162:. There are two different types of substitutional defects: Isovalent substitution and aliovalent substitution. Isovalent substitution is where the ion that is substituting the original ion is of the same oxidation state as the ion it is replacing. Aliovalent substitution is where the ion that is substituting the original ion is of a different oxidation state than the ion it is replacing. Aliovalent substitutions change the overall charge within the ionic compound, but the ionic compound must be neutral. Therefore, a charge compensation mechanism is required. Hence either one of the metals is partially or fully oxidised or reduced, or ion vacancies are created.
1875:
31:
235:
2268:
185:
311:
2280:
134:
158:. The atom is not supposed to be anywhere in the crystal, and is thus an impurity. In some cases where the radius of the substitutional atom (ion) is substantially smaller than that of the atom (ion) it is replacing, its equilibrium position can be shifted away from the lattice site. These types of substitutional defects are often referred to as
207:
Complexes can form between different kinds of point defects. For example, if a vacancy encounters an impurity, the two may bind together if the impurity is too large for the lattice. Interstitials can form 'split interstitial' or 'dumbbell' structures where two atoms effectively share an atomic site,
153:
Due to fundamental limitations of material purification methods, materials are never 100% pure, which by definition induces defects in crystal structure. In the case of an impurity, the atom is often incorporated at a regular atomic site in the crystal structure. This is neither a vacant site nor is
297:
are line defects corresponding to "adding" or "subtracting" an angle around a line. Basically, this means that if you track the crystal orientation around the line defect, you get a rotation. Usually, they were thought to play a role only in liquid crystals, but recent developments suggest that they
246:
Edge dislocations are caused by the termination of a plane of atoms in the middle of a crystal. In such a case, the adjacent planes are not straight, but instead bend around the edge of the terminating plane so that the crystal structure is perfectly ordered on either side. The analogy with a stack
106:
are lattice sites which would be occupied in a perfect crystal, but are vacant. If a neighboring atom moves to occupy the vacant site, the vacancy moves in the opposite direction to the site which used to be occupied by the moving atom. The stability of the surrounding crystal structure guarantees
82:
Point defects are defects that occur only at or around a single lattice point. They are not extended in space in any dimension. Strict limits for how small a point defect is are generally not defined explicitly. However, these defects typically involve at most a few extra or missing atoms. Larger
168:
occur in an ordered alloy or compound when atoms of different type exchange positions. For example, some alloys have a regular structure in which every other atom is a different species; for illustration assume that type A atoms sit on the corners of a cubic lattice, and type B atoms sit in the
107:
that the neighboring atoms will not simply collapse around the vacancy. In some materials, neighboring atoms actually move away from a vacancy, because they experience attraction from atoms in the surroundings. A vacancy (or pair of vacancies in an ionic solid) is sometimes called a
204:) can be considered a defect in silica. Moreover, defects can also be defined in amorphous solids based on empty or densely packed local atomic neighbourhoods, and the properties of such 'defects' can be shown to be similar to normal vacancies and interstitials in crystals.
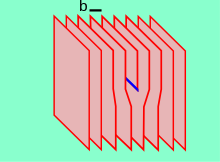
258:(b). For an edge type, b is perpendicular to the dislocation line, whereas in the cases of the screw type it is parallel. In metallic materials, b is aligned with close-packed crystallographic directions and its magnitude is equivalent to one interatomic spacing.
176:) all atoms are in rings containing six atoms. If the sheet contains regions where the number of atoms in a ring is different from six, while the total number of atoms remains the same, a topological defect has formed. An example is the
250:
The screw dislocation is more difficult to visualise, but basically comprises a structure in which a helical path is traced around the linear defect (dislocation line) by the atomic planes of atoms in the crystal lattice.
416:
simulations are widely used to study the properties of defects in solids with computer simulations. Simulating jamming of hard spheres of different sizes and/or in containers with non-commeasurable sizes using the
169:
center of the cubes. If one cube has an A atom at its center, the atom is on a site usually occupied by a B atom, and is thus an antisite defect. This is neither a vacancy nor an interstitial, nor an impurity.
524:
2145:
2140:
360:, steps between atomically flat terraces can also be regarded as planar defects. It has been shown that such defects and their geometry have significant influence on the adsorption of organic molecules
328:
occur in ordered alloys: in this case, the crystallographic direction remains the same, but each side of the boundary has an opposite phase: For example, if the ordering is usually ABABABAB (
2196:
172:
Topological defects are regions in a crystal where the normal chemical bonding environment is topologically different from the surroundings. For instance, in a perfect sheet of graphite (
389:
A successful mathematical classification method for physical lattice defects, which works not only with the theory of dislocations and other defects in crystals but also, e.g., for
464:
Hong, J.; Hu, Z.; Probert, M.; Li, K.; Lv, D.; Yang, X.; Gu, L.; Mao, N.; Feng, Q.; Xie, L.; Zhang, J.; Wu, D.; Zhang, Z.; Jin, C.; Ji, W.; Zhang, X.; Yuan, J.; Zhang, Z. (2015).
