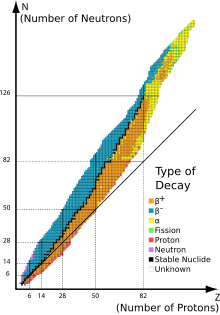338:
surrounding even-even isobars, and the last two surround the proton numbers 43 and 61 which have no beta-stable isotopes. Also, two beta-decay stable nuclides exist for odd proton numbers 1, 3, 5, 7, 17, 19, 29, 31, 35, 47, 51, 63, 77, 81, and 95; the first four cases involve very light nuclides where odd-odd nuclides are more stable than their surrounding even-even isobars, and the other numbers surround the neutron numbers 19, 21, 35, 39, 45, 61, 71, 89, 115, 123, 147 which have no beta-stable isotopes. (For
1333:
31:
1344:= 256. Black denotes the predicted beta-stability line, which is in good agreement with experimental data, though it fails to predict that Tc and Pm have no beta-stable isotope (the mass differences causing these anomalies are small). Islands of stability are predicted to center near Ds and 126, beyond which the model appears to deviate from several rules of the semi-empirical mass formula.
285:
directions, to Hf or W.) Among non-primordial nuclides, there are some other cases of theoretically possible but never-observed beta decay, notably including Rn and Cm (the most stable isotopes of their elements considering all decay modes). Finally, Ca and Zr have not been observed to undergo beta decay (which is theoretically possible for both), but double beta decay is known for both.
1890:
Gd was previously thought to be a third beta-stable isobar for mass 148, but according to current mass determinations it has a higher mass than Eu and can undergo electron capture. Nevertheless, the mass difference is very small (27.0 keV, even lower than likewise unseen electron capture of Te),
1447:-mass of the two beta-stable isobars. For example, K could either undergo electron capture or positron emission to Ar, or undergo beta minus decay to Ca: both possible products are beta-stable. The former process would produce the lighter of the two beta-stable isobars, yet the latter is more common.
337:
Two beta-decay stable nuclides exist for odd neutron numbers 1 (H and He), 3 (He and Li – the former having an extremely short half-life), 5 (Be and B), 7 (C and N), 55 (Mo and Ru), and 85 (Nd and Sm); the first four cases involve very light nuclides where odd-odd nuclides are more stable than their
1380:
closures in the region; such isotopes would decay primarily through alpha decay or spontaneous fission. Beyond the island of stability, various models that correctly predict many known beta-stable isotopes also predict anomalies in the beta-stability line that are unobserved in any known nuclides,
1360:
is small enough that such decay has never been seen. With the exception of No, no nuclides with A > 260 have been definitively identified as beta-stable. Fm is unconfirmed. Moreover, the known beta-stable nuclei for individual masses A > 257 may not represent the complete
284:
are beta decay stable, with the exception of K, V, Rb, Cd, In, La, Lu, and Re. In addition, Te and Ta have not been observed to decay, but are believed to undergo beta decay with an extremely long half-life (over 10 years). (Te can only undergo electron capture to Sb, whereas Ta can decay in both
386:= 20 (S, Cl, Ar, K, and Ca), 50 (Kr, Sr, Y, Zr, and Mo, noting also primordial but not beta-stable Rb), 58 (Mo, Ru, Rh, Pd, and Cd), 74 (Sn, Te, I, Xe, and Ba), 78 (Te, Xe, Cs, Ba, and Ce), 88 (Nd, Sm, Eu, Gd, and Dy – the last not primordial), and 90 (Nd, Sm, Eu, Gd, and Dy).
1352:
is sometimes also possible, but in all known cases it is a minor branch compared to alpha decay or spontaneous fission. Alpha decay is energetically possible for all beta-stable nuclides with A ≥ 165 with the single exception of Hg, but in most cases the
365:
and Be – the former having an extremely short half-life) and 6 (C and C). Also, the only even neutron numbers with only one beta-decay stable nuclide are 0 (H) and 2 (He); at least two beta-decay stable nuclides exist for even neutron numbers in the range 4 ≤
1381:
such as the existence of two beta-stable nuclides with the same odd mass number. This is a consequence of the fact that a semi-empirical mass formula must consider shell correction and nuclear deformation, which become far more pronounced for heavy nuclides.
1904:), and it falls within the error margin given in AME2020. Hence, Rn is probably not beta-stable, though only the alpha decay mode is experimentally known for that nuclide, and the search for beta decay yielded a lower partial half-life limit of 8 years.
304:, are known to have at least one beta-stable isotope. It is known that technetium and promethium have no beta-stable isotopes; current measurement uncertainties are not enough to say whether mendelevium has them or not.
2208:
Belli, P.; Bernabei, R.; Cappella, C.; Caracciolo, V.; Cerulli, R.; Danevich, F.A.; Di Marco, A.; Incicchitti, A.; Poda, D.V.; Polischuk, O.G.; Tretyak, V.I. (2014). "Investigation of rare nuclear decays with
1942:
Proc. Int. Symposium on Why and How should we investigate
Nuclides Far Off the Stability Line", Lysekil, Sweden, August 1966, eds. W. Forsling, C.J. Herrlander and H. Ryde, Stockholm, Almqvist & Wiksell,
1913:
While the AME2020 atomic mass evaluation gives Md a lower mass than Fm, implying beta stability, the error margin between them is larger than the mass difference. Hence, either Fm or Md could be beta-stable.
1400:), then full ionization makes decay impossible. This happens for example for Be. Moreover, sometimes the energy difference is such that while β decay violates conservation of energy for a neutral atom,
1922:
There is no known beta-stable isobar for mass 261, although they are known for the surrounding masses 260 and 262. Various models suggest that one of the undiscovered Md and No should be beta-stable.
