365:
1388:
31:
879:
394:
in the overhead stream, and therefore less steam is required. The multi-pressure configuration with split feed reduces the flow into the bottom section, which also reduces the equivalent work. Flashing feed requires less heat input because it uses the latent heat of water vapor to help strip some of
743:
capture systems. One major focus is on lowering the energy required for solvent regeneration, which has a major impact on process costs. However, there are trade-offs to consider. For example, the energy required for regeneration is typically related to the driving forces for achieving high capture
407:
The amine concentration in the absorbent aqueous solution is an important parameter in the design and operation of an amine gas treating process. Depending on which one of the following four amines the unit was designed to use and what gases it was designed to remove, these are some typical amine
384:
for specific solvents or operating conditions. Vacuum operation favors solvents with low heats of absorption while operation at normal pressure favors solvents with high heats of absorption. Solvents with high heats of absorption require less energy for stripping from temperature swing at fixed
96:
Processes within oil refineries or chemical processing plants that remove
Hydrogen Sulfide are referred to as "sweetening" processes because the odor of the processed products is improved by the absence of "sour" hydrogen sulfide. An alternative to the use of amines involves
340:
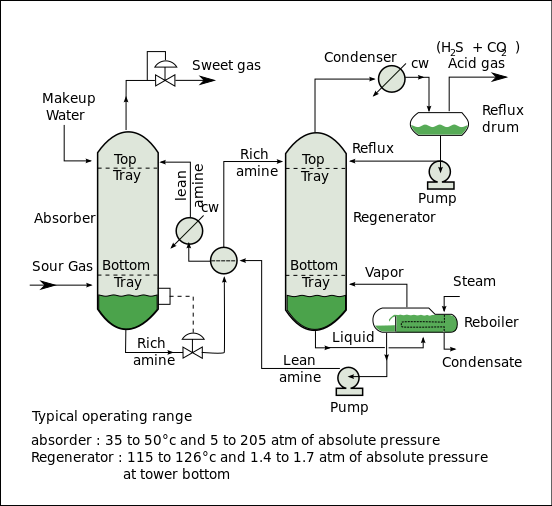
from the upflowing sour gas to produce a sweetened gas stream (i.e., a gas free of hydrogen sulfide and carbon dioxide) as a product and an amine solution rich in the absorbed acid gases. The resultant "rich" amine is then routed into the regenerator (a stripper with a
389:
at a higher pressure and does not have inefficiencies associated with multi-pressure stripper. Energy and costs are reduced since the reboiler duty cycle is slightly less than normal pressure stripper. An
Internal Exchange stripper has a smaller ratio of water vapor to
640:
production it is sometimes necessary to remove carbon dioxide from the biogas to make it comparable with natural gas. The removal of the sometimes high content of hydrogen sulfide is necessary to prevent corrosion of metallic parts after burning the bio gas.
462:
The choice of amine concentration in the circulating aqueous solution depends upon several factors and may be quite arbitrary. It is usually made simply on the basis of experience. The factors involved include whether the amine unit is treating raw
594:. In fact, the vast majority of the 64,000,000 metric tons of sulfur produced worldwide in 2005 was byproduct sulfur from refineries and other hydrocarbon processing plants. Another sulfur-removing process is the
300:
The resulting dissociated and ionized species being more soluble in solution are trapped, or scrubbed, by the amine solution and so easily removed from the gas phase. At the outlet of the amine scrubber, the
537:
in the selected amine. The choice of the type of amine will affect the required circulation rate of amine solution, the energy consumption for the regeneration and the ability to selectively remove either
1178:
191:
The chemistry involved in the amine treating of such gases varies somewhat with the particular amine being used. For one of the more common amines, monoethanolamine (MEA) denoted as
877:, Loren N. Miller & Thomas S. Zawacki, "Process for acid gas removal from gaseous mixtures", issued 21 Mar 1978, assigned to Institute of Gas Technology
744:
capacities. Thus, reducing the regeneration energy can lower the driving force and thereby increase the amount of solvent and size of absorber needed to capture a given amount of CO
399:
in the rich stream entering the stripper at the bottom of the column. The multi-pressure configuration is more attractive for solvents with a higher heats of absorption.