264:
It is the presence of dislocations and their ability to readily move (and interact) under the influence of stresses induced by external loads that leads to the characteristic
196:
solids may contain defects. These are naturally somewhat hard to define, but sometimes their nature can be quite easily understood. For instance, in ideally bonded amorphous
521:
940:
640:
Hausmann, H.; Pillukat, A.; Ehrhart, P. (1996). "Point defects and their reactions in electron-irradiated GaAs investigated by optical absorption spectroscopy".
2014:
70:, but this is usually imperfect. Several types of defects are often characterized: point defects, line defects, planar defects, bulk defects. Topological
2201:
1926:
322:
occur where the crystallographic direction of the lattice abruptly changes. This usually occurs when two crystals begin growing separately and then meet.
576:, T. Diaz de la Rubia, S. Coffa, P. A. Stolk, and C. S. Rafferty (eds), vol. 469 of MRS Symposium Proceedings, Materials Research Society, Pittsburgh,
247:
of paper is apt: if a half a piece of paper is inserted in a stack of paper, the defect in the stack is only noticeable at the edge of the half sheet.
2092:
1384:
342:
structures. They are formed by a local deviation of the stacking sequence of layers in a crystal. An example would be the ABABCABAB stacking sequence.
121:. They are generally high energy configurations. Small atoms (mostly impurities) in some crystals can occupy interstices without high energy, such as
2191:
2183:
254:
The presence of dislocation results in lattice strain (distortion). The direction and magnitude of such distortion is expressed in terms of a
2244:
2222:
963:
418:
2237:
2087:
1753:
1618:
1467:
261:
Dislocations can move if the atoms from one of the surrounding planes break their bonds and rebond with the atoms at the terminating edge.
2227:
2125:
1821:
435:
95:. These dislocations permit ionic transport through crystals leading to electrochemical reactions. These are frequently specified using
1474:
2306:
2249:
2107:
2077:
2006:
1356:
1339:
581:
284:
1959:
1075:
Cai, W.; Bulatov, V. V.; Justo, J. F.; Argon, A.S; Yip, S. (2000). "Intrinsic mobility of a dissociated dislocation in silicon".
272:
223:
are linear defects, around which the atoms of the crystal lattice are misaligned. There are two basic types of dislocations, the
1278:
Stillinger, Frank H.; Lubachevsky, Boris D. (1995). "Patterns of broken symmetry in the impurity-perturbed rigid-disk crystal".
729:
Ashkenazy, Yinon; Averback, Robert S. (2012). "Irradiation
Induced Grain Boundary Flow—A New Creep Mechanism at the Nanoscale".
2232:
2155:
2029:
1628:
821:
Nordlund, K; Ashkenazy, Y; Averback, R. S; Granato, A. V (2005). "Strings and interstitials in liquids, glasses and crystals".
775:
Mayr, S.; Ashkenazy, Y.; Albe, K.; Averback, R. (2003). "Mechanisms of radiation-induced viscous flow: Role of point defects".
1989:
2067:
901:"Control of defect binding and magnetic interaction energies in dilute magnetic semiconductors by charge state manipulation"
200:
all Si atoms have 4 bonds to O atoms and all O atoms have 2 bonds to Si atom. Thus e.g. an O atom with only one Si bond (a
2082:
2072:
1377:
997:
Waldmann, T. (2012). "The role of surface defects in large organic molecule adsorption: substrate configuration effects".
1854:
2206:
1479:
1457:
1884:
1758:
1204:
Nordlund, K.; Averback, R. (1998). "The role of self-interstitial atoms on the high temperature properties of metals".
440:
96:
1512:
1407:
1874:
2284:
2115:
1412:
2130:
2059:
1517:
1507:
405:
1643:
2272:
1996:
1892:
1765:
1728:
1522:
1502:
1370:
1680:
1239:
Sadigh, B; Lenosky, Thomas; Theiss, Silva; Caturla, Maria-Jose; Diaz De La Rubia, Tomas; Foad, Majeed (1999).