1420:
Beta decay generally causes nuclides to decay toward the isobar with the lowest mass (which is often, but not always, the one with highest binding energy) with the same mass number. Those with lower
1900:
While the AME2020 atomic mass evaluation gives Rn a lower mass than Fr, implying beta stability, it is predicted that single beta decay of Rn is energetically possible (albeit with very low
1412:, this means that Dy, Ir, Tl, At, and Am among beta-stable neutral nuclides cease to be beta-stable as bare nuclides, and are replaced by their daughters Ho, Pt, Pb, Rn, and Cm.
397:
are He, Be, Sm, Gd, and Dy. (Sm has a half-life long enough that it should barely survive as a primordial nuclide, but it has never been experimentally confirmed as such.)
374:= 4 (Li and Be), 6 (B and C), 8 (N and O), 66 (Cd and Sn, noting also primordial but not beta-stable In), 120 (Pt and Hg), and 128 (Po and Rn – both very unstable to
2192:
1959:
1348:
All beta-decay stable nuclides with A ≥ 209 are known to undergo alpha decay, though for some, spontaneous fission is the dominant decay mode.
256:
Among even mass number, five (124, 130, 136, 150, 154) have three beta-stable nuclides. None have more than three; all others have either one or two.
1877:
Zr is theoretically capable of beta decay to Nb, thus making it not a beta-stable nuclide. However, such a process has never been observed, having a
1855:
Ca is theoretically capable of beta decay to Sc, thus making it not a beta-stable nuclide. However, such a process has never been observed, having a
334:
in the special case of He. For mass 5 there are no bound isobars at all; there are bound isobars for mass 8, but the beta-stable one Be is unbound.
1404:(in which the decay electron remains bound to the daughter in an atomic orbital) is possible for the corresponding bare nucleus. Within the range
261:
2579:
2672:
1881:
greater than 2.4×10 years, longer than its double beta decay half-life, meaning that double beta decay would usually occur first.
330:, especially for the heavy elements. Possible decay modes are listed as α for alpha decay, SF for spontaneous fission, and n for
100:
2146:
Nuclear
Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment
276:
From 156 to 262, only eighteen have one, and the remaining 36 have two, though there may also exist some undiscovered ones.
1368:, though the exact location of the center of the valley of stability is model dependent. It is widely believed that an
2485:
Möller, P.; Sierk, A.J.; Ichikawa, T.; Sagawa, H. (2016). "Nuclear ground-state masses and deformations: FRDM(2012)".
2338:
Belli, P.; Bernabei, R.; Danevich, F. A.; et al. (2019). "Experimental searches for rare alpha and beta decays".
1868:×10 years, longer than its double beta decay half-life, meaning that double beta decay would usually occur first.
96:
1443:
However, there are a few odd-odd nuclides between two beta-stable even-even isobars, that predominantly decay to the
2068:
1388:
nuclei (with all electrons stripped) are somewhat different. Firstly, if a proton-rich nuclide can only decay by
1354:
2691:
2103:
Aunola, M.; Suhonen, J.; Siiskonen, T. (1999). "Shell-model study of the highly forbidden beta decay Ca → Sc".
1996:
313:
2635:
Liu, Shuo; Gao, Chao; Xu, Chang (2021). "Investigation of bound state β decay half-lives of bare atoms".
322:
is shown by arrows, i.e. arrows point towards the lightest-mass isobar. This is sometimes dominated by
318:
350 beta-decay stable nuclides are currently known. Theoretically predicted or experimentally observed
2594:
2550:
2535:
2500:
2448:
2424:"Future of superheavy element research: Which nuclei could be synthesized within the next few years?"
2357:
2232:
2153:
2114:
2077:
2037:
1968:
1377:
1369:
327:
267:
From 36 to 72, only eight (36, 40, 46, 50, 54, 58, 64, 70) have two, and the remaining 11 have one.
80:
91:
The line of beta stability can be defined mathematically by finding the nuclide with the greatest
2610:
2516:
2490:
2464:
2438:
2381:
2347:
2248:
2222:
1365:
394:
350:= 71 there is Te whose electron capture has not yet been observed, but neither are beta-stable.)
281:
1332:
2373:
1878:
1856:
1433:
1397:
319:
61:
2618:
2644:
2602:
2558:
2508:
2456:
2365:
2285:
2240:
2161:
2122:
2085:
2045:
1976:
1828:
1437:
1429:
1389:
331:
65:
2666:
2606:
2460:
2319:. 4th International Conference on the Chemistry and Physics of the Transactinide Elements
2598:
2554:
2504:
2452:
2361:
2236:
2157:
2118:
2081:
2049:
2041:
1981:
1972:
1954:
273:
From 124 to 154, only one (140) has one, five have three, and the remaining 10 have two.
79:, a term already in common use in 1965. This line lies along the bottom of the nuclear
2270:
2066:
Tretyak, V.I.; Zdesenko, Yu.G. (2002). "Tables of Double Beta Decay Data — An Update".
2022:
1813:
1762:
1712:
1703:
1680:
1598:
1425:
1392:(because the energy difference between the parent and daughter is less than 1.022
406:
103:. These nuclides are local maxima in terms of binding energy for a given mass number.
