272:
658:-canavanine by argininosuccinic acid synthetase. By these sequential reactions, the canaline-urea cycle (analogous to the ornithine-urea cycle) is formed. Every time a canavanine molecule runs through the canaline-urea cycle, the two terminal nitrogen atoms are released as urea. Urea is an important by-product of this reaction sequence because it makes ammonia (urease-mediated) that is available to support intermediary nitrogen metabolism.
437:
593:
27:
670:, a non-protein amino acid of great importance in the formation of a host of essential amino acids. In this way, the third nitrogen atom of canavanine enters into the reactions of nitrogen metabolism of the plant. As homoserine, its carbon skeleton also finds an important use.
589:
larvae fed a diet containing 2.5 mM canaline showed massive developmental aberrations, and most larvae so treated died at the pupal stage. It also exhibits potent neurotoxic effects in the moth.
311:
447:
718:
689:
286:
647:
462:
229:
93:
505:
250:
477:
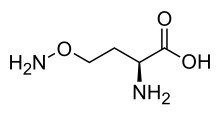
713:
708:
631:
616:
597:
419:
484:
537:
491:
133:
39:
554:
473:
267:
608:
59:
685:
682:
Plant non-protein amino and imino acids: biological, biochemical, and toxicological properties
586:
334:
153:
238:
271:
69:
113:
413:
498:
702:
574:
401:
391:
218:
582:
436:
667:
545:
533:
368:
144:
578:
577:
functionality in the side chain. This amino acid is structurally related to
592:
549:
164:
173:
569:-Canaline is the only naturally occurring amino acid known that has an
541:
381:
205:
26:
604:
124:
552:. The most common-used source for this amino acid is the jack bean,
412:
Except where otherwise noted, data are given for materials in their
295:
InChI=1/C4H10N2O3/c5-3(4(7)8)1-2-9-6/h3H,1-2,5-6H2,(H,7,8)/t3-/m0/s1
193:
591:
104:
92:
82:
603:
Its toxicity stems primarily from the fact that it readily forms
184:
430:
255:
454:
458:
646:-canavaninosuccinic acid in a reaction mediated by
217:
638:-ureidohomoserine (the corresponding analog of
68:
607:with keto acids and aldehydes, especially the
581:(it is the 5-oxa derivative) and is a potent
548:, from which it is produced by the action of
8:
654:-Canavaninosuccinic acid is cleaved to form
536:2-amino-4-(aminooxy)butyric acid)) is a non-
463:introducing citations to additional sources
406:378.1 °C (712.6 °F; 651.2 K)
270:
152:
15:
237:
662:-Canaline can be reductively cleaved to
642:-citrulline). In turn, the latter forms
453:Relevant discussion may be found on the
316:
291:
266:
172:
132:
112:
7:
619:at concentrations as low as 10 nM.
298:Key: FQPGMQABJNQLLF-VKHMYHEABX
208:
192:
47:)-2-Amino-4-aminooxy-butanoic acid
14:
648:argininosuccinic acid synthetase
615:-dependent enzymes. It inhibits
446:relies largely or entirely on a
435:
352:
346:
25:
416:(at 25 °C , 100 kPa).
634:resulting in the synthesis of
596:L-Canaline is a substrate for
358:
340:
1:
719:Non-proteinogenic amino acids
680:Rosenthal, Gerald A. (1982).
630:-Canaline is a substrate for
540:. The compound is found in
735:
684:. Boston: Academic Press.