541:
2120:
1964:
1909:
1623:
1816:
1633:
329:
1738:
2173:
1969:
1931:
1690:
1424:
934:
62:. The positions and orientations of particles, which are repeating at fixed distances determined by the
1331:
979:
1897:
1770:
1606:
1497:
1287:
1252:
1213:
1178:
1131:
1084:
1049:
1006:
912:
873:
830:
784:
738:
692:
649:
606:
477:
349:
is a defect that introduces a plane of mirror symmetry in the ordering of a crystal. For example, in
276:
39:
572:
Watkins, G. D. (1997) "Native defects and their interactions with impurities in silicon", p. 139 in
373:
Voids — small regions where there are no atoms, and which can be thought of as clusters of vacancies
1914:
1902:
1777:
1743:
1723:
1344:
413:
376:
Impurities can cluster together to form small regions of a different phase. These are often called
114:
34:
Electron microscopy of antisites (a, Mo substitutes for S) and vacancies (b, missing S atoms) in a
146:
or
Frenkel pair. This is caused when an ion moves into an interstitial site and creates a vacancy.
2163:
1974:
1919:
1462:
1303:
846:
708:
409:
350:
177:
91:: for example a vacancy in many ionic solids is called a luminescence center, a color center, or
1167:"Convergence of supercell calculations for point defects in semiconductors: vacancy in silicon"
2097:
1936:
1864:
1844:
1564:
1434:
1352:
1335:
1147:
1100:
1022:
959:
800:
754:
665:
622:
577:
503:
325:
118:
67:
63:
287:
has been used for studying the electrical activity of dislocations in semiconductors, mainly
2135:
1941:
1859:
1849:
1648:
1581:
1552:
1545:
1295:
1260:
1221:
1186:
1139:
1092:
1057:
1014:
920:
881:
838:
792:
746:
700:
657:
614:
493:
485:
87:
loops. For historical reasons, many point defects, especially in ionic crystals, are called
30:
2024:
2019:
1984:
1804:
1703:
1638:
1601:
1596:
1447:
1393:
159:
108:
1291:
1256:
1217:
1182:
1135:
1088:
1053:
1010:
916:
877:
834:
788:
742:
696:
653:
610:
481:
421:
can be an effective technique for demonstrating some types of crystallographic defects.
1834:
1799:
1787:
1782:
1748:
1718:
1708:
1667:
1611:
1535:
1489:
1323:
683:
Lieb, Klaus-Peter; Keinonen, Juhani (2006). "Luminescence of ion-irradiated α-quartz".
498:
465:
430:
357:
335:
319:
255:
143:
103:
231:
dislocation. "Mixed" dislocations, combining aspects of both types, are also common.
180:
in nanotubes, which consists of two adjacent 5-membered and two 7-membered atom rings.
2300:
1979:
1792:
1591:
1241:"Mechanism of Boron Diffusion in Silicon: An Ab Initio and Kinetic Monte Carlo Study"
850:
712:
528:
346:
339:
201:
1307:
531:, volume 25 of Landolt-Börnstein, New Series III, chapter 2, p. 88, Springer, Berlin
1685:
1675:
1569:
1452:
1166:
1119:
594:
390:
370:
Three-dimensional macroscopic or bulk defects, such as pores, cracks, or inclusions
294:
265:
242:
is shown. The dislocation line is presented in blue, the
Burgers vector b in black.
234:
796:
2168:
1839:
1713:
1540:
1264:
1225:
1096:
842:
618:
377:
314:
Origin of stacking faults: Different stacking sequences of close-packed crystals
220:
188:
Schematic illustration of defects in a compound solid, using GaAs as an example.
84:
885:
298:
might have a role also in solid materials, e.g. leading to the self-healing of
1733:
1419:
1061:
704:
280:
184:
17:
1190:
1143:
661:
137:
Schematic illustration of some simple point defect types in a monatomic solid
1442:
393:
in liquid crystals and for excitations in superfluid He, is the topological
310:
193:
126:
35:
1104:
1026:
804:
758:
626:
507:
1151:
669:
2039:
1809:
1557:
1040:
Mermin, N. (1979). "The topological theory of defects in ordered media".
445:
394:
299:
173:
122:
92:
71:
55:
353:
crystals, the stacking sequence of a twin boundary would be ABCABCBACBA.
133:
2049:
1299:
1018:
489:
288:
59:
925:
900:
750:
338:
occur in a number of crystal structures, but the common example is in
197:
1240:
864:
Hannes
Raebiger (2010). "Theory of defect complexes in insulators".