92:
64:
or theoretically higher simultaneous beta decay, as they have the lowest energy of all
57:
2685:
2614:
2520:
2385:
2252:
2105:
1955:"Nuclei Far Away from the Line of Beta Stability: Studies by On-Line Mass Separation"
1796:
1689:
1655:
1548:
1421:
1349:
417:
2468:
2563:
2311:
1901:
1782:
1671:
1639:
1557:
1516:
1500:
1393:
1364:
The general patterns of beta-stability are expected to continue into the region of
343:
17:
2369:
2244:
2648:
2126:
1647:
1589:
1492:
1373:
423:
375:
362:
323:
301:
111:
69:
30:
2289:
2166:
2141:
1832:
2512:
2423:
1721:
1580:
1508:
1401:
297:
293:
107:
45:
2377:
2313:
Decay modes and a limit of existence of nuclei in the superheavy mass region
1530:
270:
From 74 to 122, three (88, 90, 118) have one, and the remaining 22 have two.
2089:
1571:
1539:
1432:, while those with higher atomic number and lower neutron number undergo
357:≤ 102 have at least two beta-decay stable nuclides, with exactly two for
289:
41:
49:
1372:
exists along the beta stability line for isotopes of elements around
53:
2495:
2352:
2269:
Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021).
2021:
Audi, G.; Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S. (2017).
1812:
Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021).
2443:
2400:
2227:
1331:
29:
2536:"The limits of the nuclear chart set by fission and alpha decay"
2000:
1997:"Interactive Chart of Nuclides (Brookhaven National Laboratory)"
2422:
Zagrebaev, Valeriy; Karpov, Alexander; Greiner, Walter (2013).
253:
All odd mass numbers have only one beta decay stable nuclide.
60:. A subset of these nuclides are also stable with regards to
1891:
and only alpha decay has been observed experimentally for Gd.
446:
All known beta-decay stable isobars sorted by mass number
95:
for a given mass number, by a model such as the classical
2399:
Koura, H.; Katakura, J; Tachibana, T; Minato, F (2015).
393:≤ 209, the only beta-decay stable nuclides that are not
378:). Seven beta-decay stable nuclides exist for the magic
2667:
https://www-nds.iaea.org/relnsd/NdsEnsdf/masschain.html
399:
105:
2580:"Manipulation of Nuclear Lifetimes in Storage Rings"
382:= 82 (Xe, Ba, La, Ce, Pr, Nd, and Sm) and five for
2271:"The NUBASE2020 evaluation of nuclear properties"
2023:"The NUBASE2016 evaluation of nuclear properties"
1814:"The NUBASE2020 evaluation of nuclear properties"
1336:One chart of known and predicted nuclides up to
2213:crystal scintillator contaminated by radium".
27:Set of nuclides that cannot undergo beta decay
2264:
2262:
2183:
2181:
2179:
2177:
2061:
2059:
1960:Annual Review of Nuclear and Particle Science
8:
2480:
2478:
2305:
2303:
2301:
2299:
2203:
2201:
75:This set of nuclides is also known as the
2562:
2494:
2442:
2351:
2226:
2165:
1980:
1449:
1396:, the amount of decay energy needed for
444:
2191:was invoked but never defined (see the
1935:
1848:
308:List of known beta-decay stable isobars
1773:
1771:
1769:
1662:
1428:than the minimum-mass isobar undergo
7:
56:or a proton to a neutron within the
2487:Atomic Data and Nuclear Data Tables
2186:
1982:10.1146/annurev.ns.29.120179.000441
48:, that is, the transformation of a
25:
2673:Beta-decay stable nuclides up to
288:All elements up to and including
2187:Cite error: The named reference
2140:Finch, S.W.; Tornow, W. (2016).
342:= 21 the long-lived primordial
2607:10.1088/0031-8949/1995/t59/030
2461:10.1088/1742-6596/420/1/012001
2142:"Search for the β decay of Zr"
1416:Beta decay toward minimum mass
1294:
1274:
1262:
1256:
1242:
1222:
1204:
1184:
1170:
1152:
1138:
1132:
1112:
1100:
1092:
1074:
1060:
1040:
1022:
1008:
988:
970:
962:
930:
924:
904:
884:
872:
866:
840:
826:
820:
806:
774:
762:
748:
736:
728:
716:
702:
696:
684:
676:
664:
650:
638:
618:
606:
598:
592:
586:
572:
560:
546:
534:
520:
508:
494:
476:
435:
1:
2050:10.1088/1674-1137/41/3/030001
2564:10.1051/epjconf/201613103002
2403:. Japan Atomic Energy Agency
1308:
1300:
1288:
1282:
1268:
1248:
1236:
1230:
1216:
1210:
1196:
1190:
1178:
1164:
1158:
1144:
1126:
1118:
1106:
1086:
1080:
1066:
1054:
1048:
1034:
1028:
1014:
1002:
996:
982:
976:
956:
950:
944:
936:
918:
910:
898:
892:
878:
858:
852:
846:
832:
814:
800:
794:
788:
780:
768:
754:
742:
722:
710:
690:
670:
658:
644:
632:
624:
612:
580:
566:
554:
540:
528:
517:
514:
505:
502:
491:
488:
482:
479:
438:
427:
370:≤ 160, with exactly two for
353:All even proton numbers 2 ≤
2649:10.1103/PhysRevC.104.024304
2340:European Physical Journal A
2215:European Physical Journal A
97:semi-empirical mass formula
2708:
2370:10.1140/epja/i2019-12823-2
2245:10.1140/epja/i2014-14134-6
2167:10.1016/j.nima.2015.09.098
2069:At. Data Nucl. Data Tables
1478:
1470:
1462:
1454:
311:
2513:10.1016/j.adt.2015.10.002
2127:10.1209/epl/i1999-00301-2
38:Beta-decay stable isobars
2290:10.1088/1674-1137/abddae
1833:10.1088/1674-1137/abddae
2401:"Chart of the Nuclides"
1792:Majority decay (β+/EC)
1667:Minority decay (β+/EC)
1526:Minority decay (β+/EC)
1376:that are stabilized by
314:List of stable isotopes
2543:EPJ Web of Conferences
2090:10.1006/adnd.2001.0873
1953:Hansen, P. G. (1979).
