335:
348:
363:
intermediates to perform the cholesterol hydroxylation). A loop region, known as the B'-C loop, has a series of 5 residues (residues 116-120) unique to cholesterol-24 hydroxylase that contribute to the positioning of the cholesterol molecule within the active site. A single cholesterol molecule takes up the entirety of the active site, with the aliphatic tail of the cholesterol held in place by interactions with the following hydrophobic residues: Phe-121, Val-126, Ile-301, Ala-302, Ala-367, Thr-475. The active site is accessed via a single entrance created by two helices (B' and F) and the β1-sheet.
527:
379:
31:
2380:
495:(LXR), it is possible that cholesterol-24 hydroxylase may play an indirect regulatory role in the metabolism of lipids in the liver. 24S-hydroxycholesterol also regulates the rate of cholesterol synthesis in the brain, with high levels of 24S-hydroxycholesterol shown to reduce mRNA levels of the following cholesterol synthesis enzymes:
359:-rich region of 5 repeated proline residues, a structural motif absent in all other related cytochrome p450 enzymes. While the exact function of these proline residues remain highly speculative, it has been shown that the deletion of this region results in a two-fold decrease in the enzyme’s catalytic efficiency.
557:
in the encoding gene for cholesterol-24 hydroxylase have an established positive correlation with AD onset, other publications did not find such an association. Increased expression of cholesterol-24 hydroxylase has also been observed in patients of traumatic brain injury, leading to decreased levels
480:
Cholesterol-24 hydroxylase contributes to brain cholesterol homeostasis by hydroxylating cholesterol at carbon-24 to 24S-hydroxycholesterol to allow for elimination of cholesterol from the brain to the liver. Only around 6–7 mg of cholesterol, however, are hydroxylated by this enzyme on a daily
362:
Binding of cholesterol results in an enzymatic conformational change and a subsequent induced fit of the active site around the cholesterol molecule, anchoring the hydroxylation site (C-24, C-25) near the catalytic center of the enzyme (5.7Å from the iron core of the heme molecule to allow oxyferryl
490:
Cholesterol-24 hydroxylase has a variety of possible substrates, including: elongated steroid chains, cholesterol derivatives, and a variety of drug candidates. As such, it is also likely that it plays a role in lipid metabolism in the brain beyond cholesterol breakdown. Because
546:, where there is a consequent build-up of the product, 24S-hydroxycholesterol. Recent studies have shown that the increased levels of 24S-hydroxycholesterol in astrocytes may lead to a loss of glial glutamate transporters (EAAT2) and the consequent loss of the
932:
343:
Cholesterol-24 hydroxylase is similar in structure to many other cytochrome P450s, possessing, for example, the conserved stretch of 23 hydrophobic residues in the N-terminus that make up a transmembrane-anchoring domain (residues 3-27).
538:
Neuron degradation in AD has often been attributed to the imbalance in cholesterol homeostasis, and many scientists hypothesize that the lowered expression of cholesterol-24 hydroxylase may be the main cause of this imbalance.
1637:
382:
Cholesterol-24 hydroxylase mechanism. The heme molecule forms an oxyferryl intermediate that abstracts a hydrogen from cholesterol. The subsequent alkyl intermediate then reacts with the activated oxygen to form the final
782:
Bretillon L, Diczfalusy U, Bjorkhem I, Maire MA, Martine L, Joffre C, Acar N, Bron A, Creuzot-Garcher C (April 2007). "Cholesterol-24S-hydroxylase (CYP46A1) is specifically expressed in neurons of the neural retina".
338:
Cholesterol-24 hydroxylase active site and heme molecule with bound cholesterol 3-sulfate. Hydrophobic residues interacting with the aliphatic tail of the cholesterol are labelled in white. Created in pyMOL from PDB
1125:
Pikuleva IA, Norcross R, Andersson U, Shou M, Nakayama K, Bjorkhem I, Pikuleva IA (2003). "Broad substrate specificity of human cytochrome P450 46A1 which initiates cholesterol degradation in the brain".
1314:
Winblad B, Russell DW, Bjorkhem I (2001). "On the turnover of brain cholesterol in patients with
Alzheimer's disease. Abnormal induction of the cholesterol-catabolic enzyme CYP46 in glial cells".
468:
Like all other cytochrome P450s, cholesterol-24 hydroxylase utilizes an oxyferryl intermediate to hydroxylate cholesterol. The oxyferryl radical takes the hydrogen from carbon-24 to create an
1451:"Increased expression of cholesterol 24S-hydroxylase results in disruption of glial glutamate transporter EAAT2 association with lipid rafts: a potential role in Alzheimer's disease"
887:
Mast N, Andersson U, Nakayama K, Bjorkhem I, Pikuleva IA (August 2004). "Expression of human cytochrome P450 46A1 in
Escherichia coli: effects of N- and C-terminal modifications".