1334:, pp. 743–1456, World Scientific (Singapore, 1989); Paperback
2044:
1362:
522:
Properties and interactions of atomic defects in metals and alloys
309:
233:
183:
132:
29:
142:
A nearby pair of a vacancy and an interstitial is often called a
51:
1366:
117:
are atoms that occupy a site in the crystal structure at which
1120:"Vacancy formation energies for fcc and bcc transition metals"
466:"Exploring atomic defects in molybdenum disulphide monolayers"
899:
Hannes
Raebiger, Hikaru Nakayama, and Takeshi Fujita (2014).
50:
is an interruption of the regular patterns of arrangement of
1165:
Puska, M. J.; Pöykkö, S.; Pesola, M.; Nieminen, R. (1998).
595:"Direct Antisite Formation in Electron Irradiation of GaAs"
332:
crystal), an antiphase boundary takes the form of ABABBABA.
2146:
2141:
548:, J.-I. Takamura (ED.), p. 783, North Holland, Amsterdam
83:
defects in an ordered structure are usually considered
74:
establishes a mathematical method of characterization.
2034:
208:
resulting in neither atom actually occupying the site.
154:
the atom on an interstitial site and it is called a
2215:
2182:
2154:
2106:
2058:
2005:
1952:
1883:
1666:
1657:
1580:
1488:
1433:
1400:
27:Disruption of the periodicity of a crystal lattice
217:Line defects can be described by gauge theories.
546:Point Defects and Defect Interactions in Metals
558:Crawford, J. H.; Slifkin, L. M., eds. (1975).
1378:
1118:Korhonen, T; Puska, M.; Nieminen, R. (1995).
8:
939:: CS1 maint: multiple names: authors list (
574:Defects and Diffusion in Silicon Processing
66:parameters in crystals, exhibit a periodic
2212:
1663:
1485:
1430:
1385:
1371:
1363:
924:
497:
816:
814:
770:
768:
724:
722:
456:
932:
542:Atomic Defects and Diffusion in Metals
7:
2279:
1619:Phase transformation crystallography
2126:Journal of Chemical Crystallography
999:Physical Chemistry Chemical Physics
436:Crystallographic defects in diamond
385:Mathematical classification methods
271:Dislocations can be observed using
25:
593:Mattila, T; Nieminen, RM (1995).
285:Deep-level transient spectroscopy
2278:
2267:
2266:
1873:
1351:. Verlag Chemie, Weinheim 1981,
1328:Gauge Fields in Condensed Matter
954:Hirth, J. P.; Lothe, J. (1992).
419:Lubachevsky–Stillinger algorithm
273:transmission electron microscopy
2068:Bilbao Crystallographic Server
1280:Journal of Statistical Physics
958:(2 ed.). Krieger Pub Co.
1:
797:10.1103/PhysRevLett.90.055505
982:Cracked metal, heal thyself,
119:there is usually not an atom
2116:Crystal Growth & Design
1408:Timeline of crystallography
1265:10.1103/PhysRevLett.83.4341
1226:10.1103/PhysRevLett.80.4201
1097:10.1103/PhysRevLett.84.3346
619:10.1103/PhysRevLett.74.2721
401:Computer simulation methods
2323:
1927:Nuclear magnetic resonance
984:MIT news, October 9, 2013"
905:Journal of Applied Physics
886:10.1103/PhysRevB.82.073104
2262:
2131:Journal of Crystal Growth
1871:
1062:10.1103/RevModPhys.51.591
1042:Reviews of Modern Physics
843:10.1209/epl/i2005-10132-1
705:10.1080/00107510601088156
562:. New York: Plenum Press.
406:Density functional theory
2307:Crystallographic defects
1997:Single particle analysis
1855:Hermann–Mauguin notation
1191:10.1103/PhysRevB.58.1318
1144:10.1103/PhysRevB.51.9526
662:10.1103/PhysRevB.54.8527
2121:Crystallography Reviews
1965:Isomorphous replacement
1759:Lomer–Cottrell junction
599:Physical Review Letters
560:Point Defects in Solids
268:of metallic materials.