554:
MEA and DEA are primary and secondary amines. They are very reactive and can effectively remove a high volume of gas due to a high reaction rate. However, due to
380:
Alternative stripper configurations include matrix, internal exchange, flashing feed, and multi-pressure with split feed. Many of these configurations offer more
1250:
721:
into the liquid phase. Under low pressure, this transfer is hard to achieve without increasing the reboilers' heat duty, which will result in higher costs.
136:
The most commonly used amines in industrial plants are the alkanolamines DEA, MEA, and MDEA. These amines are also used in many oil refineries to remove
1186:, Polasek, J. (Bryan Research & Engineering) and Bullin, J.A. (Texas A&M University), Gas Processors Association Regional Meeting, Sept. 1994.
566:
during regeneration, which can be up to 70% of total operating costs. They are also more corrosive and chemically unstable compared to other amines.
1183:
1201:
328:
below) includes an absorber unit and a regenerator unit as well as accessory equipment. In the absorber, the downflowing amine solution absorbs
1304:
1159:
633:, amine treating is one of the commonly used processes for removing excess carbon dioxide in the final purification of the gaseous hydrogen.
101:. However, membrane separation is less attractive due to the relatively high capital and operating costs as well as other technical factors.
739:
Currently, a variety of amine mixtures are being synthesized and tested to achieve a more desirable set of overall properties for use in CO
1423:
615:
492:
1219:
858:
833:
797:
546:
alone if desired. For more information about selecting the amine concentration, the reader is referred to Kohl and
Nielsen's book.
1189:
371:
of a typical amine treating process used in petroleum refineries, natural gas processing plants and other industrial facilities.
1438:
992:
792:
526:, corrosion inhibitors are often used and that permits the use of higher concentrations of amine in the circulating solution.
602:. In some plants, more than one amine absorber unit may share a common regenerator unit. The current emphasis on removing CO
1392:
710:
Finding a suitable location (enhanced oil recovery, deep saline aquifers, basaltic rocks...) to dispose of the removed CO
920:
Oyenekan, Babatunde; Rochelle, Gary T. (2007). "Alternative
Stripper Configurations for CO2 Capture by Aqueous Amines".
468:
1428:
1243:
1236:
1418:
611:
653:
in various areas ranging from natural gas production to the food and beverage industry, and have been since 1930.
1433:
1413:
1344:
606:
from the flue gases emitted by fossil fuel power plants has led to much interest in using amines for removing CO
522:
on the surface of the steel that acts to protect the steel. When treating gases with a high percentage of CO
141:
1275:
988:
90:
1334:
1443:
1339:
1329:
1198:
656:
There are multiple classifications of amines, each of which has different characteristics relevant to CO
120:
874:
321:
1354:
1071:
762:
732:
from the inlet gas will cause degradation as well. The degraded amine is no longer able to capture CO
579:
368:
325:
1319:
1309:
98:
346:
345:) to produce regenerated or "lean" amine that is recycled for reuse in the absorber. The stripped
1314:
1259:
1103:
1040:
757:
676:
from the flue gas of a coal-fired plant, which is one of the most effective solvent to capture CO
484:
381:
199:
126:
1215:
1155:
1095:
1087:
1032:
954:
Capture
Performance of Aqueoues MEA and Mixed MEA/MDEA Solvents at the University of Regina CO
854:
829:
27:
Removal of impurities from gases by scrubbing them in aqueous solutions of various alkylamines
1079:
1024:
971:
929:
902:
62:
1324:
1205:
626:
529:
Another factor involved in choosing an amine concentration is the relative solubility of H
1075:
1015:
Abatzoglou, Nicolas; Boivin, Steve (2009). "A review of biogas purification processes".
1003:
108:
70:
1407:
1349:
1107:
772:
599:
587:
562:
per mole of amine. MEA and DEA also require a large amount of energy to strip the CO
555:
207:
86:
1044:
660:
capture. For example, monoethanolamine (MEA) reacts strongly with acid gases like CO
519:
507:
408:
concentrations, expressed as weight percent of pure amine in the aqueous solution:
114:
82:
78:
672:
concentrations. Typically, monoethanolamine (MEA) can capture 85% to 90% of the CO
1149:
1359:
893:
Baker, R. W. (2002). "Future
Directions of Membrane Gas Separation Technology".
