162:
407:
adduct. By incorporating luminescent substituents into their backbone, these compounds have proved to be sensitive ion probes, as changes in the absorption or fluorescence of the photoactive groups can be measured for very low concentrations of metal present. Some attractive examples include macrocycles, incorporating oxygen and/or nitrogen donors, that are attached to polyaromatic species such as anthracenes (via the 9 and/or 10 positions) or naphthalenes (via the 2 and 3 positions). Some modifications of dye ionophores by crown ethers exhibit
31:
259:
406:
on their side chains. Those protonated amino groups can bind to the cavity of 18-crown-6 and form stable complexes in the gas phase. Hydrogen-bonds are formed between the three hydrogen atoms of protonated amines and three oxygen atoms of 18-crown-6. These hydrogen-bonds make the complex a stable
262:
Catenane derived from cyclobis(paraquat-p-phenylene)(a cyclophane with two viologen units) and a cyclic polyether (bis(para-phenylene-34-crown-10)). Carbon atoms of the two rotaxane components are colored green and purple. Otherwise, O = red, N = blue. H atoms are omitted. The second of Nobel
136:
of the polyether influences the affinity of the crown ether for various cations. For example, 18-crown-6 has high affinity for potassium cation, 15-crown-5 for sodium cation, and 12-crown-4 for lithium cation. The high affinity of 18-crown-6 for potassium ions contributes to its toxicity. The
128:. The oxygen atoms are well situated to coordinate with a cation located at the interior of the ring, whereas the exterior of the ring is hydrophobic. The resulting cations often form salts that are soluble in nonpolar solvents, and for this reason crown ethers are useful in
1014:
Fuji, Kaoru; Tsubaki, Kazunori; Tanaka, Kiyoshi; Hayashi, Noriyuki; Otsubo, Tadamune; Kinoshita, Takayoshi (April 1999). "Visualization of
Molecular Length of α,ω-Diamines and Temperature by a Receptor Based on Phenolphthalein and Crown Ether".
137:
smallest crown ether still capable of binding cations is 8-crown-4, with the largest experimentally confirmed crown ether being 81-crown-27. Crown ethers are not the only macrocyclic ligands that have affinity for the potassium cation.
514:"Preparation and crystallinity of a large unsubstituted crown ether, cyclic heptacosa(oxyethy1ene) (cyc2o=E2, 81-crown-27), studied by Raman spectroscopy, X-ray scattering and differential scanning calorimetry"
680:
109:
sitting on a person's head. The first number in a crown ether's name refers to the number of atoms in the cycle, and the second number refers to the number of those atoms that are
745:
Ashton, P. R.; Goodnow, T. T.; Kaifer, A. E.; Reddington, M. V.; Slawin, A. M. Z.; Spencer, N.; Stoddart, J. F.; Vicent, C.; Williams, D. J. (1989). "A Catenane Made to Order".
987:
Sharghi, Hashem; Ebrahimpourmoghaddam, Sakineh (2008). "A Convenient and
Efficient Method for the Preparation of Unique Fluorophores of Lariat Naphtho-Aza-Crown Ethers".
586:
Lipkowski, J.; Fonari, M. S.; Kravtsov, V. C.; Simonov, Y. A.; Ganin, E. V.; Gemboldt, V. O. (1996). "Antimony(III) fluoride: Inclusion complexes with crown ethers".
295:
analogs. Hereby, the cation selectivity for alkali metal ions is mainly dependent on the size and charge density of the ion and the cavity size of the crown ether.
778:; Brodbelt, Jennifer S. (July 1992). "Determination of orders of relative alkali metal ion affinities of crown ethers and acyclic analogs by the kinetic method".
939:
Fabbrizzi, L.; Francese, G.; Licchelli, M.; Pallavicini, P.; Perotti, A.; Poggi, A.; Sacchi, D.; Taglietti, A. (1997). Desvergne, J. P.; Czarnik, A. W. (eds.).