412:
1543:
Ma SL, Tang NLS, Lam LCW, Chiu HFK (March 2006). "Polymorphisms of the cholesterol 24-hydroxylase (CYP46A1) gene and the risk of
Alzheimer's disease in a Chinese population".
290:
to exit the brain and pass into the bloodstream, where it can then travel to the liver to be further degraded. This enzyme has also been found at low quantities in the
178:
990:
Munro AW, Girvan HM, McLean KJ (June 2007). "Variations on a (t)heme--novel mechanisms, redox partners and catalytic functions in the cytochrome P450 superfamily".
197:
454:
431:
320:
334:
1357:
Desai P, DeKosky ST, Kamboh MI (August 2002). "Genetic variation in the cholesterol 24-hydroxylase (CYP46) gene and the risk of
Alzheimer's disease".
553:
Still, the link between expression levels of cholesterol-24 hydroxylase and
Alzheimer's Disease remain disputable. While some studies have shown that
1857:
500:
1750:
1925:
1664:
2405:
1402:
Brown J, Theisler C, Silberman S, Magnuson D, Gottardi-Littell N, Lee JM, Yager D, Crowley J, Sambamurti K, Rahman MM, Reiss AB, Eckman CB,
487:
experiments have shown that it is also capable of further metabolizing 24S-hydroxycholesterol into 24,25- and 24,27-dihydroxycholesterols.
472:
radical intermediate. The cholesterol alkyl radical then combines with the activated oxygen on the heme to create 24S-hydroxycholesterol.
347:
589:. The ability for inhibition by various xenobiotics makes this enzyme a prime candidate for drug therapy for AD or other brain injuries.
542:
On the other hand, while there is decreased expression in the neurons, there is a contrasting increase of expression in the AD patients'
1825:
1592:
1412:
1170:
561:
Cholesterol-24 hydroxylase is easily inhibited by many drugs due to its broad substrate specificity. It has been shown to metabolize
2099:
1798:
928:"Crystal structures of substrate-bound and substrate-free cytochrome P450 46A1, the principal cholesterol hydroxylase in the brain"
534:
the enzyme can metabolize. The reactions in blue are commonly observed in the brain as part of maintaining cholesterol homeostasis.
519:(AD) in humans. Studies have shown that in AD patients, there is significant decreased expression of cholesterol-24 hydroxylase in
190:
2410:
523:. As a result, there is a marked increase of cholesterol in the brain tissue, consistent with the trend observed in AD patients.
1837:
1783:
667:
157:
1598:
133:
2255:
712:
1642:
1545:
615:
2370:
1223:
2066:
1915:
1623:
1086:"Knockout of the cholesterol 24-hydroxylase gene in mice reveals a brain-specific mechanism of cholesterol turnover"
1657:
275:(CYP) superfamily of enzymes. Like many other CYP enzymes that act on cholesterol, cholesterol-24 hydroxylase is a
2240:
151:
2356:
2343:
2330:
2317:
2304:
2291:
2278:
2061:
2022:
2012:
1998:
1994:
1984:
1972:
1952:
1935:
1903:
1867:
1767:
1694:
1632:
1455:
2250:
1219:"Enhanced production of 24S-hydroxycholesterol is not sufficient to drive liver X receptor target genes in vivo"
2415:
2204:
2147:
1685:
526:
423:
218:
138:
56:
1907:
1500:
Kolsch H, Lutjohann D, Ludwig M, Schulte A, Ptok U, Jessen F, von
Bergmann K, Rao ML, Maier W, Heun R (2002).
351:
Cholester-24 hydroxylase (CYP46A1) with placement of active site shown in red. created in pyMOL from PDB 2Q9F.
287:
2152:
1698:
992:
889:
202:
2046:
1962:
1711:
558:
of cholesterol in the plasma membrane. This is hypothesized to be the brain’s typical response to injury.
554:
516:
504:
450:
378:
257:
253:
126:
397:
2173:
2092:
1842:
1815:
1650:
1166:"Structural basis of drug binding to CYP46A1, an enzyme that controls cholesterol turnover in the brain"
367:
328:
2245:
1957:
1920:
1681:
1506:
1359:
1217:
Shafaati M, Olin M, Bavner A, Pettersson H, Rozell B, Meaney S, Parini P, Bjorkhem I (October 2011).