1345:
77:line of beta stability
34:
2578:Bosch, Fritz (1995).
1808:Isotope masses from:
1335:
44:which cannot undergo
33:
1778:Minority decay (β−)
1699:Majority decay (β−)
1567:Majority decay (β−)
264:, all have only one.
2599:1995PhST...59..221B
2555:2016EPJWC.13103002M
2534:Möller, P. (2016).
2505:2016ADNDT.109....1M
2453:2013JPhCS.420a2001Z
2362:2019EPJA...55..140B
2237:2014EPJA...50..134B
2158:2016NIMPA.806...70F
2119:1999EL.....46..577A
2082:2002ADNDT..80...83T
2042:2017ChPhC..41c0001A
1973:1979ARNPS..29...69H
1402:bound-state β decay
1370:island of stability
1366:superheavy elements
447:
395:primordial nuclides
328:spontaneous fission
282:primordial nuclides
116:
81:valley of stability
18:Beta-stability line
2489:. 109–110: 1–204.
2431:Journal of Physics
2346:(8): 140–1–140–7.
2310:Koura, H. (2011).
1346:
1317:Fm (SF) → No (SF)
1312:Fm (SF) ← No (SF)
1304:Cf (SF) → Fm (SF)
445:
106:
35:
2637:Physical Review C
2278:Chinese Physics C
2030:Chinese Physics C
1879:partial half-life
1857:partial half-life
1821:Chinese Physics C
1805:
1804:
1398:positron emission
1330:
1329:
1298:Cf (SF) → Fm (α)
974:Sm → Gd ← Dy (α)
960:Nd → Sm ← Gd (α)
443:
442:
320:double beta-decay
251:
250:
62:double beta decay
16:(Redirected from
2699:
2653:
2652:
2632:
2626:
2625:
2623:
2617:. Archived from
2584:
2575:
2569:
2568:
2566:
2540:
2531:
2525:
2524:
2498:
2482:
2473:
2472:
2446:
2428:
2419:
2413:
2412:
2410:
2408:
2396:
2390:
2389:
2355:
2335:
2329:
2328:
2326:
2324:
2318:
2307:
2294:
2293:
2275:
2266:
2257:
2256:
2230:
2205:
2196:
2190:
2185:
2172:
2171:
2169:
2137:
2131:
2130:
2100:
2094:
2093:
2063:
2054:
2053:
2027:
2018:
2012:
2011:
2009:
2008:
1999:. Archived from
1993:
1987:
1986:
1984:
1950:
1944:
1940:
1923:
1920:
1914:
1911:
1905:
1898:
1892:
1888:
1882:
1875:
1869:
1867:
1866:
1859:greater than 1.1
1853:
1836:
1818:
1450:
1438:electron capture
1430:beta-minus decay
1411:
1390:electron capture
1384:The beta-stable
1292:Cf (α) ← Fm (α)
1278:Cm (α) → Cf (α)
1266:Pu (α) → Cm (α)
1260:Pu (α) ← Cm (α)
1208:Ra (α) → Th (α)
1200:Ra (α) ← Th (α)
1188:Rn (α) → Ra (α)
1182:Rn (α) ← Ra (α)
1174:Po (α) → Rn (α)
1168:Po (α) ← Rn (α)
1162:Po (α) ← Rn (α)
448:
400:
346:exists, and for
332:neutron emission
117:
101:C. F. Weizsäcker
21:
2707:
2706:
2702:
2701:
2700:
2698:
2697:
2696:
2692:Nuclear physics
2682:
2681:
2662:
2657:
2656:
2634:
2633:
2629:
2621:
2587:Physica Scripta
2582:
2577:
2576:
2572:
2538:
2533:
2532:
2528:
2484:
2483:
2476:
2426:
2421:
2420:
2416:
2406:
2404:
2398:
2397:
2393:
2337:
2336:
2332:
2322:
2320:
2316:
2309:
2308:
2297:
2273:
2268:
2267:
2260:
2212:
2207:
2206:
2199:
2188:
2175:
2139:
2138:
2134:
2102:
2101:
2097:
2065:
2064:
2057:
2025:
2020:
2019:
2015:
2006:
2004:
1995:
1994:
1990:
1952:
1951:
1947:
1941:
1937:
1932:
1927:
1926:
1921:
1917:
1912:
1908:
1899:
1895:
1889:
1885:
1876:
1872:
1865:
1862:
1861:
1860:
1854:
1850:
1845:
1816:
1811:
1434:beta-plus decay
1418:
1405:
1246:U (α) → Pu (α)
1240:U (α) ← Pu (α)
1226:Th (α) → U (α)
1220:Th (α) ← U (α)
316:
310:
89:
40:are the set of
28:
23:
22:
15:
12:
11:
5:
2705:
2703:
2695:
2694:
2684:
2683:
2680:
2679:
2669:
2661:
2660:External links
2658:
2655:
2654:
2627:
2624:on 2013-12-26.
2570:
2526:
2474:
2414:
2391:
2330:
2295:
2258:
2221:(9): 134–143.
