279:(or polymer-fiber) diaphragm separates a cathode and an anode, preventing the chlorine forming at the anode from re-mixing with the sodium hydroxide and the hydrogen formed at the cathode. This technology was also developed at the end of the nineteenth century. There are several variants of this process: the Le Sueur cell (1893), the Hargreaves-Bird cell (1901), the Gibbs cell (1908), and the Townsend cell (1904). The cells vary in construction and placement of the diaphragm, with some having the diaphragm in direct contact with the cathode.
732:
strong or too weak a solution may damage the membranes. Membrane cells typically produce caustic in the range of 30% to 33% by weight. The feed caustic flow is heated at low electrical loads to control its exit temperature. Higher loads require the caustic to be cooled, to maintain correct exit temperatures. The caustic exiting to storage is pulled from a storage tank and may be diluted for sale to customers who require weak caustic or for use on site. Another stream may be pumped into a
342:
171:
703:. The chlorine gas is compressed at this stage and may be further cooled by inter- and after-coolers. After compression it flows to the liquefiers, where it is cooled enough to liquefy. Non condensible gases and remaining chlorine gas are vented off as part of the pressure control of the liquefaction systems. These gases are routed to a gas scrubber, producing
25:
731:
Caustic, fed to the cell room flows in a loop that is simultaneously bled off to storage with a part diluted with deionized water and returned to the cell line for strengthening within the cells. The caustic exiting the cell line must be monitored for strength, to maintain safe concentrations. Too
542:
Key to the production of chlorine is the operation of the brine saturation/treatment system. Maintaining a properly saturated solution with the correct purity is vital, especially for membrane cells. Many plants have a salt pile which is sprayed with recycled brine. Others have slurry tanks that
174:
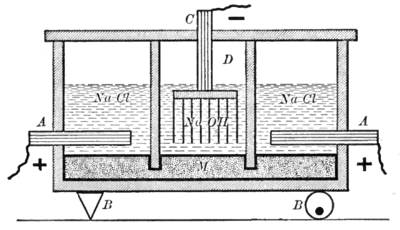
Castner–Kellner cell: Sodium chloride is electrolyzed between the "A" anode and "M" mercury cathode in the side cells, with chlorine bubbling up into the space above the NaCl and the sodium dissolving in the mercury. The sodium–mercury amalgam flows to the center cell, where it reacts with water to
507:
Small amounts of chlorine gas can be made in the laboratory by putting concentrated hydrochloric acid in a flask with a side arm and rubber tubing attached. Manganese dioxide is then added and the flask stoppered. The reaction is not greatly exothermic. As chlorine is denser than air, it can be
533:
Large-scale production of chlorine involves several steps and many pieces of equipment. The description below is typical of a membrane plant. The plant also simultaneously produces sodium hydroxide (caustic soda) and hydrogen gas. A typical plant consists of brine production/treatment, cell
429:
and is performed at high temperature (about 400 °C). The amount of extracted chlorine is approximately 80%. Due to the extremely corrosive reaction mixture, industrial use of this method is difficult and several pilot trials failed in the past. Nevertheless, recent developments are promising.
1161:
1086:
744:
Hydrogen produced as a byproduct may be vented unprocessed directly to the atmosphere or cooled, compressed and dried for use in other processes on site or sold to a customer via pipeline, cylinders or trucks. Some possible uses include the manufacture of hydrochloric acid or
1136:
1111:
331:. Sodium (or potassium) hydroxide solution is circulated through the cathode compartment, exiting at a higher concentration. A portion of the concentrated sodium hydroxide solution leaving the cell is diverted as product, while the remainder is diluted with
1165:
607:, and either have a treatment system in place, or purging of the brine loop to maintain safe levels, since chlorate anions can diffuse through the membranes and contaminate the caustic, while sulfate anions can damage the anode surface coating.
1090:
582:
After the ion exchangers, the brine is considered pure, and is transferred to storage tanks to be pumped into the cell room. The pure brine is heated to the correct temperature to control exit brine temperatures according to the
1140:
1115:
615:
The building that houses the many electrolytic cells is usually called a cell room or cell house, although some plants are built outdoors. This building contains support structures for the cells, connections for supplying
777:
Production of chlorine is extremely energy intensive. Energy consumption per unit weight of product is not far below that for iron and steel manufacture and greater than for the production of glass or cement.
