175:
20:
199:
136:
163:
242:. The individual RLi molecules are not observed. The four lithium atoms and the carbon from each alkyl group bonded to them occupy alternating vertices of the cube, with the additional atoms of the alkyl groups projecting off their respective corners.
117:
are pervasive in nature. The four iron atoms and four sulfur atoms form an alternating arrangement at the corners. The whole cluster is typically anchored by coordination of the iron atoms, usually with
174:
90:
Other compounds having different elements in the corners, various atoms or groups bonded to the corners are all part of this class of structures. Inorganic cubane-type clusters include
350:
Chakrabarty, Rajesh; Bora, Sanchay J.; Das, Birinchi K. (2007). "Synthesis, Structure, Spectral and
Electrochemical Properties, and Catalytic Use of Cobalt(III)−Oxo Cubane Clusters".
333:
Lee, S. C.; Lo, W.; Holm, R. H., "Developments in the
Biomimetic Chemistry of Cubane-Type and Higher Nuclearity Iron–Sulfur Clusters", Chem. Rev. 2014,
260:; the nitrogen atoms are the corners of the cube. Like the carbon-based cubane compounds, octaazacubane is predicted to be highly unstable due to
74:
forming the edges. Most cubanes have more complicated structures, usually with nonequivalent vertices. They may be simple covalent compounds or
126:. Some clusters arise via dimerization of square-shaped precursors. Many synthetic analogues are known including heterometallic derivatives.
487:
460:
123:
19:
386:
135:
509:
477:
114:
95:
23:
106:
91:
401:
198:
162:
35:
425:
483:
456:
417:
367:
316:
265:
236:
448:
409:
359:
334:
306:
298:
79:
57:
42:. In the idealized case, the eight vertices are symmetry equivalent and the species has O
405:
311:
286:
189:
75:
46:
443:
Stey, Thomas; Stalke, Dietmar (2009). "Lead structures in lithium organic chemistry".
503:
429:
245:
232:
71:
261:
220:
99:
452:
50:
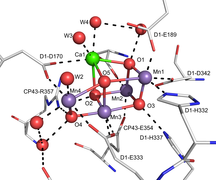
387:"Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å"
302:
287:"Identifying sequence determinants of reduction potentials of metalloproteins"
141:
110:
249:
421:
371:
320:
385:
Umena, Yasufumi; Kawakami, Keisuke; Shen, Jian-Ren; Kamiya, Nobuo (2011).
253:
224:
153:
119:
413:
149:
363:
338:
204:
53:
39:
479:
High Energy
Materials: Propellants, Explosives and Pyrotechnics
70:, cubane has carbon atoms at the corners of a cube and
223:compounds exist as clusters in solution, typically
264:at the corners, and it also does not enjoy the
122:residues. In this way, each Fe center achieves
8:
310:
49:. Such a structure is illustrated by the
66:
62:
18:
277:
128:
445:PATAI'S Chemistry of Functional Groups
285:Perrin, Jr., B.S.; Ichive, T. (2013).
105:Cubane clusters are common throughout
16:Molecular structure which forms a cube
7:
14:
124:tetrahedral coordination geometry
268:seen for its organic analogues.
197:
173:
161:
134:
34:is an arrangement of atoms in a
447:. John Wiley & Sons, Ltd.
291:Biological Inorganic Chemistry
26:, illustrative cubane cluster.
1:
476:Agrawal, Jai Prakash (2010).
453:10.1002/9780470682531.pat0298
130:Illustrative Cubane Clusters
526:
482:. Wiley-VCH. p. 498.
168:Ferredoxin (4Fe-4S-cubane)
303:10.1007/s00775-013-1004-6
96:tellurium tetrachloride
24:Tellurium tetrachloride
107:bioinorganic chemistry
92:selenium tetrachloride
27:
22:
115:iron–sulfur clusters
414:10.1038/nature09913
406:2011Natur.473...55U
352:Inorganic Chemistry
231:. Examples include
227:, with the formula
36:molecular structure
32:cubane-type cluster
510:Molecular geometry
248:is a hypothetical
78:or supramolecular
28:
489:978-3-527-62880-3
364:10.1021/ic7011759
358:(22): 9450–9462.
