162:
247:
210:
271:
17:
585:
82:
566:
127:
E 120. The traditional methods for carmine production are labour, land, and insect-intensive. Because demand for red dyes is predicted to increase, researchers are exploring metabolic engineering approaches for the production of synthetic carminic acid.
29:
are an abundant group of dyes comprising a anthraquinone unit as the shared structural element. Anthraquinone itself is colourless, but red to blue dyes are obtained by introducing electron donor groups such as
103:
for a number of structurally related dyes that use alizarin dyes (sometimes synonymous with anthraquinone dyes). It was the first natural dye for which an industrial synthesis was developed as early as 1869.
583:, Johannes Heyna, Willy Schumacher, "Verfahren zum Fixieren wasserloeslicher organischer Verbindungen auf Unterlagen faseriger Struktur", issued 1957-09-19, assigned to Hoechst AG
221:
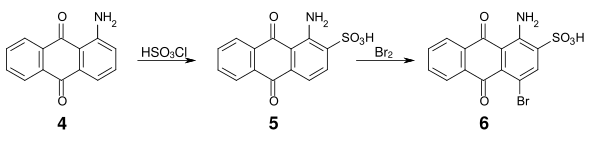
By replacing the bromine substituent with an aliphatic or aromatic amine, vibrant blue dyes are obtained. For example, bromamic acid can be condensed with 3-(2-hydroxyethylsulfonyl)-aniline (
173:
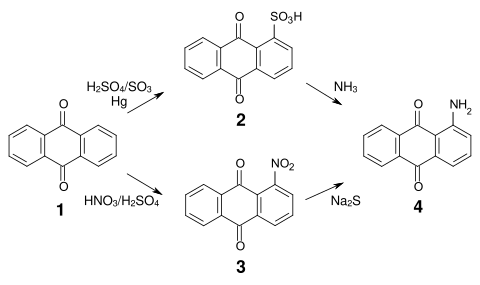
Sulfonation in α position is reversible and both the sulfonic acid groups and the nitro groups can be relatively easily replaced by amino, alkylamino, hydroxy and
38:
in the 1-, 4-, 5- or 8-position. Anthraquinone dyestuffs are structurally related to indigo dyestuffs and are classified together with these in the group of
546:
520:
321:
161:
564:, Andreas Von Der Eltz, "Verfahren zur Herstellung von C.I. Reactive Blue 19", issued 1996-04-01, assigned to Hoechst AG
246:
465:"Next-Generation Genetic and Fermentation Technologies for Safe and Sustainable Production of Food Ingredients: Colors and Flavorings"
608:
345:
188:
An important intermediate product for many acid anthraquinone dyes is bromamic acid (1-amino-4-bromoanthraquinone-2-sulfonic acid) (
367:
209:
428:
628:
270:
291:
561:
580:
145:
258:
Reactive Blue 19 is one of the oldest and still the most important reactive dyes, patented in 1949.
197:
604:
542:
516:
484:
402:
341:
317:
476:
436:
394:
262:
230:
96:
16:
480:
234:
50:
622:
601:
Color
Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments
464:
338:
Color
Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments
238:
124:
116:
112:
108:
70:
39:
31:
20:
174:
66:
62:
398:
136:
The synthesis of most anthraquinone dyes is based on anthraquinone sulfonic acid (
536:
510:
383:"The red insect dyes: carminic, kermesic and laccaic acids and their derivatives"
311:
382:
201:
54:
46:
35:
361:
265:(C.I. Vat Blue 4) - the synthesis of which was developed by René Bohn in 1901:
441:
107:
Anthraquinone dyes include red insect dyes derived from scale insects such as
488:
406:
149:
123:
with the main component carminic acid is used, for example, as an approved
92:
85:
286:) under strongly alkaline conditions at 220-235 °C, intermediate stage
182:
120:
58:
181:) is thus accessible by reaction of anthraquinone sulfonic acid with
100:
541:(in German), Berlin, Heidelberg: Springer Verlag, pp. 365 ff.,
81:
91:
One of the most important anthraquinone dyes of herbal origin is
340:(3rd ed.), Weinheim: WILEY-VCH Verlag, pp. 255 ff.,
538:
603:(3rd ed.), Weinheim: WILEY-VCH Verlag, p. 289,
535:
Heinz-Gerhard Franck, Jürgen W. Stadelhofer (1978),
512:
Industrial Dyes: Chemistry, Properties, Applications
313:
Industrial Dyes: Chemistry, Properties, Applications
261:
The first anthraquinone-based synthetic vat dye was
192:), which can be obtained from 1-aminoanthraquinone (
371:. Georg Thieme Verlag, retrieved 14. Dezember 2018.
429:"Cochineal, a red dye from bugs, moves to the lab"
229:) (oxysulfone blue), from which the reactive dye
515:, Weinheim: WILEY-VCH Verlag, pp. 200 ff.,
316:, Weinheim: WILEY-VCH Verlag, pp. 35 ff.,
463:Seo, Seung-Oh; Jin, Yong-Su (25 March 2022).
95:, which is extracted from the dyer's madder (
53:. Anthraquinone dyestuffs are represented in
8:
469:Annual Review of Food Science and Technology
269:
245:
208:
160:
45:Members of this dye group can be found in
440:
282:By dimerization of 2-aminoanthraquinone (
80:
15:
302:
185:or by reduction of nitroanthraquinone.
69:. They are characterized by very good
427:Miller, Brittney J. (25 March 2022).
7:
381:Cooksey, C. J. (17 February 2019).