624:
of each cell which vary with the electrical load on the cell room that is used to control the rate of production. Monitoring and control of the pressures in the chlorine and hydrogen headers is also done via
558:
where the calcium carbonate and magnesium hydroxide are settled out. A flocculating agent may be added just prior to the clarifier to improve settling. The decanted brine is then mechanically filtered using
534:
operations, chlorine cooling & drying, chlorine compression & liquefaction, liquid chlorine storage & loading, caustic handling, evaporation, storage & loading and hydrogen handling.
186:, was the first method used at the end of the nineteenth century to produce chlorine on an industrial scale. The "rocking" cells used have been improved over the years. Today, in the "primary cell",
293:
is produced and the brine is partially depleted. As a result, diaphragm methods produce alkali that is quite dilute (about 12%) and of lower purity than do mercury cell methods.
620:
to the cells and piping for the fluids. Monitoring and control of the temperatures of the feed caustic and brine is done to control exit temperatures. Also monitored are the
267:, mercury cells accounted for 43% of capacity in 2006 and Western European producers have committed to closing or converting all remaining chloralkali mercury plants by 2020.
338:
This method is more efficient than the diaphragm cell and produces very pure sodium (or potassium) hydroxide at about 32% concentration, but requires very pure brine.
655:
Chlorine gas exiting the cell line must be cooled and dried since the exit gas can be over 80°C and contains moisture that allows chlorine gas to be corrosive to
131:). There are three industrial methods for the extraction of chlorine by electrolysis of chloride solutions, all proceeding according to the following equations:
675:
stage that follows. Chlorine exiting is ideally between 18°C and 25°C. After cooling the gas stream passes through a series of towers with counter flowing
354:
Although a much lower production scale is involved, electrolytic diaphragm and membrane technologies are also used industrially to recover chlorine from
1162:"Integrated Pollution Prevention and Control (IPPC) - Reference Document on Best Available Techniques in the Cement and Lime Manufacturing Industries"
907:
259:, mercury-based chloralkali production was virtually phased out by 1987 (except for the last two potassium chloride units shut down in 2003). In the
723:
Liquid chlorine is typically gravity-fed to storage tanks. It can be loaded into rail or road tankers via pumps or padded with compressed dry gas.
1087:"Integrated Pollution Prevention and Control (IPPC) - Reference Document on Best Available Techniques in the Chlor-Alkali Manufacturing Industry"
46:
33:
789:
reaction cannot be reduced. Energy savings arise primarily through applying more efficient technologies and reducing ancillary energy use.
237:
at a commercially useful concentration (50% by weight). The mercury is then recycled to the primary cell by a pump situated at the bottom.
123:). These two products, as well as chlorine itself, are highly reactive. Chlorine can also be produced by the electrolysis of a solution of
1137:"Integrated Pollution Prevention and Control (IPPC) - Reference Document on Best Available Techniques in the Glass Manufacturing Industry"
599:
addition. Failure to remove chlorine can result in damage to the ion exchange units. Brine should be monitored for accumulation of both
511:
Another method for producing small amounts of chlorine gas in a lab is by adding concentrated hydrochloric acid (typically about 5M) to
1112:"Integrated Pollution Prevention and Control (IPPC) - Best Available Techniques Reference Document on the Production of Iron and Steel"
508:
easily collected by placing the tube inside a flask where it will displace the air. Once full, the collecting flask can be stoppered.
296:
Diaphragm cells are not burdened with the problem of preventing mercury discharge into the environment; they also operate at a lower
938:
1013:
855:
320:
286:
solution is continuously fed to the anode compartment and flows through the diaphragm to the cathode compartment, where the
241:
963:
683:
from the chlorine gas. After exiting the drying towers the chlorine is filtered to remove any remaining sulfuric acid.
