570:. The trial was conducted at 40 sites in the United States, the European Union, and Canada. Trial investigators evaluated participants 12 years and older with hereditary angioedema for eight weeks to determine the number of attacks for each participant. The trial enrolled only participants who had at least two attacks during the eight-week period. Participants were assigned to receive one of two doses of berotralstat or placebo once every day for 24 weeks. Neither the participants nor the investigators knew which treatment was being given until after the trial was completed. All participants could use other medications for treatment of attacks.
404:
966:
31:
946:
695:
480:
522:
InChI=1S/C30H26F4N6O/c31-24-10-9-22(28(37-17-18-7-8-18)21-5-1-3-19(11-21)15-35)13-25(24)38-29(41)26-14-27(30(32,33)34)39-40(26)23-6-2-4-20(12-23)16-36/h1-6,9-14,18,28,37H,7-8,16-17,36H2,(H,38,41)/t28-/m1/s1
134:
1044:
494:
1015:
80:
814:"Oral once-daily berotralstat for the prevention of hereditary angioedema attacks: A randomized, double-blind, placebo-controlled phase 3 trial"
853:
587:
882:
514:
630:
217:
164:
61:
1008:
558:
Berotralstat was approved for medical use in the United States in
December 2020, and in the European Union in April 2021.
859:
327:
97:
676:
383:
566:
Berotralstat was approved based on evidence from one clinical trial (Trial 1 /NCT03485911) of 120 participants with
1034:
936:
928:
for "Efficacy and Safety Study of BCX7353 as an Oral
Treatment for the Prevention of Attacks in HAE (APeX-2)" at
722:
75:
1001:
108:
746:
1039:
567:
549:
812:
Zuraw B, Lumry WR, Johnston DT, Aygören-Pürsün E, Banerji A, Bernstein JA, et al. (October 2020).
399:
973:
555:
The most common side effects include abdominal pain, vomiting, diarrhea, back pain, and heartburn.
234:
115:
929:
835:
794:
316:
190:
178:
127:
43:
717:
251:
243:
911:
825:
784:
420:
965:
403:
344:
336:
950:
985:
981:
30:
276:
1028:
699:
147:
142:
924:
773:"Oral plasma kallikrein inhibitor BCX7353 for treatment of hereditary angioedema"
830:
813:
502:
NCC1=CC(=CC=C1)N1N=C(C=C1C(=O)NC1=CC(=CC=C1F)(NCC1CC1)C1=CC=CC(=C1)C#N)C(F)(F)F
545:
456:
307:
122:
22:
839:
798:
789:
772:
612:
287:
92:
296:
262:
372:
364:
977:
479:
470:
698:
This article incorporates text from this source, which is in the
79:
355:
196:
70:
225:
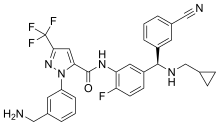
2--N--2-fluorophenyl]-5-(trifluoromethyl)pyrazole-3-carboxamide
184:
87:
388:
173:
989:
883:"Berotralstat (Oral Route) Side Effects - Mayo Clinic"
934:
771:
Hwang JR, Hwang G, Johri A, Craig T (December 2019).
625:
623:
621:
468:
455:
419:
414:
382:
354:
326:
306:
286:
261:
233:
208:
163:
158:
133:
121:
107:
60:
52:
42:
37:
275:
712:
710:
708:
671:
250:
242:
818:The Journal of Allergy and Clinical Immunology
669:
667:
665:
663:
661:
659:
657:
655:
653:
651:
631:"Orladeyo- berotralstat hydrochloride capsule"
1009:
552:(HAE) in people aged twelve years and older.
8:
96:
21:
1016:
1002:
402:
315:
29:
829:
788:
343:
335:
1045:Blood and blood forming organ drug stubs
941:
579:
519:
499:
398:
295:
222:
588:"Summary Basis of Decision - Orladeyo"
20:
7:
962:
960:
918:. U.S. National Library of Medicine.