941:
841:
785:
154:
78:
2400:
2209:
1943:
1604:
446:
2142:
1706:
1570:
1384:
1339:
810:
692:
1852:
1265:"24S-hydroxycholesterol effects on lipid metabolism genes are modeled in traumatic brain injury"
830:"cDNA cloning of cholesterol 24-hydroxylase, a mediator of cholesterol homeostasis in the brain"
1740:
1562:
1525:
1482:
1431:
1376:
1331:
1296:
1242:
1199:
1143:
1107:
1063:
1009:
969:
905:
869:
802:
764:
729:
684:
642:
496:
283:
145:
1502:"Polymorphism in the cholesterol 24S-hydroxylase gene is associated with Alzheimer's disease"
760:
628:
114:
2188:
2183:
2157:
2085:
1554:
1515:
1472:
1464:
1421:
1403:
1368:
1323:
1286:
1278:
1232:
1189:
1179:
1135:
1097:
1053:
1045:
1036:
1001:
959:
949:
897:
859:
849:
794:
756:
721:
676:
632:
624:
570:
492:
303:
90:
2235:
2219:
2132:
1830:
1627:
316:
272:
261:
61:
30:
945:
845:
2384:
2273:
2214:
2056:
2036:
2031:
1673:
1477:
1450:
1291:
1269:
1264:
1194:
1165:
1058:
1031:
964:
927:
637:
610:
491:
24S-hydroxycholesterol (main product of this enzyme) is a major activator of oxysterol
173:
1372:
1327:
2394:
2178:
2137:
1468:
1237:
1218:
864:
829:
725:
530:
Substrates metabolized by cholesterol-24 hydroxylase. The red box contains a list of
276:
1574:
1388:
1343:
814:
2127:
1875:
1871:
1282:
747:
Russell DW (2003). "The enzymes, regulation, and genetics of bile acid synthesis".
586:
566:
435:
315:
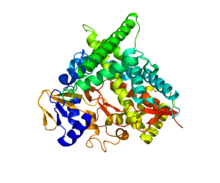
The enzymatic structure of the human cholesterol-24 hydroxylase was determined via
696:
515:
Variable expression of cholesterol-24 hydroxylase has been linked to the onset of
2351:
2286:
2122:
427:
249:
2379:
933:
Proceedings of the
National Academy of Sciences of the United States of America
2007:
1558:
901:
798:
582:
578:
550:
uptake function in the brain, another common symptom observed in AD patients.
531:
481:
basis, suggesting the existence of alternative functions – presently unknown.
926:
Mast N, White MN, Bjorkhem I, Johnson EF, Stout CD, Pikuleva IA (July 2008).
710:
Pikuleva IA (December 2006). "Cytochrome P450s and cholesterol homeostasis".
2325:
2299:
1976:
1939:
1883:
1677:
1408:"Differential expression of cholesterol hydroxylases in Alzheimer's disease"
1184:
954:
854:
611:"Cholesterol 24-hydroxylase: an enzyme of cholesterol turnover in the brain"
562:
547:
543:
245:
1566:
1529:
1486:
1435:
1426:
1407:
1380:
1335:
1300:
1246:
1203:
1147:
1111:
1102:
1085:
1067:
1013:
973:
909:
873:
806:
768:
733:
688:
680:
646:
1620:
1615:
1610:
1520:
1501:
256:. It is responsible for the majority of cholesterol turnover in the human
221:
102:
1755:
574:
483:
2051:
665:
Pikuleva IA (April 2006). "Cholesterol-metabolizing cytochromes P450".
520:
356:
121:
1139:
1049:
41:
2338:
2108:
1893:
1888:
1847:
1005:
439:
291:
241:
185:
97:
85:
73:
1084:
Lund EG, Xie C, Kotti T, Turley SD, Dietschy JM, Russell DW (2003).
2312:
2040:
1775:
1733:
1726:
1721:
1716:
525:
469:
458:
377:
346:
333:
1820:
1808:
1803:
1793:
1788:
1771:
1745:
355:
Even so, the cholesterol-24 hydroxylase C-terminus has a unique
324:
307:
expression and purification system was later developed in 2003.
109:
2081:
1646:
609:
Russell DW, Halford RW, Ramirez DMO, Shah R, Kotti T (2009).
387:
Cholesterol-24 hydroxylase catalyzes the following reaction:
1449:
Tian G, Kong Q, Lai L, Ray-Chaudhury A, Lin CLG (May 2010).
2077:
294:, where it performs the same function to a lesser degree.
266:
cholesterol,NADPH:oxygen oxidoreductase (24-hydroxylating)
1164:
Mast N, Charvet C, Pikuleva IA, Stout CD (October 2010).
401:
1032:"Hydrocarbon hydroxylation by cytochrome P450 enzymes"
323:, and was shown to be a 57kDa (500 residue) monomeric
2368:
400:
35:
Structure of cholesterol 24-hydroxylase (CYP46A1) in
2264:
2228:
2197:
2166:
2115:
2021:
1993:
1971:
1934:
1902:
1866:
1766:
1693:
196:
184:
172:
167:
144:
132:
120:
108:
96:
84:
72:
67:
55:
50:
23:
406:
301:) was first accomplished in 1999 and an extensive
279:that hydroxylates the side-chain of cholesterol.
1263:Cartagena CM, Burns MP, Rebeck GW (March 2010).
1159:
1157:
286:than cholesterol, it can more easily pass the
2093:
1658:
604:
602:
8:
1611:UniProt entry on Cholesterol-24 hydroxylase
1079:
1077:
985:
983:
828:Lund EG, Guileyardo JM, Russell DW (1999).