290:is obtained in two steps, which is
481:10.1146/annurev-food-052720-012228
253:Synthesis of C.I. Reactive Blue 19
14:
168:Synthesis of 1-aminoanthraquinone
225:) to form the vibrant blue dye (
387:Biotechnic & Histochemistry
294:and oxidized to indanthrone 5.
1:
399:10.1080/10520295.2018.1511065
599:Zollinger, Heinrich (2003),
336:Zollinger, Heinrich (2003),
132:Synthetic anthraquinone dyes
509:Hunger, Klaus, ed. (2003),
310:Hunger, Klaus, ed. (2003),
645:
216:Synthesis of bromamic acid
77:Natural anthraquinone dyes
442:10.1146/knowable-032522-1
292:cyclized intramolecularly
140:) or nitroanthraquinone (
277:Synthesis of indanthrone
144:), which is obtained by
362:Anthrachinon-Farbstoffe
196:) by sulfonation with
177:. Aminoanthraquinone (
88:
23:
231:C.I. Reactive Blue 19
84:
19:
198:chlorosulfonic acid
99:). Alizarin is the
629:Anthraquinone dyes
233:is obtained after
152:of anthraquinone (
89:
27:Anthraquinone dyes
24:
548:978-3-662-07876-1
522:978-3-662-01950-4
433:Knowable Magazine
323:978-3-662-01950-4
278:
254:
217:
169:
636:
614:
613:
596:
590:
589:
588:
584:
577:
571:
570:
569:
565:
558:
552:
551:
532:
526:
525:
506:
500:
499:
497:
495:
460:
454:
453:
451:
449:
444:
424:
418:
417:
415:
413:
378:
372:
357:
351:
350:
333:
327:
326:
307:
276:
273:
252:
249:
215:
212:
167:
164:
644:
643:
639:
638:
637:
635:
634:
633:
619:
618:
617:
611:
598:
597:
593:
586:
579:
578:
574:
567:
560:
559:
555:
549:
534:
533:
529:
523:
508:
507:
503:
493:
491:
462:
461:
457:
447:
445:
426:
425:
421:
411:
409:
380:
379:
375:
358:
354:
348:
335:
334:
330:
324:
309:
308:
304:
300:
200:and subsequent
134:
119:. The colorant
97:Rubia tinctorum
79:
12:
11:
5:
642:
640:
632:
631:
621:
620:
616:
615:
609:
591:
572:
553:
547:
527:
521:
501:
475:(1): 463–488.
455:
419:
393:(2): 100–107.
373:
352:
346:
328:
322:
301:
299:
296:
280:
279:
274:
256:
255:
250:
235:esterification
219:
218:
213:
171:
170:
165:
133:
130:
78:
75:
71:light fastness
61:, but also in
51:synthetic dyes
49:as well as in
13:
10:
9:
6:
4:
3:
2:
641:
630:
627:
626:
624:
612:
610:3-906390-23-3
606:
602:
595:
592:
582:
576:
573:
563:
557:
554:
550:
544:
540:
539:
531:
528:
524:
518:
514:
513:
505:
502:
490:
486:
482:
478:
474:
470:
466:
459:
456:
443:
438:
434:
430:
423:
420:
408:
404:
400:
396:
392:
388:
384:
377:
374:
370:
369:
364:
363:
356:
353:
349:
347:3-906390-23-3
343:
339:
332:
329:
325:
319:
315:
314:
306:
303:
297:
295:
293:
289:
285:
275:
272:
268:
267:
266:
264:
259:
251:
248:
244:
243:
242:
240:
239:sulfuric acid
236:
232:
228:
224:
214:
211:
207:
206:
205:
203:
199:
195:
191:
186:
184:
180:
176:
175:alkoxy groups
166:
163:
159:
158:
157:
155:
151:
147:
143:
139:
131:
129:
126:
125:food colorant
122:
118:
117:laccaic acids
114:
113:kermesic acid
110:
109:carminic acid
105:
102:
98:
94:
87:
83:
76:
74:
72:
68:
67:disperse dyes
64:
60:
56:
52:
48:
43:
41:
40:carbonyl dyes
37:
33:
28:
22:
21:Anthraquinone
18:
600:
594:
575:
556:
537:
530:
511:
504:
492:. Retrieved
472:
468:
458:
446:. Retrieved
432:
422:
410:. Retrieved
390:
386:
376:
368:Römpp Online
366:
360:
355:
337:
331:
312:
305:
287:
283:
281:
260:
257:
226:
222:
220:
193:
189:
187:
178:
172:
153:
141:
137:
135:
106:
90:
47:natural dyes
44:
36:amino groups
26:
25:
263:indanthrone
202:bromination
146:sulfonation
562:DE 4422160
298:References
581:DE 965902
489:1941-1413
407:1052-0295
359:Entry on
150:nitration
623:Category
494:28 March
448:28 March
412:28 March
93:alizarin
86:Alizarin
63:reactive
183:ammonia
121:carmine
55:mordant
32:hydroxy
607:
587:
568:
545:
519:
487:
405:
365:. at:
344:
320:
115:, and
101:eponym
237:with
605:ISBN
543:ISBN
517:ISBN
496:2022
485:ISSN
450:2022
414:2022
403:ISSN
342:ISBN
318:ISBN
65:and
57:and
477:doi
437:doi
395:doi
156:).
148:or
59:vat
34:or
625::
483:.
473:13
471:.
467:.
435:.
431:.
401:.
391:94
389:.
385:.
241:.
204:.
111:,
73:.
42:.
498:.
479::
452:.
439::
416:.
397::
288:3
284:1
227:8
223:7
194:4
190:6
179:4
154:1
142:3
138:2
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.