767:
595:
464:
203:
185:
1364:
1091:
1036:
1270:
1083:
664:
and has a fast reaction time and an ability to remove high percentages of CO
1099:
364:
17:
724:
Primary and secondary amines, for example, MEA and DEA, will react with CO
1299:
782:
630:
488:
342:
180:
174:
137:
694:
Oxygen content of the gas can cause amine degradation and acid formation
30:
777:
622:
53:, refers to a group of processes that use aqueous solutions of various
975:
933:
906:
1228:
637:
591:
471:
by-product gases that contain relatively low concentrations of both H
1028:
578:
S, much of which often comes from a sulfur-removing process called
787:
479:
or whether the unit is treating gases with a high percentage of CO
363:
58:
54:
29:
1179:
Description of Gas
Sweetening Equipment and Operating Conditions
1288:
1232:
1124:
Folger, P. (2009). "Carbon
Capture: a Technology Assessment".
958:
Capture
Technology Development Plant and the Boundary Dam CO
483:
such as the offgas from the steam reforming process used in
439:
Methyldiethanolamine: About 30 to 55 % for removing H
736:, which decreases the overall carbon capture efficiency.
586:
S-rich stripped gas stream is then usually routed into a
717:
The partial pressure is the driving force to transfer CO
687:
Low pressure gas increases difficulty of transferring CO
1151:
Carbon
Dioxide Sequestration and Related Technologies
950:
Idem, Raphael (2006). "Pilot Plant Studies of the CO
621:
In the specific case of the industrial synthesis of
558:, the loading capacity is limited to 0.5 mol CO
1126:Congressional Research Service Report for Congress
683:Challenges of carbon capture using amine include:
598:which recovers sulfur in any form as concentrated
1143:
1141:
1139:
574:In oil refineries, that stripped gas is mostly H
429:Diethanolamine: About 20 to 25 % removing H
414:Monoethanolamine: About 20 % for removing H
385:capacity. The matrix stripper recovers 40% of CO
104:Many different amines are used in gas treating:
1199:Description of the classic book on gas treating
1058:Rochelle, G. T. (2009). "Amine Scrubbing for CO
1244:
701:degradation of primary (and secondary) amines
449:Diglycolamine: About 50 % for removing H
8:
210:to form a positively charged ammonium group
1154:. John Wiley & Sons. pp. 128–131.
851:Petroleum Refining Technology and Economics
629:process of hydrocarbons to produce gaseous
1285:
1251:
1237:
1229:
1148:Wu, Ying; Carroll, John J. (5 July 2011).
945:
943:
422:, and about 32 % for removing only CO
320:A typical amine gas treating process (the
1119:
1117:
1004:Discussion of recovered byproduct sulfur
510:. However, in an amine treating unit, CO
352:
331:
308:
234:
163:
155:
132:Aminoethoxyethanol (Diglycolamine) (DGA)
809:
819:
817:
815:
813:
506:are acid gases and hence corrosive to
148:Description of a typical amine treater
34:Amine gas plant at a natural gas field
1184:Selecting Amines for Sweetening Units
1017:Biofuels, Bioproducts and Biorefining
824:Arthur Kohl; Richard Nielson (1997).
748:, thus, increasing the capital cost.
349:from the regenerator is concentrated
7:
1387:
853:(2nd ed.). Marcel Dekker, Inc.
1214:(Fifth ed.). Gulf Publishing.
849:Gary, J.H.; Handwerk, G.E. (1984).
616:conventional coal-fired power plant
514:is the stronger acid of the two. H
25:
1194:Sulfur and Carbon Dioxide Removal
828:(5th ed.). Gulf Publishing.
798:Solid sorbents for carbon capture
140:from liquid hydrocarbons such as
1386:
728:and form degradation products. O
57:(commonly referred to simply as
993:United States Geological Survey
793:Ionic liquids in carbon capture
77:) from gases. It is a common
1210:Arthur Kohl; Richard Nielsen.
1190:Natural Gas Supply Association
962:Capture Demonstration Plant".