2100:
2086:
2078:
1665:
1651:
1643:
660:
658:
656:
321:Stanford Synchrotron Radiation Lightsource
164:
1519:
1476:
1425:
1290:
1236:
1193:
1183:
1101:
1057:
963:
953:
863:
853:
636:
399:
1030:Ortiz de Montellano PR (February 2010).
1025:
1023:
761:10.1146/annurev.biochem.72.121801.161712
629:10.1146/annurev.biochem.78.072407.103859
2375:
921:
919:
598:
282:Because 24S-hydroxycholesterol is more
1258:
1256:
414:(24S)-24-hydroxycholesterol + NADP + H
297:Genetic cloning of the encoding gene (
20:
7:
1858:24-hydroxycholesterol 7α-hydroxylase
1633:Review on Cholesterol-24 Hydroxylase
1546:International Psychogeriatrics / IPA
1413:The Journal of Biological Chemistry
1171:The Journal of Biological Chemistry
407:{\displaystyle \rightleftharpoons }
39:. Based on PyMOL rendering of PDB
14:
327:-containing protein bound to the
2378:
1469:10.1111/j.1471-4159.2010.06661.x
1238:10.1111/j.1365-2796.2011.02389.x
726:10.1016/j.pharmthera.2006.05.014
29:
1838:Cholesterol 7 alpha-hydroxylase
1784:Flavin-containing monooxygenase
713:Pharmacology & Therapeutics
668:Drug Metabolism and Disposition
271:This enzyme is a member of the
1283:10.1016/j.brainres.2009.12.080
1:
1373:10.1016/s0304-3940(02)00443-3
1328:10.1016/S0304-3940(01)02277-7
616:Annual Review of Biochemistry
2406:Genes on human chromosome 14
1638:Heme hydroxylation mechanism
1621:RCSB Protein Data Bank Entry
1224:Journal of Internal Medicine
834:Proc. Natl. Acad. Sci. U.S.A
230:cholesterol 24-monooxygenase
2067:Deoxyhypusine monooxygenase
391:cholesterol + NADPH + H + O
226:cholesterol 24S-hydroxylase
2432:
1603:gene details page in the
224:), also commonly known as
215:Cholesterol 24-hydroxylase
24:Cholesterol 24-hydroxylase
2256:Michaelis–Menten kinetics
2062:Ecdysone 20-monooxygenase
2013:Stearoyl-CoA desaturase-1
1985:Dopamine beta-hydroxylase
1953:Phenylalanine hydroxylase
1559:10.1017/S1041610205003108
1456:Journal of Neurochemistry
902:10.1016/j.abb.2004.05.012
799:10.1080/02713680701231857
163:
28:
2148:Diffusion-limited enzyme
264:of this enzyme class is
2411:NADPH-dependent enzymes
1185:10.1074/jbc.M110.143313
993:Natural Product Reports
955:10.1073/pnas.0803717105
890:Arch. Biochem. Biophys.
855:10.1073/pnas.96.13.7238
2047:Squalene monooxygenase
1963:Tryptophan hydroxylase
1712:HIF prolyl-hydroxylase
1427:10.1074/jbc.M402324200
1103:10.1074/jbc.M303415200
681:10.1124/dmd.105.008789
535:
451:24S-hydroxycholesterol
408:
384:
352:
340:
258:central nervous system
254:24S-hydroxycholesterol
2241:Eadie–Hofstee diagram
2174:Allosteric regulation
1843:Methane monooxygenase
1816:Nitric oxide synthase
1521:10.1038/sj.mp.4001109
529:
511:Clinical significance
409:
381:
368:allosteric regulatory
350:
337:
329:endoplasmic reticulum
2251:Lineweaver–Burk plot
1958:Tyrosine hydroxylase
1682:steroid hydroxylases
1597:genome location and
1507:Molecular Psychiatry
1360:Neuroscience Letters
786:Current Eye Research
573:, methoxyresorufin,
398:
1908:iron–sulfur protein
1616:HMDB Database entry
1605:UCSC Genome Browser
1420:(33): 34674–34681.
1178:(41): 31783–31795.
946:2008PNAS..105.9546M
846:1999PNAS...96.7238L
517:Alzheimer's disease
426:of this enzyme are
366:There are no known
311:Molecular structure
288:blood–brain barrier
2210:Enzyme superfamily
2143:Enzyme promiscuity
1707:Prolyl hydroxylase
1626:2014-02-28 at the
749:Annu. Rev. Biochem
536:
404:
385:
353:
341:
248:the conversion of
2366:
2365:
2075:
2074:
1741:Lysyl hydroxylase
1140:10.1021/bi035512f
1050:10.1021/cr9002193
940:(28): 9546–9551.