751:Union Register of medicinal products
146:
371:
363:
266:
14:
681:U.S. Food and Drug Administration
964:
944:
693:
677:"Drug Trials Snapshot: Orladeyo"
443:
437:
431:
527:Key:UXNXMBYCBRBRFD-MUUNZHRXSA-N
854:"Orladeyo: FDA-Approved Drugs"
747:"Orladeyo Product information"
449:
425:
1:
988:. You can help Knowledge by
860:Food and Drug Administration
540:, sold under the brand name
548:used to prevent attacks of
128:Plasma kallikrein inhibitor
1061:
959:
831:10.1016/j.jaci.2020.10.015
415:Chemical and physical data
723:European Medicines Agency
510:
490:
213:
28:
976:article relating to the
916:Drug Information Portal
922:Clinical trial number
790:10.2217/imt-2019-0128
568:hereditary angioedema
550:hereditary angioedema
982:blood forming organs
25:
930:ClinicalTrials.gov
887:www.mayoclinic.org
727:. 24 February 2021
1035:CYP2D6 inhibitors
997:
996:
824:(1): 164–172.e9.
783:(17): 1439–1444.
683:. 3 December 2020
613:Product monograph
594:. 23 October 2014
535:
534:
481:Interactive image
384:CompTox Dashboard
200:
188:
176:
90:
73:
56:BCX7353, BCX-7353
1052:
1018:
1011:
1004:
968:
961:
949:
948:
947:
940:
919:
898:
897:
895:
893:
879:
873:
872:
870:
868:
850:
844:
843:
833:
809:
803:
802:
792:
768:
762:
761:
759:
757:
743:
737:
736:
734:
732:
714:
703:
697:
696:
692:
690:
688:
673:
646:
645:
643:
641:
627:
616:
610:
604:
603:
601:
599:
584:
483:
463:
451:
445:
439:
433:
427:
407:
406:
392:
390:
375:
367:
347:
339:
319:
299:
279:
269:
268:
254:
246:
198:
195:
186:
183:
175:
172:
150:
100:
89:
86:
83:
72:
69:
33:
26:
24:
1060:
1059:
1055:
1054:
1053:
1051:
1050:
1049:
1025:
1024:
1023:
1022:
957:
955:
945:
943:
935:
910:
907:
902:
901:
891:
889:
881:
880:
876:
866:
864:
852:
851:
847:
811:
810:
806:
770:
769:
765:
755:
753:
745:
744:
740:
730:
728:
718:"Orladeyo EPAR"
716:
715:
706:
694:
686:
684:
675:
674:
649:
639:
637:
629:
628:
619:
611:
607:
597:
595:
586:
585:
581:
576:
564:
531:
528:
523:
518:
517:
506:
503:
498:
497:
486:
461:
448:
442:
436:
430:
410:
400:DTXSID401336676
386:
378:
350:
322:
302:
282:
265:
257:
229:
226:
221:
220:
204:
154:
110:
103:
17:
12:
11:
5:
1058:
1056:
1048:
1047:
1042:
1037:
1027:
1026:
1021:
1020:
1013:
1006:
998:
995:
994:
969:
954:
953:
933:
932:
920:
912:"Berotralstat"
906:
905:External links
903:
900:
899:
874:
845:
804:
763:
738:
704:
647:
617:
605:
578:
577:
575:
572:
563:
560:
533:
532:
530:
529:
526:
524:
521:
513:
512:
511:
508:
507:
505:
504:
501:
493:
492:
491:
488:
487:
485:
484:
476:
474:
466:
465:
459:
453:
452:
446:
440:
434:
428:
423:
417:
416:
412:
411:
409:
408:
395:
393:
380:
379:
377:
376:
368:
360:
358:
352:
351:
349:
348:
340:
332:
330:
324:
323:
321:
320:
312:
310:
304:
303:
301:
300:
292:
290:
284:
283:
281:
280:
272:
270:
259:
258:
256:
255:
247:
239:
237:
231:
230:
228:
227:
224:
216:
215:
214:
211:
210:
206:
205:
203:
202:
193:
181:
169:
167:
161:
160:
156:
155:
153:
152:
139:
137:
131:
130:
125:
119:
118:
113:
111:administration
105:
104:
102:
101:
84:
66:
64:
58:
57:
54:
50:
49:
46:
40:
39:
35:
34:
15:
13:
10:
9:
6:
4:
3:
2:
1057:
1046:
1043:
1041:
1038:
1036:
1033:
1032:
1030:
1019:
1014:
1012:
1007:
1005:
1000:
999:
993:
991:
987:
983:
979:
975:
970:
967:
963:
958:
952:
942:
938:
931:
927:
926:
921:
917:
913:
909:
908:
904:
888:
884:
878:
875:
863:
861:
855:
849:
846:
841:
837:
832:
827:
823:
819:
815:
808:
805:
800:
796:
791:
786:
782:
778:
777:Immunotherapy
774:
767:
764:
752:
748:
742:
739:
726:
724:
719:
713:
711:
709:
705:
701:
700:public domain
682:
678:
672:
670:
668:
666:
664:
662:
660:
658:
656:
654:
652:
648:
636:
632:
626:
624:
622:
618:
614:
609:
606:
593:
592:Health Canada
589:
583:
580:
573:
571:
569:
561:
559:
556:
553:
551:
547:
543:
539:
525:
520:
516:
509:
500:
496:
489:
482:
478:
477:
475:
472:
467:
460:
458:
454:
424:
422:
418:
413:
405:
401:
397:
396:
394:
385:
381:
374:
370:as HCl:
369:
366:
362:
361:
359:
357:
353:
346:
342:as HCl:
341:
338:
334:
333:
331:
329:
325:
318:
314:
313:
311:
309:
305:
298:
294:
293:
291:
289:
285:
278:
274:
273:
271:
264:
260:
253:
249:as HCl:
248:
245:
241:
240:
238:
236:
232:
223:
219:
212:
207:
201: Rx-only
194:
192:
182:
180:
171:
170:
168:
166:
162:
157:
149:
144:
141:
140:
138:
136:
132:
129:
126:
124:
120:
117:
114:
112:
106:
99:
94:
85:
82:
77:
68:
67:
65:
63:
59:
55:
51:
47:
45:
41:
38:Clinical data
36:
32:
27:
19:
1040:Orphan drugs
990:expanding it
971:
956:
923:
915:
890:. Retrieved
886:
877:
865:. Retrieved
857:
848:
821:
817:
807:
780:
776:
766:
754:. Retrieved
750:
741:
729:. Retrieved
721:
685:. Retrieved
680:
638:. Retrieved
634:
608:
596:. Retrieved
591:
582:
565:
557:
554:
541:
538:Berotralstat
537:
536:
252:1809010-52-3
244:1809010-50-1
165:Legal status
159:Legal status
98:Berotralstat
62:License data
23:Berotralstat
18:
925:NCT03485911
867:25 December
687:25 December
640:25 December
598:28 November
464: g·mol
209:Identifiers
53:Other names
44:Trade names
1029:Categories
574:References
546:medication
469:3D model (
457:Molar mass
345:88SH1NBL2B
337:XZA0KB1BDQ
308:ChemSpider
235:CAS Number
218:IUPAC name
123:Drug class
16:Medication
277:137528262
109:Routes of
951:Medicine
840:33098856
799:31635497
635:DailyMed
542:Orladeyo
317:81368516
288:DrugBank
135:ATC code
116:By mouth
93:DailyMed
48:Orladeyo
892:3 March
756:3 March
731:12 July
615:hres.ca
562:History
544:, is a
462:562.573
421:Formula
297:DB15982
263:PubChem
177::
151:)
145: (
143:B06AC06
95::
78::
937:Portal
838:
797:
495:SMILES
373:D11674
365:D11673
191:℞-only
189:
179:℞-only
91:
81:by INN
74:
984:is a
978:blood
972:This
862:(FDA)
858:U.S.
725:(EMA)
515:InChI
471:JSmol
986:stub
980:and
974:drug
894:2021
869:2020
836:PMID
795:PMID
758:2023
733:2021
689:2020
642:2020
600:2022
356:KEGG
328:UNII
826:doi
822:148
785:doi
389:EPA
267:CID
148:WHO
76:EMA
1031::
914:.
885:.
856:.
834:.
820:.
816:.
793:.
781:11
779:.
775:.
749:.
720:.
707:^
679:.
650:^
633:.
620:^
590:.
435:26
429:30
197:EU
185:US
174:CA
88:US
71:EU
1017:e
1010:t
1003:v
992:.
939::
896:.
871:.
842:.
828::
801:.
787::
760:.
735:.
702:.
691:.
644:.
602:.
473:)
450:O
447:6
444:N
441:4
438:F
432:H
426:C
391:)
387:(
199::
187::
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.