2210:
2197:
2173:
2132:
2095:
2055:
2013:
1988:
1945:
1934:
1933:
1931:
1928:
1925:
1924:
1915:
1906:
1893:
1883:
1870:
1863:
1847:
1846:
1844:
1841:
1840:
1839:
1838:
1837:
1803:
1802:
1799:
1793:
1789:
1788:
1785:
1779:
1775:
1774:
1772:
1770:
1768:
1765:
1760:
1756:
1755:
1752:
1749:
1746:
1743:
1740:
1737:
1734:
1731:
1728:
1727:
1724:
1718:
1715:
1709:
1706:
1700:
1696:
1695:
1692:
1686:
1683:
1677:
1674:
1668:
1664:
1663:
1661:
1658:
1653:
1650:
1645:
1642:
1637:
1633:
1632:
1629:
1626:
1623:
1620:
1617:
1614:
1611:
1608:
1605:
1604:
1601:
1595:
1592:
1586:
1583:
1577:
1574:
1568:
1564:
1563:
1560:
1554:
1551:
1545:
1542:
1536:
1533:
1527:
1523:
1522:
1519:
1514:
1511:
1506:
1503:
1498:
1495:
1490:
1486:
1485:
1482:
1479:
1477:
1474:
1471:
1469:
1466:
1463:
1461:
1458:
1455:
1453:
1426:neutron number
1417:
1414:
1328:
1327:
1325:
1323:
1320:
1318:
1315:
1313:
1310:
1306:
1305:
1302:
1299:
1296:
1293:
1290:
1287:
1284:
1280:
1279:
1276:
1273:
1270:
1267:
1264:
1261:
1258:
1254:
1253:
1250:
1247:
1244:
1241:
1238:
1235:
1232:
1228:
1227:
1224:
1221:
1218:
1215:
1212:
1209:
1206:
1202:
1201:
1198:
1195:
1192:
1189:
1186:
1183:
1180:
1176:
1175:
1172:
1169:
1166:
1163:
1160:
1157:
1154:
1150:
1149:
1146:
1143:
1140:
1137:
1134:
1131:
1128:
1124:
1123:
1120:
1117:
1114:
1111:
1108:
1105:
1102:
1098:
1097:
1094:
1091:
1088:
1085:
1082:
1079:
1076:
1072:
1071:
1068:
1065:
1062:
1059:
1056:
1053:
1050:
1046:
1045:
1042:
1039:
1036:
1033:
1030:
1027:
1024:
1020:
1019:
1016:
1013:
1010:
1007:
1004:
1001:
998:
994:
993:
990:
987:
984:
981:
978:
975:
972:
968:
967:
964:
961:
958:
955:
952:
949:
946:
942:
941:
938:
935:
932:
929:
926:
923:
920:
916:
915:
912:
909:
906:
903:
900:
897:
894:
890:
889:
886:
883:
880:
877:
874:
871:
868:
864:
863:
860:
857:
854:
851:
848:
845:
842:
838:
837:
834:
831:
828:
825:
822:
819:
816:
812:
811:
808:
805:
802:
799:
796:
793:
790:
786:
785:
782:
779:
776:
773:
770:
767:
764:
760:
759:
756:
753:
750:
747:
744:
741:
738:
734:
733:
730:
727:
724:
721:
718:
715:
712:
708:
707:
704:
701:
698:
695:
692:
689:
686:
682:
681:
678:
675:
672:
669:
666:
663:
660:
656:
655:
652:
649:
646:
643:
640:
637:
634:
630:
629:
626:
623:
620:
617:
614:
611:
608:
604:
603:
600:
597:
594:
591:
588:
585:
582:
578:
577:
574:
571:
568:
565:
562:
559:
556:
552:
551:
548:
545:
542:
539:
536:
533:
530:
526:
525:
522:
519:
516:
513:
510:
507:
504:
500:
499:
496:
493:
490:
487:
484:
481:
478:
474:
473:
470:
467:
464:
461:
458:
455:
452:
441:
440:
437:
434:
430:
429:
426:
420:
413:
412:
409:
403:
309:
306:
278:
277:
274:
271:
268:
265:
249:
248:
245:
242:
239:
235:
234:
232:
229:
226:
222:
221:
219:
216:
213:
209:
208:
206:
203:
200:
196:
195:
192:
189:
186:
182:
181:
179:
176:
173:
169:
168:
166:
163:
160:
156:
155:
153:
150:
147:
143:
142:
140:
138:
135:
131:
130:
127:
124:
121:
110:stable / even
93:binding energy
88:
85:
68:with the same
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
2704:
2693:
2690:
2689:
2687:
2678:
2676:
2670:
2668:
2665:Decay-Chains
2664:
2663:
2659:
2650:
2646:
2643:(2): 024304.
2642:
2638:
2631:
2628:
2620:
2616:
2612:
2608:
2604:
2600:
2596:
2592:
2588:
2581:
2574:
2571:
2565:
2560:
2556:
2552:
2549:: 03002:1–8.
2548:
2544:
2537:
2530:
2527:
2522:
2518:
2514:
2510:
2506:
2502:
2497:
2492:
2488:
2481:
2479:
2475:
2470:
2466:
2462:
2458:
2454:
2450:
2445:
2440:
2437:(1): 012001.
2436:
2432:
2425:
2418:
2415:
2402:
2395:
2392:
2387:
2383:
2379:
2375:
2371:
2367:
2363:
2359:
2354:
2349:
2345:
2341:
2334:
2331:
2315:
2314:
2306:
2304:
2302:
2300:
2296:
2291:
2287:
2284:(3): 030001.