501:squalene synthase
497:HMG CoA reductase
493:liver X receptors
212:
211:
208:
207:
127:metabolic pathway
2423:
2383:
2382:
2374:
2246:Hanes–Woolf plot
2189:Enzyme activator
2184:Enzyme inhibitor
2158:Enzyme catalysis
2102:
2095:
2088:
2079:
1667:
1660:
1653:
1644:
1579:
1578:
1540:
1534:
1533:
1523:
1497:
1491:
1490:
1480:
1446:
1440:
1439:
1429:
1399:
1393:
1392:
1354:
1348:
1347:
1311:
1305:
1304:
1294:
1260:
1251:
1250:
1240:
1214:
1208:
1207:
1197:
1187:
1161:
1152:
1151:
1134:(48): 14284–92.
1122:
1116:
1115:
1105:
1081:
1072:
1071:
1061:
1037:Chemical Reviews
1027:
1018:
1017:
1006:10.1039/b604190f
987:
978:
977:
967:
957:
923:
914:
913:
884:
878:
877:
867:
857:
825:
819:
818:
779:
773:
772:
744:
738:
737:
707:
701:
700:
662:
651:
650:
640:
606:
571:dextromethorphan
413:
411:
410:
405:
374:Enzyme mechanism
165:
44:
33:
21:
2431:
2430:
2426:
2425:
2424:
2422:
2421:
2420:
2416:Cytochrome P450
2391:
2390:
2389:
2377:
2369:
2367:
2362:
2274:Oxidoreductases
2260:
2236:Enzyme kinetics
2224:
2220:List of enzymes
2193:
2162:
2133:Catalytic triad
2111:
2106:
2076:
2071:
2025:- miscellaneous
2017:
1989:
1967:
1930:
1898:
1862:
1853:14α-demethylase
1762:
1689:
1674:Oxidoreductases
1671:
1628:Wayback Machine
1588:
1583:
1582:
1542:
1541:
1537:
1499:
1498:
1494:
1448:
1447:
1443:
1406:(August 2004).
1401:
1400:
1396:
1356:
1355:
1351:
1313:
1312:
1308:
1262:
1261:
1254:
1216:
1215:
1211:
1163:
1162:
1155:
1124:
1123:
1119:
1096:(25): 22980–8.
1083:
1082:
1075:
1029:
1028:
1021:
989:
988:
981:
925:
924:
917:
886:
885:
881:
840:(13): 7238–43.
827:
826:
822:
781:
780:
776:
746:
745:
741:
709:
708:
704:
664:
663:
654:
608:
607:
600:
595:
513:
478:
462:
443:
417:
396:
395:
394:
376:
317:crystallography
313:
273:cytochrome P450
262:systematic name
46:
40:
17:
12:
11:
5:
2429:
2427:
2419:
2418:
2413:
2408:
2403:
2393:
2392:
2388:
2387:
2364:
2363:
2361:
2360:
2347:
2334:
2321:
2308:
2295:
2282:
2268:
2266:
2262:
2261:
2259:
2258:
2253:
2248:
2243:
2238:
2232:
2230:
2226:
2225:
2223:
2222:
2217:
2212:
2207:
2201:
2199:
2198:Classification
2195:
2194:
2192:
2191:
2186:
2181:
2176:
2170:
2168:
2164:
2163:
2161:
2160:
2155:
2150:
2145:
2140:
2135:
2130:
2125:
2119:
2117:
2113:
2112:
2107:
2105:
2104:
2097:
2090:
2082:
2073:
2072:
2070:
2069:
2064:
2059:
2054:
2049:
2044:
2037:Heme oxygenase
2034:
2032:Cyclooxygenase
2028:
2026:
2019:
2018:
2016:
2015:
2010:
2004:
2002:
1991:
1990:
1988:
1987:
1981:
1979:
1969:
1968:
1966:
1965:
1960:
1955:
1949:
1947:
1932:
1931:
1929:
1928:
1923:
1918:
1912:
1910:
1900:
1899:
1897:
1896:
1891:
1886:
1880:
1878:
1864:
1863:
1861:
1860:
1855:
1850:
1845:
1840:
1835:
1834:
1833:
1828:
1823:
1813:
1812:
1811:
1806:
1801:
1796:
1791:
1780:
1778:
1764:
1763:
1761:
1760:
1759:
1758:
1753:
1743:
1738:
1737:
1736:
1731:
1730:
1729:
1724:
1719:
1703:
1701:
1699:2-oxoglutarate
1691:
1690:
1672:
1670:
1669:
1662:
1655:
1647:
1641:
1640:
1635:
1630:
1618:
1613:
1608:
1587:
1586:External links
1584:
1581:
1580:
1535:
1514:(8): 899–902.
1492:
1463:(4): 978–989.
1441:
1394:
1349:
1316:Neurosci. Lett
1306:
1270:Brain Research
1252:
1231:(4): 377–387.
1209:
1153:
1117:
1073:
1044:(2): 932–948.
1019:
1000:(3): 585–609.
979:
915:
879:
820:
793:(4): 361–366.
774:
739:
720:(3): 761–773.
702:
675:(4): 513–520.