383:, and potassium can change by multiple magnitudes, which is attributed to the high differences in their charge density. Between the cations of potassium,
247:
cations. He proceeded to report systematic studies of the synthesis and binding properties of crown ethers in a seminal series of papers. The fields of
846:
255:, and other emerging disciplines benefited from the discovery of crown ethers. Pedersen particularly popularized the dibenzo crown ethers.
896:
Shannon, R. D. (1976-09-01). "Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides".
971:
387:, and cesium changes in affinities are less notable, as their charge density varies less than the alkali metals in earlier periods.
512:
Yang, Zhao; Yu, Ga-Er; Cooke, Jennifer; Ali-Abid, Ziad; Viras, Kyriakos; Matsuura, Hiroatsu; Ryan, Anthony J; Booth, Colin (1996).
1069:
543:
Marczenko, K. M.; Mercier, H. P. A.; Schrobilgen, G. J. (2018). "A Stable Crown-Ether
Complex with a Noble-gas Compound".
408:
455:
van der Ham, Alex; Hansen, Thomas; Lodder, Gerrit; Codée, Jeroen D. C.; Hamlin, Trevor A.; Filippov, Dmitri V. (2019).
861:
Frensdorff, Hans K. (February 1971). "Stability constants of cyclic polyether complexes with univalent cations".
156:) interactions, between the Lewis basic oxygen atoms of the crown ether and the electrophilic Lewis acid center.
161:
272:
129:
101: = 6). The term "crown" refers to the resemblance between the structure of a crown ether bound to a
252:
223:
on each molecule. This linking defines a polydentate ligand that could partially envelop the cation and, by
694:
Down, J. L.; Lewis, J.; Moore, B.; Wilkinson, G. (1959). "761. The solubility of alkali metals in ethers".
394:
can also bind to protonated amines and form very stable complexes in both solution and the gas phase. Some
1084:
1055:
513:
905:
775:
675:
212:
292:
125:
30:
811:
603:
568:
288:
178:
963:
1032:
967:
921:
878:
842:
803:
795:
723:
560:
494:
476:
264:
248:
54:
46:
1024:
996:
955:
913:
870:
834:
787:
754:
719:"Macrocyclic Polyethers: Dibenzo-18-Crown-6 Polyether and Dicyclohexyl-18-Crown-6 Polyether"
699:
657:
630:
595:
552:
525:
484:
468:
425:
208:
196:
106:
58:
207:, discovered a simple method of synthesizing a crown ether when he was trying to prepare a
420:
182:
954:
Bouas-Laurent, H.; Desvergne, J. P.; Fages, F.; Marsau, P. (1993). A. W., Czarnik (ed.).
909:
227:
of the phenolic hydroxyls, neutralize the bound dication. He was surprised to isolate a
956:
838:
489:
456:
284:
275:
for the discovery of the synthetic routes to, and binding properties of, crown ethers.
263:
Prizes in
Chemistry involving crown ethers was awarded for the design and synthesis of
74:
943:. NATO ASI Series C. Vol. 492. Dordrecht: Kluwer Academic Publishers. p. 75.
1078:
791:
291:, crown ethers exhibit stronger affinities for diverse cations than their divided or
815:
607:
572:
678:, Stewart, D. G.; Waddan, D. Y. & Borrows, E. T., issued 1957-10-23
435:
267:. Many of these "machines" incorporate crown ethers as essential design components.
244:
153:
648:
Pedersen, C. J. (1967). "Cyclic polyethers and their complexes with metal salts".
621:
Pedersen, C. J. (1967). "Cyclic polyethers and their complexes with metal salts".
718:
395:
149:
142:
962:. ACS Symposium Series 538. Washington, DC: American Chemical Society. p.
457:"Computational and NMR Studies on the Complexation of Lithium Ion to 8-Crown-4"
258:
917:
391:
228:
224:
174:
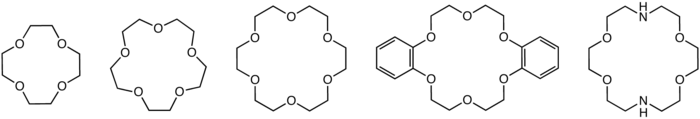
170:
166:
145:
also display a marked preference for the potassium cation over other cations.
