incoming nucleophilic attack happens at one of the termini of the π-system in the figure below:
299:
284:
274:
430:
55:
66:. They describe how and where unsaturated hydrocarbon generally become more susceptibile to
577:
547:
518:
489:
452:
396:
373:
350:
296:
When asymmetrical allyl ligands are present attack occurs at the more substituted position.
538:
509:
387:
364:
438:
63:
39:
17:
594:
456:
47:
100:
85:
97:
Nucleophiles preferentially add to acyclic polyenes rather than cyclic polyenes.
79:
148:
were a π-ligand, it would be activated toward nucleophilic attack as well.
581:
493:
354:
551:
522:
400:
377:
51:
298:
264:
255:
has larger lobes on the ends rather than the internal positions.
78:
Nucleophilic attack is preferred on even-numbered polyenes (even
165:
54:. The rules were published in 1978 by organometallic chemists
323:
316:
283:
273:
244:
125:
99:
84:
171:
259:
Effects of types of ligands on regiochemistry of attack
157:
425:
423:
421:
419:
417:
229:
117:Simplified: even before odd and open before closed
8:
263:Nucleophilic attack at terminal position of
212:
206:
198:
197:
182:
179:
174:
170:
164:
163:
158:
156:
569:Journal of the American Chemical Society
481:Journal of the American Chemical Society
342:Journal of the American Chemical Society
413:
217:
187:
7:
25:
50:containing multiple unsaturated
292:Effects of asymmetrical ligands
1:
27:Organometallic chemistry rule
457:10.1016/0040-4020(78)87001-X
617:
474:(1986). "Metal carbonyl ν
36:Green–Davies–Mingos rules
32:organometallic chemistry
328:
321:
303:
288:
278:
249:
231:
130:
104:
89:
18:Addition to pi ligands
439:Mingos, D. Michael P.
327:
320:
302:
287:
277:
248:
232:
129:
103:
88:
44:nucleophilic addition
435:Green, Malcolm L. H.
155:
601:Reaction mechanisms
582:10.1021/ja00283a033
494:10.1021/ja00270a037
355:10.1021/ja00335a084
173:
70:upon complexation.
68:nucleophilic attack
472:Angelici Robert J.
431:Davies, Stepehn G.
329:
322:
304:
289:
279:
269:π accepting ligand
250:
227:
224:
194:
159:
131:
105:
90:
576:(23): 7346–7355.
552:10.1021/om0003032
546:(14): 2716–2723.
523:10.1021/om960950m
488:(10): 2735–2742.
470:Bush Russell C.;
451:(20): 3047–3077.
401:10.1021/om700539e
395:(22): 5386–5394.
378:10.1021/om0207459
372:(18): 3649–3658.
349:(23): 7272–7273.
310:Uses in synthesis
225:
216:
205:
195:
186:
167:
162:
56:Stephen G. Davies
16:(Redirected from
608:
586:
585:
562:
556:
555:
533:
527:
526:
517:(5): 1058–1064.
504:
498:
497:
467:
461:
460:
427:
404:
381:
358:
236:
234:
233:
228:
226:
214:
213:
211:
210:
203:
202:
196:
184:
183:
181:
180:
178:
172:
169:
168:
160:
21:
616:
615:
611:
610:
609:
607:
606:
605:
591:
590:
589:
564:
563:
559:
539:Organometallics
535:
534:
530:
510:Organometallics
506:
505:
501:
477:
469:
468:
464:
429:
428:
415:
411:
388:Organometallics
384:
365:Organometallics
361:
337:
334:
332:Internal attack
312:
294:
261:
153:
152:
147:
110:
95:
76:
48:metal complexes
46:to 18-electron
28:
23:
22:
15:
12:
11:
5:
614:
612:
604:
603:
593:
592:
588:
587:
557:
528:
499:
475:
462:
412:
410:
407:
406:
405:
382:
359:
333:
330:
311:
308:
293:
290:
260:
257:
252:
251:
238:
237:
223:
220:
209:
201:
193:
190:
177:
143:
133:
132:
109:
106:
94:
91:
75:
72:
64:Michael Mingos
40:regiochemistry
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
613:
602:
599:
598:
596:
583:
579:
575:
571:
570:
561:
558:
553:
549:
545:
541:
540:
532:
529:
524:
520:
516:
512:
511:
503:
500:
495:
491:
487:
483:
482:
473:
466:
463:
458:
454:
450:
446:
445:
440:
436:
432:
426:
424:
422:
420:
418:
414:
408:
402:
398:
394:
390:
389:
383:
379:
375:
371:
367:
366:
360:
356:
352:
348:
344:
343:
336:
335:
331:
326:
319:
315:
309:
307:
301:
297:
291:
286:
282:
276:
272:
270:
267:ligands when
266:
258:
256:
247:
243:
242:
241:
221:
218:
207:
199:
191:
188:
175:
151:
150:
149:
146:
140:
137:
128:
124:
123:
122:
119:
118:
114:
107:
102:
98:
92:
87:
83:
81:
73:
71:
69:
65:
61:
60:Malcolm Green
57:
53:
49:
45:
41:
37:
33:
19:
573:
567:
560:
543:
537:
531:
514:
508:
502:
485:
479:
465:
448:
442:
392:
386:
369:
363:
346:
340:
313:
305:
295:
280:
271:is present.
262:
253:
239:
144:
141:
138:
134:
120:
116:
115:
111:
96:
77:
38:predict the
35:
29:
444:Tetrahedron
409:References
219:δ
208:≡
200:−
189:δ
176:−
80:hapticity
595:Category
52:ligands
108:Rule 3
93:Rule 2
74:Rule 1
62:, and
34:, the
265:allyl
42:for
578:doi
574:108
548:doi
519:doi
490:doi
486:108
453:doi
397:doi
374:doi
351:doi
347:106
82:).
30:In
597::
572:.
544:19
542:.
515:16
513:.
484:.
476:CO
449:34
447:.
437:;
433:;
416:^
393:26
391:.
370:22
368:.
345:.
58:,
584:.
580::
554:.
550::
525:.
521::
496:.
492::
459:.
455::
403:.
399::
380:.
376::
357:.
353::
222:+
215:O
204:C
192:+
185:M
166:n
161:L
145:n
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.