652:
597:
596:
594:
591:
512:
509:
477:
474:
460:
441:
420:
419:
415:
403:
392:
375:
372:
312:
309:
210:
209:
206:
205:
200:
194:
193:
188:
182:
181:
176:
170:
169:
161:
160:
149:
142:
141:
136:
130:
129:
124:
118:
117:
112:
106:
105:
100:
94:
93:
88:
82:
81:
76:
70:
69:
65:
64:
59:
53:
52:
48:
47:
34:
26:
25:
16:Protein family
15:
13:
10:
9:
6:
4:
3:
2:
2428:
2417:
2414:
2412:
2409:
2407:
2404:
2402:
2399:
2398:
2396:
2386:
2381:
2376:
2372:
2358:
2354:
2353:
2348:
2345:
2341:
2340:
2335:
2332:
2328:
2327:
2322:
2319:
2315:
2314:
2309:
2306:
2302:
2301:
2296:
2293:
2289:
2288:
2283:
2280:
2276:
2275:
2270:
2269:
2267:
2263:
2257:
2254:
2252:
2249:
2247:
2244:
2242:
2239:
2237:
2234:
2233:
2231:
2227:
2221:
2218:
2216:
2215:Enzyme family
2213:
2211:
2208:
2206:
2203:
2202:
2200:
2196:
2190:
2187:
2185:
2182:
2180:
2179:Cooperativity
2177:
2175:
2172:
2171:
2169:
2165:
2159:
2156:
2154:
2151:
2149:
2146:
2144:
2141:
2139:
2138:Oxyanion hole
2136:
2134:
2131:
2129:
2126:
2124:
2121:
2120:
2118:
2114:
2110:
2103:
2098:
2096:
2091:
2089:
2084:
2083:
2080:
2068:
2065:
2063:
2060:
2058:
2055:
2053:
2050:
2048:
2045:
2042:
2038:
2035:
2033:
2030:
2029:
2027:
2024:
2020:
2014:
2011:
2009:
2006:
2005:
2003:
2000:
1996:
1992:
1986:
1983:
1982:
1980:
1978:
1974:
1970:
1964:
1961:
1959:
1956:
1954:
1951:
1950:
1948:
1945:
1944:BH4 dependent
1941:
1937:
1933:
1927:
1924:
1922:
1919:
1917:
1914:
1913:
1911:
1909:
1905:
1901:
1895:
1892:
1890:
1887:
1885:
1882:
1881:
1879:
1877:
1873:
1869:
1865:
1859:
1856:
1854:
1851:
1849:
1846:
1844:
1841:
1839:
1836:
1832:
1829:
1827:
1824:
1822:
1819:
1818:
1817:
1814:
1810:
1807:
1805:
1802:
1800:
1797:
1795:
1792:
1790:
1787:
1786:
1785:
1782:
1781:
1779:
1777:
1773:
1769:
1765:
1757:
1754:
1752:
1749:
1748:
1747:
1744:
1742:
1739:
1735:
1732:
1728:
1725:
1723:
1720:
1718:
1715:
1714:
1713:
1710:
1709:
1708:
1705:
1704:
1702:
1700:
1696:
1692:
1687:
1683:
1679:
1675:
1668:
1663:
1661:
1656:
1654:
1649:
1648:
1645:
1639:
1636:
1634:
1631:
1629:
1625:
1622:
1619:
1617:
1614:
1612:
1609:
1606:
1602:
1601:
1596:
1595:
1590:
1589:
1585:
1576:
1572:
1568:
1564:
1560:
1556:
1552:
1548:
1547:
1539:
1536:
1531:
1527:
1522:
1517:
1513:
1509:
1508:
1503:
1496:
1493:
1488:
1484:
1479:
1474:
1470:
1466:
1462:
1458:
1457:
1452:
1445:
1442:
1437:
1433:
1428:
1423:
1419:
1415:
1414:
1409:
1405:
1398:
1395:
1390:
1386:
1382:
1378:
1374:
1370:
1366:
1362:
1361:
1353:
1350:
1345:
1341:
1337:
1333:
1329:
1325:
1322:(1–2): 45–8.
1321:
1317:
1310:
1307:
1302:
1298:
1293:
1288:
1284:
1280:
1276:
1272:
1271:
1266:
1259:
1257:
1253:
1248:
1244:
1239:
1234:
1230:
1226:
1225:
1220:
1213:
1210:
1205:
1201:
1196:
1191:
1186:
1181:
1177:
1173:
1172:
1167:
1160:
1158:
1154:
1149:
1145:
1141:
1137:
1133:
1129:
1121:
1118:
1113:
1109:
1104:
1099:
1095:
1091:
1090:J. Biol. Chem
1087:
1080:
1078:
1074:
1069:
1065:
1060:
1055:
1051:
1047:
1043:
1039:
1038:
1033:
1026:
1024:
1020:
1015:
1011:
1007:
1003:
999:
995:
994:
986:
984:
980:
975:
971:
966:
961:
956:
951:
947:
943:
939:
935:
934:
929:
922:
920:
916:
911:
907:
903:
899:
896:(1): 99–108.