34:
1036:
925:
882:
799:
480:
240:
236:
232:
138:
38:
17:
1000:
807:
758:
564:
556:
498:
472:
243:
represented a new class of complexing agents that were capable of binding
703:
529:
430:
384:
220:
216:
118:
114:
70:
874:
661:
634:
599:
376:
200:
1028:
399:
380:
204:
133:
110:
102:
299:
Comparison of Cavity Size with
Effective Ion Radii of Alkali Metals
403:
257:
160:
62:
29:
411:
that are dependent on the chain lengths of chained cations.
958:
375:
Affinities of a given crown ether towards the cations of
117:
of ethylene oxide; an important group are derived from
89:. Important members of this series are the tetramer (
780:
Journal of the
American Society for Mass Spectrometry
390:
Apart from its high affinity for potassium cations,
124:
Crown ethers strongly bind certain cations, forming
235:cations. Citing earlier work on the dissolution of
829:Christensen, J.J.; Izatt, R.M. (1978), "PREFACE",
747:Angewandte Chemie International Edition in English
148:Crown ethers have been shown to coordinate to
77:, the repeating unit being ethyleneoxy, i.e.,
27:Ring molecules with several ether (–O–) groups
8:
941:Chemosensors of Ion and Molecule Recognition
831:Synthetic Multidentate Macrocyclic Compounds
69:). The most common crown ethers are cyclic
239:in 16-crown-4, he realized that the cyclic
113:. Crown ethers are much broader than the
488:
1017:Journal of the American Chemical Society
863:Journal of the American Chemical Society
650:Journal of the American Chemical Society
623:Journal of the American Chemical Society
297:
84:
80:
447:
770:
768:
7:
215:. His strategy entailed linking two
165:Structures of common crown ethers:
152:through electrostatic, σ-hole (see
839:10.1016/b978-0-12-377650-1.50005-8
97: = 5), and the hexamer (
25:
898:Acta Crystallographica Section A
696:Journal of the Chemical Society
93: = 4), the pentamer (
1:
717:Pedersen, Charles J. (1988).
792:10.1016/1044-0305(92)85031-e
518:J. Chem. Soc., Faraday Trans
833:, Elsevier, pp. ix–x,
1101:
1054:Pedersen, Charles (1987).
733:, vol. 6, p. 395
918:10.1107/s0567739476001551
271:Pedersen shared the 1987
273:Nobel Prize in Chemistry
253:phase transfer catalysts
231:that strongly complexed
130:phase transfer catalysis
409:extinction coefficients
313:Effective Ion Radius/Å
1001:10.1002/hlca.200890148
989:Helvetica Chimica Acta
759:10.1002/anie.198913961
557:10.1002/anie.201806640
473:10.1002/cphc.201900496
268:
185:
42:
261:
164:
33:
704:10.1039/jr9590003767
588:J. Chem. Crystallogr
545:Angew. Chem. Int. Ed
530:10.1039/FT9969203173
402:, contain a primary
279:Affinity for cations
910:1976AcCrA..32..751S
875:10.1021/ja00732a007
662:10.1021/ja00986a052
635:10.1021/ja01002a035
551:(38): 12448–12452.
310:Favored Alkali Ion
300:
219:groups through one
61:containing several
600:10.1007/BF01670315
298:
289:macrocyclic effect
269:
265:molecular machines
186:
179:dibenzo-18-crown-6
57:that consist of a
55:chemical compounds
43:
37:coordinating to a
1029:10.1021/ja9836444
1023:(15): 3807–3808.
848:978-0-12-377650-1
776:Liou, Chien-Chung
753:(10): 1396–1399.
731:Collected Volumes
724:Organic Syntheses
656:(10): 2495–2496.
629:(26): 7017–7036.
524:(17): 3173–3182.
467:(16): 2103–2109.
