149:
193:
133:
179:
213:
163:
418:
Brossi, A.; Schenker, F.; Leimgruber, W. (January 1964), "Synthesen in der
Isochinolinreihe Neue Synthesen der Kaktusalkaloide Anhalamin, Anhalidin, rac . Anhalonidin und rac . Pellotin",
372:
Chan, Camilla B.; Poulie, Christian B. M.; Wismann, Simon S.; Soelberg, Jens; Kristensen, Jesper L. (2021-08-27), "The
Alkaloids from Lophophora diffusa and Other "False Peyotes"",
199:
271:
unit is formed from a hydroxyl and a methoxy group. Further branching of the biosynthetic pathways occurs through the methylation of the
192:
344:). The other alkaloids predominantly exhibit much less pronounced pharmacological effects and may have anticonvulsant properties.
132:
148:
296:
178:
255:
scaffold can also be constructed from the intermediates of mescaline biosynthesis by a ring closure, resulting in
52:, which alone contains over 40 known compounds. The alkaloids can be categorized into two groups, derived from
162:
292:
252:
106:, the content and variety of cactus alkaloids are significantly lower, but some contain compounds such as
57:
31:
336:
139:
62:
82:
123:
115:
446:
280:
169:
76:
264:
394:
35:
423:
377:
111:
212:
53:
440:
308:
268:
260:
225:
87:
381:
331:
272:
244:
304:
300:
256:
229:
119:
95:
48:
427:
345:
327:
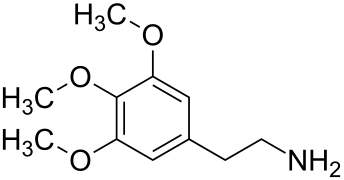
316:
312:
276:
248:
184:
154:
107:
71:
67:
23:
46:
Cactus alkaloids are found in the cactus family, particularly in the genus
291:
Various synthetic methods for cactus alkaloids are known. In the case of
240:
236:
232:
217:
27:
211:
220:
is the starting point for the biosynthesis of cactus alkaloids
348:
was briefly used as a sedative in the early 20th century.
334:
and is responsible for the hallucinogenic properties of
295:
compounds, the ring system is usually built up by a
86:, the primary alkaloid is pellotine, followed by
422:, vol. 47, no. 7, pp. 2089–2098,
376:, vol. 84, no. 8, pp. 2398–2407,
263:. Anhalonidine is the biosynthetic precursor of
8:
243:. By introducing a third hydroxy group and
60:, respectively. The primary alkaloid in
357:
128:
114:, mescaline, or pellotine. A number of
275:from dopamine. This pathway leads to
7:
367:
365:
363:
361:
228:of cactus alkaloids starts from the
14:
315:can be synthesized starting from
16:Chemical compounds found in cacti
191:
177:
161:
147:
131:
102:. In species outside the genus
42:Occurrence and Representatives
1:
297:Bischler-Napieralski reaction
247:of all three hydroxy groups,
382:10.1021/acs.jnatprod.1c00381
395:"Mescaline in Trichocereus"
374:Journal of Natural Products
235:and proceeds initially via
463:
66:(in terms of quantity) is
30:. Structurally, they are
428:10.1002/hlca.19640470752
118:are found in the genus
32:tetrahydroisoquinolines
420:Helvetica Chimica Acta
323:Pharmacological Effect
293:tetrahydroisoquinoline
253:tetrahydroisoquinoline
221:
58:tetrahydroisoquinoline
337:Lophophora williamsii
215:
140:Lophophora williamsii
63:Lophophora williamsii
399:The Mescaline Garden
279:and subsequently to
200:Echinopsis pachanoi
222:
170:Lophophora diffusa
116:psychoactive cacti
77:Lophophora diffusa
83:Lophophora fricii
74:. In the species
36:phenylethylamines
454:
431:
430:
415:
409:
408:
406:
405:
391:
385:
384:
369:
319:, among others.
195:
181:
165:
151:
135:
124:San Pedro cactus
112:N-methyltyramine
20:Cactus alkaloids
462:
461:
457:
456:
455:
453:
452:
451:
437:
436:
435:
434:
417:
416:
412:
403:
401:
393:
392:
388:
371:
370:
359:
354:
325:
289:
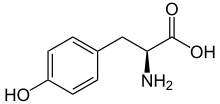
216:The amino acid
210:
203:
196:
187:
182:
173:
166:
157:
152:
143:
136:
44:
17:
12:
11:
5:
460:
458:
450:
449:
439:
438:
433:
432:
410:
386:
356:
355:
353:
350:
324:
321:
288:
285:
209:
206:
205:
204:
197:
190:
188:
183:
176:
174:
167:
160:
158:
153:
146:
144:
137:
130:
122:, such as the
70:, followed by
54:phenethylamine
43:
40:
26:that occur in
15:
13:
10:
9:
6:
4:
3:
2:
459:
448:
445:
444:
442:
429:
425:
421:
414:
411:
400:
396:
390:
387:
383:
379:
375:
368:
366:
364:
362:
358:
351:
349:
347:
343:
339:
338:
333:
329:
322:
320:
318:
314:
310:
306:
302:
298:
294:
286:
284:
282:
278:
274:
270:
267:, in which a
266:
262:
258:
254:
251:is formed. A
250:
246:
242:
238:
234:
231:
227:
219:
214:
207:
202:
201:
194:
189:
186:
180:
175:
172:
171:
164:
159:
156:
150:
145:
142:
141:
134:
129:
127:
125:
121:
117:
113:
109:
105:
101:
97:
93:
89:
85:
84:
79:
78:
73:
69:
65:
64:
59:
55:
51:
50:
41:
39:
37:
33:
29:
25:
21:
419:
413:
402:. Retrieved
398:
389:
373:
341:
335:
326:
309:anhalonidine
290:
269:benzodioxole
261:anhalonidine
226:biosynthesis
223:
208:Biosynthesis
198:
168:
138:
103:
99:
91:
88:anhalonidine
81:
75:
61:
47:
45:
19:
18:
332:psychedelic
281:lophophorin
273:amino group
245:methylation
404:2024-08-30
352:References
305:anhalidine
301:Anhalamine
265:anhalonine
257:anhalamine
230:amino acid
120:Echinopsis
104:Lophophora
100:L. diffusa
96:anhalamine
49:Lophophora
447:Alkaloids
346:Pellotine
328:Mescaline
317:mescaline
313:pellotine
287:Synthesis
277:pellotine
249:mescaline
185:Pellotine
155:Mescaline
108:hordenine
92:L. fricii
72:pellotine
68:mescaline
24:alkaloids
441:Category
241:dopamine
237:tyramine
233:tyrosine
218:tyrosine
342:peyote
311:, and
28:cactus
330:is a
259:and
239:and
224:The
94:and
80:and
56:and
34:and
22:are
424:doi
378:doi
98:in
90:in
443::
397:.
360:^
307:,
303:,
299:.
283:.
110:,
38:.
426::
407:.
380::
340:(
126:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.