895:
892:
891:
883:
880:
875:
871:
866:
861:
856:
851:
847:
843:
839:
835:
831:
824:
821:
816:
812:
808:
804:
800:
796:
792:
788:
787:
778:
775:
770:
766:
762:
758:
754:
750:
743:
740:
735:
731:
727:
723:
719:
715:
714:
706:
703:
698:
694:
690:
686:
682:
678:
674:
670:
669:
661:
659:
657:
653:
648:
644:
639:
634:
630:
626:
623:: 1017–1040.
622:
618:
617:
612:
605:
603:
599:
592:
590:
588:
584:
580:
576:
572:
568:
564:
559:
556:
555:polymorphisms
551:
549:
545:
540:
533:
528:
524:
522:
518:
510:
508:
506:
502:
498:
494:
488:
486:
485:
475:
473:
471:
466:
464:
456:
452:
448:
444:
437:
433:
429:
425:
390:
389:
388:
380:
373:
371:
369:
364:
360:
358:
349:
345:
336:
332:
330:
326:
322:
318:
310:
308:
306:
305:
300:
295:
293:
289:
285:
280:
278:
277:monooxygenase
274:
269:
267:
263:
259:
255:
251:
247:
243:
239:
235:
231:
227:
223:
220:
216:
204:
201:
199:
195:
192:
189:
187:
183:
180:
177:
175:
171:
166:
162:
159:
156:
153:
150:
147:
143:
140:
137:
135:
131:
128:
125:
123:
119:
116:
113:
111:
107:
104:
103:NiceZyme view
101:
99:
95:
92:
89:
87:
83:
80:
77:
75:
71:
66:
63:
60:
58:
54:
49:
43:
38:
32:
27:
22:
19:
2352:Translocases
2349:
2336:
2323:
2310:
2297:
2287:Transferases
2284:
2271:
2128:Binding site
1876:flavoprotein
1680:, including
1678:dioxygenases
1599:
1593:
1553:(1): 37–45.
1550:
1544:
1538:
1511:
1505:
1495:
1460:
1454:
1444:
1417:
1411:
1397:
1364:
1358:
1352:
1319:
1315:
1309:
1274:
1268:
1228:
1222:
1212:
1175:
1169:
1131:
1128:Biochemistry
1127:
1120:
1093:
1089:
1041:
1035:
997:
991:
937:
931:
893:
888:
882:
837:
833:
823:
790:
784:
777:
752:
748:
742:
717:
711:
705:
672:
666:
620:
614:
587:testosterone
567:progesterone
560:
552:
541:
537:
514:
489:
482:
479:
467:
445:, and its 3
421:
386:
365:
361:
354:
342:
331:in neurons.
314:
302:
298:
296:
281:
270:
265:
237:
233:
229:
225:
214:
213:
91:BRENDA entry
37:Homo sapiens
36:
18:
2123:Active site
1367:(1): 9–12.
532:xenobiotics
428:cholesterol
250:cholesterol
79:IntEnz view
51:Identifiers
2401:EC 1.14.13
2395:Categories
2326:Isomerases
2300:Hydrolases
2167:Regulation
2008:Tyrosinase
1975:: reduced
1938:: reduced
1906:: reduced
1870:: reduced
755:: 137–74.
593:References
583:phenacetin
579:diclofenac
544:astrocytes
507:synthase.
424:substrates
222:1.14.13.98
148:structures
115:KEGG entry
62:1.14.13.98
2205:EC number
1977:ascorbate
1940:pteridine
1404:Wolozin B
563:bufuralol
548:glutamate
402:⇌
246:catalyzes
68:Databases
2229:Kinetics
2153:Cofactor
2116:Activity
1624:Archived
1575:29923846
1567:16734927
1530:12232784
1487:20193040
1436:15148325
1389:24909348
1381:12123847
1344:37449539
1336:11698143
1301:20053345
1277:: 1–12.
1247:21486371
1204:20667828
1148:14640697
1112:12686551
1068:19769330
1014:17534532
974:18621681
910:15234274
874:10377398
815:26919085
807:17453958
769:12543708
734:16872679
689:16434543
647:19489738
575:cortisol
484:In vitro
476:Function
447:products
383:product.
240:, is an
203:proteins
191:articles
179:articles
152:RCSB PDB
45:.
2385:Biology
2339:Ligases
2109:Enzymes
2023:1.14.99
2001:: other
1995:1.14.18
1973:1.14.17
1936:1.14.16
1904:1.14.15
1868:1.14.14
1768:1.14.13
1695:1.14.11
1600:CYP46A1
1594:CYP46A1
1478:3010752
1292:2826556
1195:2951250
1059:2820140
965:2474539
942:Bibcode
842:Bibcode
638:2837268
521:neurons
370:sites.