1:
590:to convert it into elemental
91:natural gas processing plants
649:Amines are used to remove CO
172:are commonly referred to as
1460:
1424:Carbon capture and storage
645:Carbon capture and storage
612:carbon capture and storage
1382:
1295:
1284:
1266:
1305:Atmospheric distillation
989:Sulfur production report
1084:10.1126/science.1176731
704:High energy consumption
691:from the gas into amine
188:processing industries.
142:liquified petroleum gas
1439:Natural gas technology
1276:List of oil refineries
372:
93:and other industries.
85:, and is also used in
35:
1340:Hydrodesulphurisation
707:Very large facilities
376:Alternative processes
367:
223:can be expressed as:
33:
1355:Solvent deasphalting
763:Hydrodesulfurization
668:, even at the low CO
580:hydrodesulfurization
369:Process flow diagram
305:is thus depleted in
121:Methyldiethanolamine
1320:Catalytic reforming
1310:Vacuum distillation
1076:2009Sci...325.1652R
1070:(5948): 1652–1654.
964:Ind. Eng. Chem. Res
895:Ind. Eng. Chem. Res
99:membrane technology
1429:Chemical processes
1370:Amine gas treating
1315:Catalytic cracking
1260:Petroleum refining
1204:2008-03-16 at the
758:Ammonia production
518:S forms a film of
485:ammonia production
469:petroleum refinery
373:
324:, as shown in the
200:acid-base reaction
127:Diisopropanolamine
39:Amine gas treating
36:
1419:Biogas technology
1401:
1400:
1378:
1377:
1161:978-0-470-93876-8
976:10.1021/ie050569e
934:10.1002/aic.11316
907:10.1021/ie0108088
382:energy efficiency
152:Gases containing
16:(Redirected from
1451:
1434:Gas technologies
1414:Acid gas control
1390:
1389:
1286:
1253:
1246:
1239:
1230:
1225:
1212:Gas Purification
1166:
1165:
1145:
1134:
1133:
1121:
1112:
1111:
1055:
1049:
1048:
1012:
1006:
1001:
995:
986:
980:
979:
970:(8): 2414–2420.
947:
938:
937:
928:(12): 3144–154.
917:
911:
910:
901:(6): 1393–1411.
890:
884:
883:
882:
878:
871:
865:
864:
846:
840:
839:
826:Gas Purification
821:
356:
335:
322:Girbotol process
312:
296:
295:
294:
284:
283:
275:
274:
273:
265:
264:
247:
246:
238:
220:
219:
167:
159:
115:Monoethanolamine
63:hydrogen sulfide
51:acid gas removal
41:, also known as
21:
1459:
1458:
1454:
1453:
1452:
1450:
1449:
1448:
1404:
1403:
1402:
1397:
1374:
1291:
1280:
1262:
1257:
1222:
1209:
1206:Wayback Machine
1192:Scroll down to
1175:
1170:
1169:
1162:
1147:
1146:
1137:
1123:
1122:
1115:
1061:
1057:
1056:
1052:
1029:10.1002/bbb.117
1014:
1013:
1009:
1002:
998:
987:
983:
961:
957:
953:
949:
948:
941:
919:
918:
914:
892:
891:
887:
880:
873:
872:
868:
861:
848:
847:
843:
836:
823:
822:
811:
806:
754:
747:
742:
735:
731:
727:
720:
713:
700:
690:
679:
675:
671:
667:
663:
659:
652:
647:
627:steam reforming
609:
605:
585:
577:
572:
565:
561:
552:
545:
541:
536:
532:
525:
517:
513:
505:
501:
482:
478:
474:
456:
452:
446:
442:
436:
432:
425:
421:
417:
405:
398:
393:
388:
378:
360:
354:
350:
339:
333:
329:
316:
310:
306:
293:
290:
289:
288:
286:
282:
279:
278:
277:
272:
269:
268:
267:
263:
260:
259:
258:
256:
254:
245:
242:
241:
240:
236:
232:
230:
218:
215:
214:
213:
196:
171:
165:
161:
157:
153:
150:
76:
68:
43:amine scrubbing
28:
23:
22:
15:
12:
11:
5:
1457:
1455:
1447:
1446:
1441:
1436:
1431:
1426:
1421:
1416:
1406:
1405:
1399:
1398:
1396:
1395:
1383:
1380:
1379:
1376:
1375:
1373:
1372:
1367:
1362:
1357:
1352:
1347:
1342:
1337:
1335:Polymerisation
1332:
1327:
1322:
1317:
1312:
1307:
1302:
1296:
1293:
1292:
1289:
1282:
1281:
1279:
1278:
1273:
1267:
1264:
1263:
1258:
1256:
1255:
1248:
1241:
1233:
1227:
1226:
1220:
1196:
1187:
1181:
1174:
1173:External links
1171:
1168:
1167:
1160:
1135:
1113:
1059:
1050:
1007:
996:
981:
959:
955:
951:
939:
912:
885:
866:
859:
841:
834:
808:
807:
805:
802:
801:
800:
795:
790:
785:
780:
775:
770:
765:
760:
753:
750:
745:
740:
733:
729:
725:
718:
715:
714:
711:
708:
705:
702:
698:
695:
692:
688:
677:
673:
669:
665:
661:
657:
650:
646:
643:
607:
603:
583:
575:
571:
568:
563:
559:
551:
548:
543:
539:
534:
530:
523:
515:
511:
503:
499:
480:
476:
472:
460:
459:
458:
457:
454:
450:
447:
444:
440:
437:
434:
430:
427:
423:
419:
415:
404:
401:
396:
391:
386:
377:
374:
358:
337:
314:
298:
297:
291:
280:
270:
261:
252:
249:
243:
228:
216:
202:involving the
194:
169:
149:
146:
134:
133:
130:
124:
118:
112:
109:Diethanolamine
74:
71:carbon dioxide
66:
47:gas sweetening
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1456:
1445:
1442:
1440:
1437:
1435:
1432:
1430:
1427:
1425:
1422:
1420:
1417:
1415:
1412:
1411:
1409:
1394:
1385:
1384:
1381:
1371:
1368:
1366:
1363:
1361:
1358:
1356:
1353:
1351:
1350:Hydrocracking
1348:
1346:
1343:
1341:
1338:
1336:
1333:
1331:
1330:Isomerisation
1328:
1326:
1323:
1321:
1318:
1316:
1313:
1311:
1308:
1306:
1303:
1301:
1298:
1297:
1294:
1287:
1283:
1277:
1274:
1272:
1269:
1268:
1265:
1261:
1254:
1249:
1247:
1242:
1240:
1235:
1234:
1231:
1223:
1221:0-88415-220-0
1217:
1213:
1207:
1203:
1200:
1197:
1195:
1191:
1188:
1185:
1182:
1180:
1177:
1176:
1172:
1163:
1157:
1153:
1152:
1144:
1142:
1140:
1136:
1131:
1127:
1120:
1118:
1114:
1109:
1105:
1101:
1097:
1093:
1089:
1085:
1081:
1077:
1073:
1069:
1065:
1054:
1051:
1046:
1042:
1038:
1034:
1030:
1026:
1022:
1018:
1011:
1008:
1005:
1000:
997:
994:
990:
985:
982:
977:
973:
969:
965:
946:
944:
940:
935:
931:
927:
923:
922:AIChE Journal
916:
913:
908:
904:
900:
896:
889:
886:
876:
870:
867:
862:
860:0-8247-7150-8
856:
852:
845:
842:
837:
835:0-88415-220-0
831:
827:
820:
818:
816:
814:
810:
803:
799:
796:
794:
791:
789:
786:
784:
781:
779:
776:
774:
773:Claus process
771:
769:
766:
764:
761:
759:
756:
755:
751:
749:
737:
722:
709:
706:
703:
696:
693:
686:
685:
684:
681:
654:
644:
642:
639:
634:
632:
628:
624:
619:
617:
613:
601:
600:sulfuric acid
597:
593:
589:
588:Claus process
581:
569:
567:
557:
556:stoichiometry
549:
547:
542:S alone or CO
527:
521:
509:
496:
494:
490:
486:
470:
466:
448:
438:
428:
413:
412:
411:
410:
409:
402:
400:
383:
375:
370:
366:
362:
348:
344:
327:
323:
318:
304:
303:sweetened gas
250:
226:
225:
224:
222:
209:
208:electron pair
206:of the amine
205:
201:
197:
189:
187:
183:
182:
177:
176:
147:
145:
143:
139:
131:
128:
125:
122:
119:
116:
113:
110:
107:
106:
105:
102:
100:
94:
92:
88:
87:petrochemical
84:
80:
72:
64:
60:
56:
52:
48:
44:
40:
32:
19:
1444:Oil refining
1369:
1211:
1193:
1150:
1129:
1125:
1067:
1063:
1053:
1023:(1): 42–71.