373:
372:
249:organic synthesis
47:organic chemistry
16:(Redirected from
1092:
1066:
1060:
1041:
1040:
1011:
1005:
1004:
995:(7): 1363–1373.
984:
978:
977:
961:
951:
945:
944:
936:
930:
929:
893:
887:
886:
858:
852:
851:
826:
820:
819:
772:
763:
762:
742:
736:
734:
727:
714:
708:
707:
691:
685:
684:
683:
679:
672:
666:
665:
645:
639:
638:
618:
612:
611:
583:
577:
576:
540:
534:
533:
509:
503:
502:
492:
452:
426:Thia-crown ether
301:
213:divalent cations
209:complexing agent
197:Charles Pedersen
88:
68:
21:
1100:
1099:
1095:
1094:
1093:
1091:
1090:
1089:
1075:
1074:
1070:Molecular crown
1058:
1056:"Nobel Lecture"
1053:
1050:
1045:
1044:
1013:
1012:
1008:
986:
985:
981:
974:
953:
952:
948:
938:
937:
933:
895:
894:
890:
860:
859:
855:
849:
828:
827:
823:
774:
773:
766:
744:
743:
739:
729:
716:
715:
711:
693:
692:
688:
681:
674:
673:
669:
647:
646:
642:
620:
619:
615:
585:
584:
580:
542:
541:
537:
511:
510:
506:
454:
453:
449:
444:
421:Aza-crown ether
417:
281:
193:
183:aza-crown ether
86:
82:
78:
66:
28:
23:
22:
15:
12:
11:
5:
1098:
1096:
1088:
1087:
1077:
1076:
1073:
1072:
1067:
1049:
1048:External links
1046:
1043:
1042:
1006:
979:
972:
946:
931:
904:(5): 751–767.
888:
869:(3): 600–606.
853:
847:
821:
786:(5): 543–548.
764:
737:
709:
686:
667:
640:
613:
578:
535:
504:
446:
445:
443:
440:
439:
438:
433:
428:
423:
416:
413:
371:
370:
367:
364:
361:
357:
356:
353:
350:
347:
343:
342:
339:
336:
333:
329:
328:
325:
322:
319:
315:
314:
311:
308:
307:Cavity Size/Å
305:
285:chelate effect
280:
277:
192:
189:
188:
187:
75:ethylene oxide
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1097:
1086:
1083:
1082:
1080:
1071:
1068:
1064:
1057:
1052:
1051:
1047:
1038:
1034:
1030:
1026:
1022:
1018:
1010:
1007:
1002:
998:
994:
990:
983:
980:
975:
973:9780841227286
969:
965:
960:
959:
950:
947:
942:
935:
932:
927:
923:
919:
915:
911:
907:
903:
899:
892:
889:
884:
880:
876:
872:
868:
864:
857:
854:
850:
844:
840:
836:
832:
825:
822:
817:
813:
809:
805:
801:
797:
793:
789:
785:
781:
777:
771:
769:
765:
760:
756:
752:
748:
741:
738:
732:
726:
725:
720:
713:
710:
705:
701:
697:
690:
687:
677:
671:
668:
663:
659:
655:
651:
644:
641:
636:
632:
628:
624:
617:
614:
609:
605:
601:
597:
593:
589:
582:
579:
574:
570:
566:
562:
558:
554:
550:
546:
539:
536:
531:
527:
523:
519:
515:
508:
505:
500:
496:
491:
486:
482:
478:
474:
470:
466:
462:
458:
451:
448:
441:
437:
434:
432:
429:
427:
424:
422:
419:
418:
414:
412:
410:
405:
401:
397:
393:
388:
386:
382:
378:
368:
365:
362:
359:
358:
354:
351:
348:
345:
344:
340:
337:
334:
331:
330:
326:
323:
320:
317:
316:
312:
309:
306:
303:
302:
296:
294:
290:
286:
278:
276:
274:
266:
260:
256:
254:
250:
246:
242:
238:
234:
230:
226:
222:
218:
214:
210:
206:
202:
198:
190:
184:
180:
176:
172:
168:
163:
159:
158:
157:
155:
151:
146:
144:
140:
135:
131:
127:
122:
120:
116:
112:
108:
104:
100:
96:
92:
76:
72:
64:
60:
56:
52:
48:
40:
36:
32:
19:
1085:Crown ethers
1062:
1020:
1016:
1009:
992:
988:
982:
957:
949:
940:
934:
901:
897:
891:
866:
862:
856:
830:
824:
783:
779:
750:
746:
740:
730:
722:
712:
695:
689:
670:
653:
649:
643:
626:
622:
616:
591:
587:
581:
548:
544:
538:
521:
517:
507:
464:
461:ChemPhysChem
460:
450:
436:Metallacrown
389:
374:
304:Crown Ether
282:
270:
245:alkali metal
199:, who was a
194:
154:halogen bond
147:
123:
98:
94:
90:
51:crown ethers
50:
44:
18:Crown ethers
1063:Nobel Prize
594:(12): 823.