357:proline
319:at the
304:E. coli
299:CYP46A1
238:CYP46A1
139:profile
122:MetaCyc
2371:Portal
2313:Lyases
1872:flavin
1751:ALKBH1
1591:Human
1573:
1565:
1528:
1485:
1475:
1434:
1387:
1379:
1342:
1334:
1299:
1289:
1245:
1202:
1192:
1146:
1110:
1066:
1056:
1012:
972:
962:
908:
872:
862:
813:
805:
767:
732:
697:304953
695:
687:
645:
635:
585:, and
503:, and
457:, and
438:, and
422:The 4
292:retina
260:. The
242:enzyme
186:PubMed
168:Search
158:PDBsum
98:ExPASy
86:BRENDA
74:IntEnz
57:EC no.
2265:Types
2041:HMOX1
1776:NADPH
1734:P4HTM
1727:EGLN3
1722:EGLN2
1717:EGLN1
1688:1.14)
1571:S2CID
1385:S2CID
1340:S2CID
865:22064
811:S2CID
693:S2CID
470:alkyl
432:NADPH
284:polar
244:that
236:, or
234:CYP46
134:PRIAM
2357:list
2350:EC7
2344:list
2337:EC6
2331:list
2324:EC5
2318:list
2311:EC4
2305:list
2298:EC3
2292:list
2285:EC2
2279:list
2272:EC1
2057:21A2
2052:17A1
1926:11A1
1921:11B2
1916:11B1
1884:19A1
1831:NOS3
1826:NOS2
1821:NOS1
1809:FMO5
1804:FMO4
1799:FMO3
1794:FMO2
1789:FMO1
1772:NADH
1746:AlkB
1563:PMID
1526:PMID
1483:PMID
1432:PMID
1377:PMID
1332:PMID
1297:PMID
1275:1319
1243:PMID
1200:PMID
1144:PMID
1108:PMID
1064:PMID
1010:PMID
970:PMID
906:PMID
870:PMID
803:PMID
765:PMID
730:PMID
685:PMID
643:PMID
455:NADP
449:are
339:2Q9F
325:heme
198:NCBI
155:PDBe
110:KEGG
42:2Q9F
1894:2E1
1889:2D6
1874:or
1848:3A4
1774:or
1756:FTO
1555:doi
1516:doi
1473:PMC
1465:doi
1461:113
1422:doi
1418:279
1369:doi
1365:328
1324:doi
1320:314
1287:PMC
1279:doi
1233:doi
1229:270
1190:PMC
1180:doi
1176:285
1136:doi
1098:doi
1094:278
1054:PMC
1046:doi
1042:110
1002:doi
960:PMC
950:doi
938:105
898:doi
894:428
860:PMC
850:doi
795:doi
757:doi
722:doi
718:112
677:doi
633:PMC
625:doi
505:FPP
252:to
174:PMC
146:PDB
2397::
1999:19
1770::
1697::
1686:EC
1676::
1569:.
1561:.
1551:18
1549:.
1524:.
1510:.
1504:.
1481:.
1471:.
1459:.
1453:.
1430:.
1416:.
1410:.
1383:.
1375:.
1363:.
1338:.
1330:.
1318:.
1295:.
1285:.
1273:.
1267:.
1255:^
1241:.
1227:.
1221:.
1198:.
1188:.
1174:.
1168:.
1156:^
1142:.
1132:42
1130:.
1106:.
1092:.
1088:.
1076:^
1062:.
1052:.
1040:.
1034:.
1022:^
1008:.
998:24
996:.
982:^
968:.
958:.
948:.
936:.
930:.
918:^
904:.
868:.
858:.
848:.
838:96
836:.
832:.
809:.
801:.
791:32
789:.
763:.
753:72
751:.
728:.
716:.
691:.
683:.
673:34
671:.
655:^
641:.
631:.
621:78
619:.
613:.
601:^
581:,
577:,
569:,
565:,
499:,
465:.
453:,
434:,
430:,
268:.
232:,
228:,
219:EC
2373::
2359:)
2355:(
2346:)
2342:(
2333:)
2329:(
2320:)
2316:(
2307:)
2303:(
2294:)
2290:(
2281:)
2277:(
2101:e
2094:t
2087:v
2043:)
2039:(
1997:-
1946:)
1942:(
1684:(
1666:e
1659:t
1652:v
1607:.
1577:.
1557::
1532:.
1518::
1512:7
1489:.
1467::
1438:.
1424::
1391:.
1371::
1346:.
1326::
1303:.
1281::
1249:.
1235::
1206:.
1182::
1150:.
1138::
1114:.
1100::
1070:.
1048::
1016:.
1004::
976:.
952::
944::
912:.
900::
876:.
852::
844::
817:.
797::
771:.
759::
736:.
724::
699:.
679::
649:.
627::
463:O
461:2
459:H
442:2
440:O
436:H
418:O
416:2
393:2
217:(
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.