1020:
1016:
1010:
999:
984:
967:
963:
925:
921:
915:
898:
894:
888:
869:
850:
844:
825:
738:
723:
716:
682:
655:
648:
635:
620:
573:
553:
528:
520:iron sulfide
508:carbon steel
497:
493:power plants
461:
406:
379:
347:overhead gas
326:flow diagram
319:
302:
299:
211:
192:
190:
179:
173:
151:
135:
103:
95:
79:unit process
61:) to remove
50:
46:
42:
38:
37:
1360:Visbreaking
768:WSA Process
610:(see also:
596:WSA Process
550:MEA and DEA
465:natural gas
204:protonation
186:hydrocarbon
55:alkylamines
18:Amine plant
1408:Categories
1345:Sweetening
1325:Alkylation
1062:Capture".
875:US 4080424
804:References
625:, for the
489:flue gases
181:acid gases
175:sour gases
138:sour gases
83:refineries
1300:Desalting
1290:Processes
1271:Petroleum
1108:206521374
1092:0036-8075
1037:1932-104X
582:. This H
1202:Archived
1132:: 26–44.
1100:19779188
1045:84907789
783:Rectisol
752:See also
631:hydrogen
533:S and CO
502:S and CO
475:S and CO
453:S and CO
443:S and CO
433:S and CO
418:S and CO
343:reboiler
160:or both
89:plants,
81:used in
1393:Commons
1072:Bibcode
1064:Science
991:by the
778:Selexol
636:In the
623:ammonia
487:or the
184:in the
144:(LPG).
69:S) and
1391:
1365:Coking
1218:
1158:
1106:
1098:
1090:
1043:
1035:
881:
857:
832:
638:biogas
592:sulfur
498:Both H
403:Amines
395:the CO
357:and CO
336:and CO
313:and CO
198:, the
168:and CO
129:(DIPA)
123:(MDEA)
59:amines
1104:S2CID
1041:S2CID
788:Amine
491:from
276:⇌ RNH
239:⇌ RNH
117:(MEA)
111:(DEA)
1216:ISBN
1156:ISBN
1096:PMID
1088:ISSN
1033:ISSN
855:ISBN
830:ISBN
614:and
570:Uses
248:+ HS
212:(RNH
49:and
1208:by
1080:doi
1068:325
1025:doi
972:doi
930:doi
903:doi
618:).
467:or
317:.
287:HCO
251:RNH
227:RNH
193:RNH
178:or
73:(CO
1410::
1138:^
1128:.
1116:^
1102:.
1094:.
1086:.
1078:.
1066:.
1039:.
1031:.
1019:.
968:45
966:.
942:^
926:53
924:.
899:41
897:.
812:^
697:CO
680:.
495:.
390:CO
361:.
285:+
266:CO
255:+
231:+
65:(H
45:,
1252:e
1245:t
1238:v
1224:.
1164:.
1130:5
1110:.
1082::
1074::
1060:2
1047:.
1027::
1021:3
978:.
974::
960:2
956:2
952:2
936:.
932::
909:.
905::
863:.
838:.
746:2
741:2
734:2
730:2
726:2
719:2
712:2
699:2
689:2
678:2
674:2
670:2
666:2
662:2
658:2
651:2
608:2
604:2
584:2
576:2
564:2
560:2
544:2
540:2
538:H
535:2
531:2
524:2
516:2
512:2
504:2
500:2
481:2
477:2
473:2
455:2
451:2
445:2
441:2
435:2
431:2
426:.
424:2
420:2
416:2
397:2
392:2
387:2
359:2
355:S
353:2
351:H
338:2
334:S
332:2
330:H
315:2
311:S
309:2
307:H
292:3
281:3
271:3
262:2
257:H
253:2
244:3
237:S
235:2
233:H
229:2
221:)
217:3
195:2
170:2
166:S
164:2
162:H
158:S
156:2
154:H
75:2
67:2
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.