2283:
2279:
2272:
2265:
2263:
2259:
2254:
2250:
2246:
2242:
2238:
2234:
2229:
2224:
2220:
2216:
2204:
2202:
2198:
2194:
2184:
2182:
2180:
2178:
2174:
2168:
2163:
2159:
2155:
2151:
2147:
2143:
2136:
2133:
2128:
2124:
2120:
2116:
2112:
2108:
2107:
2099:
2096:
2091:
2087:
2083:
2079:
2076:(1): 83–116.
2075:
2071:
2070:
2062:
2060:
2056:
2051:
2047:
2043:
2039:
2036:(3): 030001.
2035:
2031:
2024:
2017:
2014:
2003:on 2020-07-25
2002:
1998:
1992:
1989:
1983:
1978:
1974:
1970:
1966:
1962:
1961:
1956:
1949:
1946:
1939:
1936:
1929:
1919:
1916:
1910:
1907:
1903:
1897:
1894:
1887:
1884:
1880:
1874:
1871:
1858:
1852:
1849:
1842:
1834:
1830:
1827:(3): 030001.
1826:
1822:
1815:
1810:
1809:
1807:
1806:
1800:
1798:
1794:
1791:
1790:
1786:
1784:
1780:
1777:
1776:
1766:
1764:
1761:
1758:
1757:
1753:
1750:
1747:
1744:
1741:
1738:
1735:
1732:
1730:
1729:
1725:
1723:
1719:
1716:
1714:
1710:
1707:
1705:
1701:
1698:
1697:
1693:
1691:
1687:
1684:
1682:
1678:
1675:
1673:
1669:
1666:
1665:
1659:
1657:
1654:
1651:
1649:
1646:
1643:
1641:
1638:
1635:
1634:
1630:
1627:
1624:
1621:
1618:
1615:
1612:
1609:
1607:
1606:
1602:
1600:
1596:
1593:
1591:
1587:
1584:
1582:
1578:
1575:
1573:
1569:
1566:
1565:
1561:
1559:
1555:
1552:
1550:
1546:
1544:39.9623831225
1543:
1541:
1537:
1534:
1532:
1528:
1525:
1524:
1520:
1518:
1515:
1512:
1510:
1507:
1504:
1502:
1499:
1496:
1494:
1491:
1488:
1487:
1483:
1480:
1475:
1472:
1467:
1464:
1459:
1456:
1452:
1451:
1448:
1446:
1441:
1439:
1435:
1431:
1427:
1423:
1422:atomic number
1415:
1413:
1409:
1403:
1399:
1395:
1391:
1387:
1386:fully ionized
1382:
1379:
1375:
1371:
1367:
1362:
1359:
1357:
1351:
1350:Cluster decay
1343:
1339:
1334:
1326:
1324:
1321:
1319:
1316:
1314:
1311:
1307:
1303:
1297:
1291:
1285:
1281:
1277:
1271:
1265:
1259:
1255:
1251:
1245:
1239:
1233:
1229:
1225:
1219:
1213:
1207:
1203:
1199:
1193:
1187:
1181:
1177:
1173:
1167:
1161:
1155:
1151:
1147:
1141:
1135:
1129:
1125:
1121:
1115:
1109:
1103:
1099:
1095:
1089:
1083:
1077:
1073:
1069:
1063:
1057:
1051:
1047:
1043:
1037:
1031:
1025:
1021:
1017:
1011:
1005:
999:
995:
991:
985:
979:
973:
969:
965:
959:
953:
947:
943:
939:
933:
927:
921:
917:
914:Xe → Ba ← Ce
913:
907:
901:
896:Te → Xe ← Ba
895:
891:
887:
881:
876:Sn → Te ← Xe
875:
869:
865:
861:
855:
849:
843:
839:
835:
829:
823:
817:
813:
809:
803:
797:
791:
787:
783:
777:
771:
765:
761:
757:
751:
745:
739:
735:
731:
725:
719:
713:
709:
705:
699:
693:
687:
683:
679:
673:
667:
661:
657:
653:
647:
641:
635:
631:
627:
621:
615:
609:
605:
601:
595:
589:
583:
579:
575:
569:
563:
557:
553:
549:
543:
537:
531:
527:
523:
511:
501:
497:
485:
475:
471:
468:
465:
462:
459:
456:
453:
450:
449:
432:
431:
425:
421:
419:
415:
414:
410:
408:
404:
402:
401:
398:
396:
392:
387:
385:
381:
377:
373:
369:
364:
360:
356:
351:
349:
345:
341:
335:
333:
329:
325:
321:
315:
307:
305:
303:
299:
295:
291:
286:
283:
275:
272:
269:
266:
263:
259:
258:
257:
254:
246:
243:
240:
237:
236:
233:
230:
227:
224:
223:
220:
217:
214:
211:
210:
207:
204:
201:
198:
197:
193:
190:
187:
184:
183:
180:
177:
174:
171:
170:
167:
164:
161:
158:
157:
154:
151:
148:
145:
144:
141:
139:
136:
133:
132:
128:
125:
122:
119:
118:
115:
114:
109:
104:
102:
99:developed by
98:
94:
86:
84:
82:
78:
73:
71:
67:
63:
59:
55:
51:
47:
43:
39:
32:
19:
2674:
2640:
2636:
2630:
2619:the original
2590:
2586:
2573:
2546:
2542:
2529:
2486:
2434:
2430:
2417:
2405:. Retrieved
2394:
2343:
2339:
2333:
2321:. Retrieved
2312:
2281:
2277:
2218:
2214:
2149:
2145:
2135:
2110:
2104:
2098:
2073:
2067:
2033:
2029:
2016:
2005:. Retrieved
2001:the original
1991:
1964:
1958:
1948:
1938:
1918:
1909:
1902:decay energy
1896:
1886:
1873:
1851:
1824:
1820:
1801:145.9131169
1726:242.0588358
1694:242.0587426
1576:35.967545106
1562:149.9172755
1444:
1442:
1419:
1407:
1385:
1383:
1363:
1355:
1347:
1341:
1337:
1090:Os ← Pt (α)
1038:Yb ← Hf (α)
966:Sm ← Gd (α)
954:Nd → Sm (α)
948:Nd → Sm (α)
940:Nd (α) ← Sm
390:
388:
383:
379:
371:
367:
358:
354:
352:
347:
339:
336:
317:
287:
279:
255:
252:
112:
90:
87:Introduction
76:
74:
37:
36:
2593:: 221–229.