396:amino acids
360:21-crown-7
346:18-crown-6
332:15-crown-5
318:12-crown-4
283:Due to the
217:catecholate
203:working at
150:Lewis acids
143:valinomycin
53:are cyclic
442:References
398:, such as
392:18-crown-6
349:1.34-1.55
335:0.86-0.92
241:polyethers
229:by-product
225:ionization
175:18-crown-6
171:15-crown-5
167:12-crown-4
139:Ionophores
35:18-crown-6
1037:0002-7863
926:0567-7394
883:0002-7863
800:1044-0305
676:GB 785229
481:1439-7641
321:0.6-0.75
237:potassium
233:potassium
195:In 1967,
181:, and an
134:denticity
126:complexes
115:oligomers
71:oligomers
39:potassium
1079:Category
816:36106963
808:24234497
698:: 3767.
608:93153773
573:49589053
565:29953704
499:31282054
431:Cryptand
415:See also
385:rubidium
363:1.7-2.1
221:hydroxyl
141:such as
119:catechol
105:, and a
65:groups (
906:Bibcode
490:6772996
377:lithium
293:acyclic
201:chemist
191:History
1035:
970:
924:
881:
845:
814:
806:
798:
682:
606:
571:
563:
497:
487:
479:
400:lysine
381:sodium
205:DuPont
132:. The
111:oxygen
103:cation
67:R−O−R’
1059:(PDF)
812:S2CID
604:S2CID
569:S2CID
404:amine
369:1.67
355:1.38
341:1.02
327:0.76
107:crown
63:ether
1033:ISSN
968:ISBN
922:ISSN
879:ISSN
843:ISBN
804:PMID
796:ISSN
561:PMID
495:PMID
477:ISSN
287:and
211:for
59:ring
1025:doi
1021:121
997:doi
914:doi
871:doi
835:doi
788:doi
755:doi
700:doi
658:doi
631:doi
596:doi
553:doi
526:doi
485:PMC
469:doi
366:Cs
338:Na
324:Li
79:−CH
73:of
45:In
41:ion
1081::
1061:.
1031:.
1019:.
993:91
991:.
966:.
964:59
920:.
912:.
902:32
900:.
877:.
867:93
865:.
841:,
810:.
802:.
794:.
782:.
767:^
751:28
749:.
728:;
721:.
654:89
652:.
627:89
625:.
602:.
592:26
590:.
567:.
559:.
549:57
547:.
522:92
520:.
516:.
493:.
483:.
475:.
465:20
463:.
459:.
379:,
352:K
251:,
177:,
173:,
169:,
121:.
87:O−
83:CH
49:,
1065:.
1039:.
1027::
1003:.
999::
976:.
928:.
916::
908::
885:.
873::
837::
818:.
790::
784:3
761:.
757::
735:.
706:.
702::
664:.
660::
637:.
633::
610:.
598::
575:.
555::
532:.
528::
501:.
471::
99:n
95:n
91:n
85:2
81:2
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.