632:ornithine aminotransferase
617:ornithine aminotransferase
611:cofactor of many vitamin B
598:Ornithine aminotransferase
410:
327:
307:
282:
52:
38:
33:
24:
538:proteinogenic amino acid
600:
595:
555:Canavalia ensiformis
459:improve this article
609:pyridoxal phosphate
376: g·mol
21:
601:
420:Infobox references
16:
714:Toxic amino acids
709:Alpha-Amino acids
524:
523:
509:
428:Chemical compound
426:
425:
251:CompTox Dashboard
94:Interactive image
726:
695:
665:
661:
657:
653:
645:
641:
637:
629:
587:Tobacco hornworm
568:
529:
519:
516:
510:
508:
467:
439:
431:
375:
360:
354:
348:
342:
335:Chemical formula
275:
274:
259:
257:
241:
221:
210:
196:
176:
156:
136:
116:
96:
72:
29:
22:
19:
734:
733:
729:
728:
727:
725:
724:
723:
699:
698:
692:
679:
676:
663:
659:
655:
651:
643:
639:
635:
627:
625:
623:Plant nutrition
614:
566:
564:
527:
520:
514:
511:
468:
466:
452:
440:
429:
422:
417:
373:
363:
357:
351:
345:
337:
323:
320:
315:
314:
303:
300:
299:
296:
290:
289:
278:
260:
253:
244:
224:
211:
199:
179:
159:
139:
119:
99:
86:
75:
62:
48:
17:
12:
11:
5:
732:
730:
722:
721:
716:
711:
701:
700:
697:
696:
690:
675:
672:
624:
621:
612:
563:
560:
522:
521:
457:. Please help
443:
441:
434:
427:
424:
423:
418:
414:standard state
411:
408:
407:
404:
398:
397:
394:
388:
387:
384:
378:
377:
371:
365:
364:
361:
355:
349:
343:
338:
333:
330:
329:
325:
324:
322:
321:
318:
310:
309:
308:
305:
304:
302:
301:
297:
294:
293:
285:
284:
283:
280:
279:
277:
276:
268:DTXSID60197925
263:
261:
249:
246:
245:
243:
242:
234:
232:
226:
225:
223:
222:
214:
212:
204:
201:
200:
198:
197:
189:
187:
181:
180:
178:
177:
169:
167:
161:
160:
158:
157:
149:
147:
141:
140:
138:
137:
129:
127:
121:
120:
118:
117:
109:
107:
101:
100:
98:
97:
89:
87:
80:
77:
76:
74:
73:
65:
63:
58:
55:
54:
50:
49:
42:
36:
35:
31:
30:
13:
10:
9:
6:
4:
3:
2:
731:
720:
717:
715:
712:
710:
707:
706:
704:
693:
691:0-12-597780-8
687:
683:
678:
677:
673:
671:
669:
649:
633:
622:
620:
618:
610:
606:
599:
594:
590:
588:
584:
580:
576:
575:hydroxylamine
572:
561:
559:
557:
556:
551:
547:
544:that contain
543:
539:
535:
531:
518:
515:February 2015
507:
504:
500:
497:
493:
490:
486:
483:
479:
476: –
475:
471:
470:Find sources:
464:
460:
456:
450:
449:
448:single source
444:This article
442:
438:
433:
432:
421:
415:
409:
405:
403:
402:Boiling point
400:
399:
395:
393:
392:Melting point
390:
389:
385:
383:
380:
379:
372:
370:
367:
366:
339:
336:
332:
331:
326:
319:O=C(O)(N)CCON
317:
313:
306:
292:
288:
281:
273:
269:
265:
264:
262:
252:
248:
247:
240:
236:
235:
233:
231:
228:
227:
220:
216:
215:
213:
207:
203:
202:
195:
191:
190:
188:
186:
183:
182:
175:
171:
170:
168:
166:
163:
162:
155:
151:
150:
148:
146:
143:
142:
135:
134:ChEMBL1231652
131:
130:
128:
126:
123:
122:
115:
111:
110:
108:
106:
103:
102:
95:
91:
90:
88:
84:
79:
78:
71:
67:
66:
64:
61:
57:
56:
51:
46:
41:
37:
32:
28:
23:
681:
626:
602:
570:
565:
553:
526:
525:
512:
502:
495:
488:
481:
469:
445:
53:Identifiers
44:
583:insecticide
386:1.298 g/mL
328:Properties
114:CHEBI:41401
703:Categories
674:References
668:homoserine
546:canavanine
534:IUPAC name
485:newspapers
474:"Canaline"
369:Molar mass
239:T7H2XP1ZNS
145:ChemSpider
81:3D model (
60:CAS Number
40:IUPAC name
20:-Canaline
579:ornithine
530:-Canaline
455:talk page
562:Toxicity
550:arginase
165:DrugBank
70:496-93-5
573:-alkyl
542:legumes
499:scholar
396:213 °C
382:Density
374:134.135
206:PubChem
174:DB02821
688:
605:oximes
501:
494:
487:
480:
472:
312:SMILES
219:441443
194:C08270
154:390176
125:ChEMBL
34:Names
506:JSTOR
492:books
287:InChI
105:ChEBI
83:JSmol
686:ISBN
478:news
230:UNII
185:KEGG
650:.
461:by
256:EPA
209:CID
705::
585:.
558:.
350:10
43:(2
694:.
666:-
664:l
660:l
656:l
652:l
644:l
640:l
636:l
628:l
613:6
571:O
567:l
532:(
528:l
517:)
513:(
503:·
496:·
489:·
482:·
465:.
451:.
362:3
359:O
356:2
353:N
347:H
344:4
341:C
258:)
254:(
85:)
45:S
18:l
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.