554:
and magnesium. The reactions are often carried out in a series of reactors before the treated brine is sent to a large
245:
183:
195:
1068:. Lenntech Water treatment & air purification Holding B.V., Rotterdamseweg 402 M, 2629 HH Delft, The Netherlands
837:. Lenntech Water treatment & air purification Holding B.V., Rotterdamseweg 402 M, 2629 HH Delft, The Netherlands
733:
327:. Saturated sodium (or potassium) chloride solution is passed through the anode compartment, leaving at a lower
790:
696:
736:
set to produce commercial 50% caustic. Rail cars and tanker trucks are loaded at loading stations via pumps.
785:
is an indispensable raw material for the production of chlorine, the energy consumption corresponding to the
316:
Development of this technology began in the 1970s. The electrolysis cell is divided into two "sections" by a
38:
992:
914:
700:
522:
626:
834:
664:
493:
708:
704:
512:
435:
418:
287:
222:
128:
104:
592:
564:
124:
1203:
746:
450:
386:
355:
202:
anodes) are placed in a sodium (or potassium) chloride solution flowing over a liquid mercury
179:
226:
1047:
889:
786:
640:
617:
596:
544:
255:
It is estimated that there are still around 100 mercury-cell plants operating worldwide. In
112:
365:) also enables chlorine to be produced, in this case as a by-product of the manufacture of
240:
The mercury process is the least energy-efficient of the three main technologies (mercury,
1065:
750:
644:
584:
516:
332:
324:
96:
76:
1188:
942:
1017:
206:. When a potential difference is applied and current flows, chlorine is released at the
859:
668:
632:
587:. Brine exiting the cell room must be treated to remove residual chlorine and control
501:
394:
263:, there will be only five mercury plants remaining in operation by the end of 2008. In
229:" or "secondary cell"), where it is usually converted back to mercury by reaction with
793:
of the overall process thus depend largely on the way the electricity is produced. If
1197:
798:
676:
328:
260:
341:
300:, resulting in an energy savings over the mercury cell method, but large amounts of
672:
660:
568:
108:
92:
72:
707:, or used in the production of hydrochloric acid (by combustion with hydrogen) or
170:
880:
Landolt, D.; Ibl, N. (1972). "Anodic chlorate formation on platinized titanium".
659:
piping. Cooling the gas allows for a large amount of moisture from the brine to
802:
782:
692:
643:
to the cells. As the current is increased, flow rates for brine and caustic and
560:
548:
1051:
1038:
López, Núria (2008). "Mechanism of HCl oxidation (Deacon process) over RuO2".
794:
572:
362:
305:
967:
766:
754:
636:
555:
497:
382:
370:
249:
234:
218:
801:
or other low carbon sources are used, emissions will be much lower than if
24:
381:
Before electrolytic methods were used for chlorine production, the direct
712:
680:
600:
595:
stage. This can be accomplished via dechlorination towers with acid and
576:
431:
426:
276:
207:
199:
191:
187:
116:
67:
1189:
Production of chlorine gas and demonstration of its oxidizing properties
71:
gas can be produced by extracting from natural materials, including the
893:
621:
604:
551:
358:
solutions, producing hydrogen (but no caustic alkali) as a co-product.
297:
203:
525:
can be used to generate chlorine gas when added to hydrochloric acid.
762:
390:
317:
290:
264:
214:
543:
are fed raw salt and recycled brine. The raw brine is treated with
445:
Another earlier process to produce chlorine was to heat brine with
366:
340:
301:
256:
230:
210:
169:
100:
80:
127:, in which case the co-products are hydrogen and caustic potash (
758:
656:
446:
283:
18:
575:. At several points in this process the brine is tested for
588:
908:"Regional Awareness-raising Workshop on Mercury Pollution"
393:(frequently through exposure to air) was exercised in the
107:. The production of chlorine results in the co-products
639:
power source. Plant load is controlled by varying the
496:
was the first to isolate chlorine in a laboratory. The
835:"Electrolytic Processes for Chlorine and Caustic Soda"
647:
are increased, while lowering the feed temperatures.
335:
and passed through the electrolysis apparatus again.
225:. This flows continuously into a separate reactor ("
175:
produce sodium hydroxide and regenerate the mercury.