2323:18 November
1787:145.913041
1708:151.9197910
1685:157.9241039
1676:151.9197324
1660:242.0595474
1652:157.9255315
1644:151.9217935
1603:149.918659
1585:39.96259098
1535:35.96708076
1521:149.919747
1505:39.96399848
1497:35.96830698
1424:and higher
1374:copernicium
1078:W → Os (α)
1070:W ← Os (α)
1058:Hf ← W (α)
376:alpha decay
324:alpha decay
302:mendelevium
70:mass number
2671:(Russian)
2496:1508.06294
2407:30 October
2353:1908.11458
2189:AME2020 II
2113:(5): 577.
2007:2009-06-19
1967:: 69–119.
1930:References
1767:145.914696
1717:157.924409
1594:107.904184
1553:107.903892
1513:107.905956
312:See also:
298:promethium
294:technetium
46:beta decay
2615:250860726
2521:118707897
2444:1207.5700
2386:201664098
2378:1434-601X
2253:118513731
2228:1407.5844
2193:help page
2152:: 70–74.
1720:82.7% to
1688:17.3% to
1679:0.01% to
1538:11.2% to
292:, except
2686:Category
2469:55434734
1711:0.6% to
1648:Tb-158m1
1640:Eu-152m1
1322:No (SF)
1136:Hg → Pb
1116:Pt → Hg
1110:Pt ← Hg
1096:Os → Pt
1044:Yb → Hf
1026:Er → Yb
1018:Er ← Yb
1006:Dy ← Er
1000:Dy ← Er
992:Gd → Dy
986:Gd ← Dy
980:Gd ← Dy
934:Ce → Nd
922:Ba ← Ce
908:Xe → Ba
902:Xe ← Ba
888:Te → Xe
882:Te ← Xe
870:Sn → Te
862:Sn ← Te
850:Cd → Sn
844:Cd → Sn
836:Cd ← Sn
830:Pd → Cd
824:Pd ← Cd
818:Pd ← Cd
810:Ru → Pd
804:Ru ← Pd
798:Mo → Ru
792:Mo → Ru
784:Mo ← Ru
778:Zr → Mo
772:Zr ← Mo
752:Kr → Sr
746:Kr ← Sr
740:Se → Kr
732:Se → Kr
726:Se ← Kr
720:Ge → Se
714:Ge ← Se
700:Zn → Ge
680:Ni ← Zn
662:Fe ← Ni
648:Cr ← Fe
636:Ti ← Cr
622:Ca → Ti
602:Ar ← Ca
290:nobelium
42:nuclides
2595:Bibcode
2551:Bibcode
2501:Bibcode
2449:Bibcode
2358:Bibcode
2233:Bibcode
2154:Bibcode
2115:Bibcode
2078:Bibcode
2038:Bibcode
1969:Bibcode
1795:63% to
1781:37% to
1759:Parent
1751:Nuclide
1745:Nuclide
1739:Nuclide
1733:Nuclide
1702:72% to
1670:28% to
1636:Parent
1628:Nuclide
1622:Nuclide
1616:Nuclide
1610:Nuclide
1597:89% to
1588:97% to
1579:89% to
1570:98% to
1556:11% to
1517:Eu-150m
1489:Parent
1481:Nuclide
1473:Nuclide
1465:Nuclide
1457:Nuclide
1340:= 149,
1309:Fm (α)
1301:Fm (α)
1295:Es (α)
1289:Cf (α)
1286:Cf (α)
1283:Cf (α)
1275:Bk (α)
1272:Cm (α)
1269:Cm (α)
1263:Am (α)
1257:Am (α)
1252:Pu (α)
1249:Pu (α)
1243:Np (α)
1223:Pa (α)
1217:Th (α)
1214:Th (α)
1211:Th (α)
1205:Ac (α)
1197:Ra (α)
1194:Ra (α)
1191:Ra (α)
1185:Fr (α)
1179:Rn (α)
1171:At (α)
1165:Po (α)
1159:Po (α)
1156:Po (α)
1153:Bi (α)
963:Eu (α)
951:Sm (α)
590:S ← Ar
498:Be (α)
489:He (n)
472:Even A
439:Even A
262:2 to 34
225:212-262
212:194-210
199:156-192
185:118-154
108:β decay
66:isobars
58:nucleus
50:neutron
2613:
2519:
2467:
2384:
2376:
2251:
1797:Nd-146
1783:Sm-146
1763:Pm-146
1722:Cm-242
1713:Dy-158
1704:Gd-152
1690:Pu-242
1681:Gd-158
1672:Sm-152
1656:Am-242
1599:Gd-150
1590:Cd-108
1558:Sm-150
1549:Pd-108
1547:3% to
1529:2% to
1509:Ag-108
1445:higher
1358:-value
1237:U (α)
1234:U (α)
1231:U (α)
466:Even A
460:Even A
454:Even A
433:Odd Z
428:Odd A
411:Odd N
300:, and
172:74-116
129:Three
54:proton
2622:(PDF)
2611:S2CID
2583:(PDF)
2539:(PDF)
2517:S2CID
2491:arXiv
2465:S2CID
2439:arXiv
2427:(PDF)
2382:S2CID
2348:arXiv
2317:(PDF)
2274:(PDF)
2249:S2CID
2223:arXiv
2026:(PDF)
1843:Notes
1817:(PDF)
1754:Mass
1631:Mass
1581:Ca-40
1572:Ar-36
1540:Ar-40
1493:Cl-36
1484:Mass
1410:≤ 270
1378:shell
1361:set.