339:10.1021/cr4004067
266:kinetic stability
80:cluster compounds
517:
494:
493:
473:
467:
466:
440:
434:
433:
391:
382:
376:
375:
347:
341:
331:
325:
324:
314:
282:
201:
177:
165:
138:
69:
58:chemical formula
525:
524:
520:
519:
518:
516:
515:
514:
500:
499:
498:
497:
490:
475:
474:
470:
463:
442:
441:
437:
400:(7345): 55–60.
389:
384:
383:
379:
349:
348:
344:
332:
328:
284:
283:
279:
274:
259:
230:
215:
214:
210:
202:
193:
187:
183:
178:
169:
166:
157:
147:
139:
88:
68:
64:
60:
45:
17:
12:
11:
5:
523:
521:
513:
512:
502:
501:
496:
495:
488:
468:
461:
435:
377:
342:
326:
297:(6): 599–608.
276:
275:
273:
270:
257:
256:with formula N
228:
217:
216:
212:
208:
203:
196:
194:
190:Photosystem II
185:
181:
179:
172:
170:
167:
160:
158:
145:
140:
133:
131:
87:
84:
76:macromolecular
72:covalent bonds
43:
15:
13:
10:
9:
6:
4:
3:
2:
522:
511:
508:
507:
505:
491:
485:
481:
480:
472:
469:
464:
462:9780470682531
458:
454:
450:
446:
439:
436:
431:
427:
423:
419:
415:
411:
407:
403:
399:
395:
388:
381:
378:
373:
369:
365:
361:
357:
353:
346:
343:
340:
336:
330:
327:
322:
318:
313:
308:
304:
300:
296:
292:
288:
281:
278:
271:
269:
267:
263:
255:
251:
247:
246:Octaazacubane
243:
241:
240:-butyllithium
239:
234:
233:methyllithium
226:
222:
206:
200:
195:
191:
176:
171:
164:
159:
155:
151:
143:
137:
132:
129:
127:
125:
121:
116:
112:
108:
103:
101:
97:
93:
85:
83:
81:
77:
73:
59:
55:
52:
48:
41:
38:that forms a
37:
33:
25:
21:
478:
471:
444:
438:
397:
393:
380:
355:
351:
345:
329:
294:
290:
280:
262:angle strain
244:
237:
221:alkyllithium
218:
113:containing
104:
100:sodium silox
89:
31:
29:
111:Ferredoxins
51:hydrocarbon
272:References
188:cubane in
142:Das cubane
430:205224374
250:allotrope
225:tetramers
504:Category
422:21499260
372:17910439
321:23690205
254:nitrogen
219:Several
154:pyridine
120:cysteine
86:Examples
47:symmetry
402:Bibcode
312:3723707
152:; py =
150:acetate
148:(OAc =
56:. With
486:
459:
428:
420:
394:Nature
370:
319:
309:
205:Cubane
98:, and
54:cubane
426:S2CID
390:(PDF)
484:ISBN
457:ISBN
418:PMID
368:PMID
317:PMID
238:tert
235:and
180:CaMn
40:cube
449:doi
410:doi
398:473
360:doi
335:doi
307:PMC
299:doi
252:of
207:, C
506::
455:.
424:.
416:.
408:.
396:.
392:.
366:.
356:46
354:.
315:.
305:.
295:18
293:.
289:.
144:,
109:.
102:.
94:,
82:.
30:A
492:.
465:.
451::
432:.
412::
404::
374:.
362::
337::
323:.
301::
258:8
229:4
213:8
211:H
209:8
192:.
186:4
184:O
182:3
156:)
146:4
67:8
65:H
63:8
61:C
44:h
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.