679:. These towers progressively remove any remaining
361:
Furthermore, electrolysis of fused chloride salts (
727:Caustic handling, evaporation, storage and loading
663:out of the gas stream. Cooling also improves the
966:. Salt Manufacturers' Association. Archived from
434:patented a catalyst for the Deacon process using
345:Membrane cell process for chloralkali production
233:, producing hydrogen and sodium (or potassium)
417:This reaction is accomplished with the use of
221:) dissolves in the mercury cathode forming an
8:
691:Several methods of compression may be used:
248:) and there are also concerns about mercury
986:
984:
308:to the commercial concentration of 50%.
49:of all important aspects of the article.
813:
304:are required if the caustic has to be
150:Overall process: 2 NaCl (or KCl) + 2 H
45:Please consider expanding the lead to
1164:. European Commission. Archived from
1139:. European Commission. Archived from
1114:. European Commission. Archived from
1089:. European Commission. Archived from
888:(3). Chapman and Hall Ltd.: 201–210.
271:Diaphragm cell electrolysis (bipolar)
182:cell electrolysis, also known as the
7:
591:levels before being returned to the
91:Chlorine can be manufactured by the
882:Journal of Applied Electrochemistry
275:In diaphragm cell electrolysis, an
14:
993:"When the Industry Charged Ahead"
23:
37:may be too short to adequately
824:, 1970 ed., Dover publications
529:Membrane industrial production
47:provide an accessible overview
1:
1016:. Euro Chlor. Archived from
941:. Euro Chlor. Archived from
858:. Euro Chlor. Archived from
687:Compression and liquefaction
567:before entering a series of
492:Using this process, chemist
350:Other electrolytic processes
964:"The Electrolysis of Brine"
135:Cathode: 2 H (aq) + 2 e → H
1220:
1052:10.1016/j.jcat.2008.01.020
734:multiple effect evaporator
312:Membrane cell electrolysis
166:Mercury cell electrolysis
103:), which is known as the
791:Greenhouse gas emissions
547:and sodium hydroxide to
500:can be recovered by the
1066:"The Chlorine Industry"
627:pressure control valves
196:conductive metal oxides
184:Castner–Kellner process
995:. Chemistry Chronicles
913:. UNEP. Archived from
523:Potassium permanganate
346:
176:
16:Production of chlorine
344:
323:membrane acting as a
173:
142:Anode: 2 Cl (aq) → Cl
1040:Journal of Catalysis
494:Carl Wilhelm Scheele
719:Storage and loading
709:ethylene dichloride
705:sodium hypochlorite
513:sodium hypochlorite
436:ruthenium(IV) oxide
419:copper(II) chloride
129:potassium hydroxide
105:Chloralkali process
894:10.1007/BF02354977
773:Energy consumption
711:(by reaction with
651:Cooling and drying
635:is supplied via a
571:to further remove
347:
177:
162:+ 2 NaOH (or KOH)
125:potassium chloride
83:) and other ways.
991:Kiefer, David M.
822:General Chemistry
747:hydrogen peroxide
740:Hydrogen handling
451:manganese dioxide
387:hydrogen chloride
356:hydrochloric acid
190:anodes clad with
64:
63:
1211:
1177:
1176:
1174:
1173:
1158:
1152:
1151:
1149:
1148:
1133:
1127:
1126:
1124:
1123:
1108:
1102:
1101:
1099:
1098:
1083:
1077:
1076:
1074:
1073:
1062:
1056:
1055:
1035:
1029:
1028:
1026:
1025:
1010:
1004:
1003:
1001:
1000:
988:
979:
978:
976:
975:
960:
954:
953:
951:
950:
939:"Diaphragm cell"
935:
929:
928:
926:
925:
919:
912:
904:
898:
897:
877:
871:
870:
868:
867:
852:
846:
845:
843:
842:
831:
825:
820:Pauling, Linus,
818:
618:electrical power
597:sodium bisulfite
545:sodium carbonate
325:cation exchanger
113:sodium hydroxide
59:
56:
50:
27:
19:
1219:
1218:
1214:
1213:
1212:
1210:
1209:
1208:
1194:
1193:
1185:
1180:
1171:
1169:
1160:
1159:
1155:
1146:
1144:
1135:
1134:
1130:
1121:
1119:
1110:
1109:
1105:
1096:
1094:
1085:
1084:
1080:
1071:
1069:
1064:
1063:
1059:
1037:
1036:
1032:
1023:
1021:
1014:"Membrane cell"
1012:
1011:
1007:
998:
996:
990:
989:
982:
973:
971:
962:
961:
957:
948:
946:
937:
936:
932:
923:
921:
917:
910:
906:
905:
901:
879:
878:
874:
865:
863:
854:
853:
849:
840:
838:
833:
832:
828:
819:
815:
811:
787:electrochemical
775:
751:desulfurization
742:
729:
721:
689:
653:
645:deionized water
613:
601:chlorate anions
585:electrical load
540:
531:
517:sodium chlorate
488:
484:
480:
476:
472:
468:
464:
460:
441:
424:
412:
408:
404:
379:
352:
333:deionized water
314:
273:
168:
161:
157:
153:
145:
138:
122:
97:sodium chloride
89:
77:sodium chloride
60:
54:
51:
44:
32:This article's
28:
17:
12:
11:
5:
1217:
1215:
1207:
1206:
1196:
1195:
1192:
1191:
1184:
1183:External links
1181:
1179:
1178:
1153:
1128:
1103:
1078:
1057:
1030:
1005:
980:
955:
930:
899:
872:
856:"Mercury cell"
847:
826:
812:
810:
807:
774:
771:
757:, or use as a
741:
738:
728:
725:
720:
717:
688:
685:
652:
649:
633:Direct current
612:
609:
605:sulfate anions
579:and strength.
569:ion exchangers
539:
536:
530:
527:
502:Weldon process
490:
489:
486:
482:
478:
474:
470:
466:
462:
458:
439:
422:
415:
414:
410:
406:
402:
395:Deacon process
378:
375:
351:
348:
313:
310:
272:
269:
167:
164:
159:
155:
151:
148:
147:
143:
140:
136:
120:
88:
87:Gas extraction
85:
62:
61:
41:the key points
31:
29:
22:
15:
13:
10:
9:
6:
4:
3:
2:
1216:
1205:
1202:
1201:
1199:
1190:
1187:
1186:
1182:
1168:on 2010-07-01
1167:
1163:
1157:
1154:
1143:on 2010-07-01
1142:
1138:
1132:
1129:
1118:on 2007-09-28
1117:
1113:
1107:
1104:
1093:on 2010-07-01
1092:
1088:
1082:
1079:
1067:
1061:
1058:
1053:
1049:
1045:
1041:
1034:
1031:
1020:on 2007-08-14
1019:
1015:
1009:
1006:
994:
987:
985:
981:
970:on 2007-05-14
969:
965:
959:
956:
945:on 2007-09-27
944:
940:
934:
931:
920:on 2007-10-29
916:
909:
903:
900:
895:
891:
887:
883:
876:
873:
862:on 2011-09-18
861:
857:
851:
848:
836:
830:
827:
823:
817:
814:
808:
806:
804:
800:
799:nuclear power
796:
792:
788:
784:
779:
772:
770:
768:
764:
760:
756:
752:
749:, as well as
748:
739:
737:
735:
726:
724:
718:
716:
714:
710:
706:
702:
698:
697:reciprocating
694:
686:
684:
682:
678:
677:sulfuric acid
674:
670:
666:
662:
658:
650:
648:
646:
642:
638:
634:
630:
628:
623:
619:
610:
608:
606:
602:
598:
594:
590:
586:
580:
578:
574:
570:
566:
562:
557:
553:
550:
546:
537:
535:
528:
526:
524:
520:
518:
514:
509:
505:
503:
499:
495:
456:
455:
454:
452:
448:
443:
437:
433:
428:
420:
400:
399:
398:
396:
392:
388:
384:
377:Other methods
376:
374:
372:
368:
364:
363:Downs process
359:
357:
349:
343:
339:
336:
334:
330:
329:concentration
326:
322:
319:
311:
309:
307:
303:
299:
294:
292:
289:
285:
280:
278:
270:
268:
266:
262:
261:United States
258:
253:
251:
247:
243:
238:
236:
232:
228:
224:
220:
216:
212:
209:
205:
201:
197:
193:
189:
185:
181:
172:
165:
163:
141:
134:
133:
132:
130:
126:
118:
114:
110:
106:
102:
98:
94:
86:
84:
82:
78:
74:
70:
69:
58:
55:December 2023
48:
42:
40:
35:
30:
26:
21:
20:
1170:. Retrieved
1166:the original
1156:
1145:. Retrieved
1141:the original
1131:
1120:. Retrieved
1116:the original
1106:
1095:. Retrieved
1091:the original
1081:
1070:. Retrieved
1060:
1043:
1039:
1033:
1022:. Retrieved
1018:the original
1008:
997:. Retrieved
972:. Retrieved
968:the original
958:
947:. Retrieved
943:the original
933:
922:. Retrieved
915:the original
902:
885:
881:
875:
864:. Retrieved
860:the original
850:
839:. Retrieved
829:
821:
816:
803:fossil fuels
780:
776:
743:
730:
722:
690:
673:liquefaction
667:of both the
654:
631:
614:
581:
565:leaf filters
561:sand filters
541:
532:
521:
510:
506:
491:
444:
416:
380:
360:
353:
337:
315:
295:
281:
274:
254:
239:
178:
149:
115:, NaOH) and
109:caustic soda
93:electrolysis
90:
73:electrolysis
66:
65:
52:
36:
34:lead section
783:electricity
701:centrifugal
693:liquid ring
669:compression
549:precipitate
457:2 NaCl + 2H
1172:2007-09-02
1147:2007-09-02
1122:2007-09-02
1097:2007-09-02
1072:2007-03-17
1024:2007-08-15
999:2007-03-17
974:2007-03-17
949:2007-08-15
924:2007-10-28
866:2007-08-15
841:2007-03-17
809:References
805:are used.
795:hydropower
767:fuel cells
665:efficiency
593:saturation
573:impurities
519:solution.
369:sodium or
306:evaporated
198:(formerly
99:solution (
79:solution (
1046:: 29–39.
755:petroleum
637:rectified
611:Cell room
556:clarifier
498:manganese
430:Recently
401:4 HCl + O
383:oxidation
371:magnesium
321:permeable
250:emissions
242:diaphragm
235:hydroxide
219:potassium
146:(g) + 2 e
39:summarize
1204:Chlorine
1198:Category
713:ethylene
681:moisture
671:and the
661:condense
622:voltages
577:hardness
432:Sumitomo
427:catalyst
367:metallic
277:asbestos
246:membrane
208:titanium
200:graphite
192:platinum
188:titanium
117:hydrogen
68:Chlorine
763:boilers
641:current
552:calcium
425:) as a
298:voltage
288:caustic
227:denuder
223:amalgam
204:cathode
180:Mercury
781:Since
485:O + Cl
477:+ MnSO
405:→ 2 Cl
391:oxygen
318:cation
291:alkali
265:Europe
215:sodium
154:O → Cl
119:gas (H
918:(PDF)
911:(PDF)
699:, or
538:Brine
481:+ 2 H
465:+ MnO
421:(CuCl
409:+ 2 H
389:with
302:steam
257:Japan
231:water
211:anode
101:brine
95:of a
81:brine
75:of a
759:fuel
657:iron
603:and
469:→ Na
449:and
447:acid
438:(RuO
284:salt
282:The
244:and
217:(or
213:and
1048:doi
1044:255
890:doi
765:or
761:in
753:of
715:).
563:or
515:or
442:).
385:of
194:or
158:+ H
139:(g)
1200::
1042:.
983:^
884:.
797:,
769:.
695:,
629:.
589:pH
504:.
473:SO
461:SO
453:.
397::
373:.
252:.
1175:.
1150:.
1125:.
1100:.
1075:.
1054:.
1050::
1027:.
1002:.
977:.
952:.
927:.
896:.
892::
886:2
869:.
844:.
487:2
483:2
479:4
475:4
471:2
467:2
463:4
459:2
440:2
423:2
413:O
411:2
407:2
403:2
160:2
156:2
152:2
144:2
137:2
121:2
111:(
57:)
53:(
43:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.