469:Odd A
463:Odd A
457:Odd A
451:Odd A
436:Odd A
422:Even
416:Even
405:Even
361:= 4 (
260:From
238:Total
159:60-72
146:36-58
52:to a
2677:=118
2409:2018
2374:ISSN
2325:2018
1943:1967
1864:−0.6
1748:Mass
1742:Mass
1736:Mass
1625:Mass
1619:Mass
1613:Mass
1531:S-36
1501:K-40
1476:Mass
1468:Mass
1460:Mass
1406:2 ≤
389:For
280:All
134:2-34
2645:doi
2641:104
2603:doi
2591:T59
2559:doi
2547:131
2509:doi
2457:doi
2435:420
2366:doi
2286:doi
2241:doi
2209:BaF
2162:doi
2150:806
2123:doi
2106:EPL
2086:doi
2046:doi
1977:doi
1829:doi
1436:or
1394:MeV
1148:Pb
1145:Pb
1142:Pb
1139:Tl
1133:Tl
1130:Hg
1127:Hg
1122:Hg
1119:Hg
1113:Au
1107:Pt
1104:Pt
1101:Ir
1093:Ir
1087:Os
1084:Os
1081:Os
1075:Re
1061:Ta
1055:Hf
1052:Hf
1049:Hf
1041:Lu
1035:Yb
1032:Yb
1029:Yb
1023:Tm
1015:Er
1012:Er
1009:Ho
1003:Dy
997:Dy
989:Tb
983:Gd
977:Gd
971:Eu
957:Sm
945:Nd
937:Nd
931:Pr
928:Ce
925:La
919:Ba
911:Ba
905:Cs
899:Xe
893:Xe
879:Te
873:Sb
867:Sb
859:Sn
856:Sn
853:Sn
847:Sn
841:In
833:Cd
827:Ag
821:Ag
815:Pd
807:Rh
801:Ru
795:Ru
789:Mo
781:Mo
775:Nb
769:Zr
766:Zr
758:Sr
755:Sr
749:Rb
743:Kr
737:Br
729:Br
723:Se
717:As
711:Ge
706:Ge
703:Ga
697:Ga
694:Zn
691:Zn
688:Zn
685:Cu
677:Cu
674:Ni
671:Ni
668:Ni
665:Co
659:Fe
654:Fe
651:Mn
645:Cr
642:Cr
633:Ti
628:Ti
625:Ti
619:Sc
616:Ca
613:Ca
610:Ca
596:Ar
593:Cl
587:Cl
570:Si
567:Si
564:Si
561:Al
558:Mg
555:Mg
550:Mg
547:Na
544:Ne
541:Ne
538:Ne
503:Be
495:Li
492:Li
486:He
483:He
326:or
126:Two
123:One
120:βDS
2688::
2639:.
2609:.
2601:.
2589:.
2585:.
2557:.
2545:.
2541:.
2515:.
2507:.
2499:.
2477:^
2463:.
2455:.
2447:.
2433:.
2429:.
2380:.
2372:.
2364:.
2356:.
2344:55
2342:.
2298:^
2282:45
2280:.
2276:.
2261:^
2247:.
2239:.
2231:.
2219:50
2217:.
2200:^
2195:).
2176:^
2160:.
2148:.
2144:.
2121:.
2111:46
2109:.
2084:.
2074:80
2072:.
2058:^
2044:.
2034:41
2032:.
2028:.
1975:.
1965:29
1963:.
1957:.
1825:45
1823:.
1819:.
1440:.
1067:W
1064:W
885:I
763:Y
639:V
607:K
599:K
584:S
581:S
576:S
573:P
535:F
532:O
529:O
524:O
521:N
518:N
515:C
512:C
509:B
506:B
480:H
477:H
363:Be
296:,
247:5
244:76
241:50
231:19
205:14
194:5
191:12
178:20
137:17
83:.
72:.
2675:Z
2651:.
2647::
2605::
2597::
2567:.
2561::
2553::
2523:.
2511::
2503::
2493::
2471:.
2459::
2451::
2441::
2411:.
2388:.
2368::
2360::
2350::
2327:.
2292:.
2288::
2255:.
2243::
2235::
2225::
2211:2
2170:.
2164::
2156::
2129:.
2125::
2117::
2092:.
2088::
2080::
2052:.
2048::
2040::
2010:.
1985:.
1979::
1971::
1835:.
1831::
1408:A
1356:Q
1342:N
1338:Z
424:A
418:Z
407:N
391:A
384:N
380:N
372:N
368:N
359:Z
355:Z
348:N
344:K
340:N
228:7
218:3
215:6
202:5
188:2
175:2
165:2
162:5
152:6
149:6
113:A
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.