48:crystallographic defect
1634:Spinodal decomposition
1332:"Stresses and defects"
956:Theory of dislocations
330:hexagonal close-packed
315:
243:
189:
138:
43:
2174:Gregori Aminoff Prize
1970:Molecular replacement
1349:Solid State Reactions
980:"Chandler, David L.,
540:Siegel, R. W. (1982)
470:Nature Communications
313:
237:
187:
156:substitutional defect
136:
33:
1480:Structure prediction
685:Contemporary Physics
441:Kröger–Vink notation
326:Antiphase boundaries
277:field ion microscopy
227:dislocation and the
115:Interstitial defects
97:Kröger–Vink notation
40:molybdenum disulfide
1744:Cottrell atmosphere
1724:Partial dislocation
1468:Restriction theorem
1345:Hermann Schmalzried
1292:1995JSP....78.1011S
1257:1999PhRvL..83.4341S
1218:1998PhRvL..80.4201N
1183:1998PhRvB..58.1318P
1136:1995PhRvB..51.9526K
1089:2000PhRvL..84.3346C
1054:1979RvMP...51..591M
1011:2012PCCP...1410726W
917:2014JAP...115a2008R
878:2010PhRvB..82g3104R
835:2005EL.....71..625N
789:2003PhRvL..90e5505M
743:2012NanoL..12.4084A
697:2006ConPh..47..305L
654:1996PhRvB..54.8527H
611:1995PhRvL..74.2721M
520:Ehrhart, P. (1991)
482:2015NatCo...6.6293H
414:kinetic Monte Carlo
2164:Carl Hermann Medal
1975:Molecular dynamics
1822:Defects in diamond
1817:Stone–Wales defect
1463:Reciprocal lattice
1425:Biocrystallography
1300:10.1007/BF02183698
1286:(3–4): 1011–1026.
1019:10.1039/C2CP40800G
490:10.1038/ncomms7293
410:molecular dynamics
351:cubic close-packed
316:
244:
190:
178:Stone Wales defect
139:
60:crystalline solids
44:
42:. Scale bar: 1 nm.
2294:
2293:
2258:
2257:
1865:Thermal ellipsoid
1830:
1829:
1739:Frank–Read source
1699:
1698:
1565:Aperiodic crystal
1531:
1530:
1413:Crystallographers
1251:(21): 4341–4344.
1212:(19): 4201–4204.
1130:(15): 9526–9532.
1083:(15): 3346–3349.
965:978-0-89464-617-1
926:10.1063/1.4838016
866:Physical Review B
751:10.1021/nl301554k
648:(12): 8527–8539.
642:Physical Review B
605:(14): 2721–2724.
68:crystal structure
16:(Redirected from
2314:
2282:
2281:
2270:
2269:
2213:
2136:Kristallografija
1990:Gerchberg–Saxton
1885:Characterisation
1877:
1860:Structure factor
1664:
1649:Ostwald ripening
1486:
1431:
1387:
1380:
1373:
1364:
1312:
1311:
1275:
1269:
1268:
1236:
1230:
1229:
1201:
1195:
1194:
1177:(3): 1318–1325.
1162:
1156:
1155:
1115:
1109:
1108:
1072:
1066:
1065:
1037:
1031:
1030:
1005:(30): 10726–31.
994:
988:
987:
976:
970:
969:
951:
945:
944:
938:
930:
928:
896:
890:
889:
861:
855:
854:
818:
809:
808:
772:
763:
762:
726:
717:
716:
680:
674:
673:
637:
631:
630:
590:
584:
570:
564:
563:
555:
549:
538:
532:
518:
512:
511:
501:
461:
320:Grain boundaries
240:edge dislocation
166:Antisite defects
21:
2322:
2321:
2317:
2316:
2315:
2313:
2312:
2311:
2297:
2296:
2295:
2290:
2254:
2211:
2178:
2150:
2102:
2054:
2025:CrystalExplorer
2001:
1985:Phase retrieval
1948:
1879:
1878:
1869:
1826:
1805:Schottky defect
1704:Perfect crystal
1695:
1691:Abnormal growth
1653:
1639:Supersaturation
1602:Miscibility gap
1583:
1576:
1527:
1484:
1448:Bravais lattice
1429:
1396:
1394:Crystallography
1391:
1320:
1318:Further reading
1315:
1277:
1276:
1272:
1245:Phys. Rev. Lett
1238:
1237:
1233:
1206:Phys. Rev. Lett
1203:
1202:
1198:
1164:
1163:
1159:
1117:
1116:
1112:
1077:Phys. Rev. Lett
1074:
1073:
1069:
1039:
1038:
1034:
996:
995:
991:
978:
977:
973:
966:
953:
952:
948:
931:
898:
897:
893:
863:
862:
858:
820:
819:
812:
777:Phys. Rev. Lett
774:
773:
766:
728:
727:
720:
682:
681:
677:
639:
638:
634:
592:
591:
587:
571:
567:
557:
556:
552:
539:
535:
519:
515:
463:
462:
458:
454:
427:
403:
387:
367:
358:single crystals
336:Stacking faults
308:
215:
160:off-center ions
109:Schottky defect
104:Vacancy defects
80:
28:
23:
22:
15:
12:
11:
5:
2320:
2318:
2310:
2309:
2299:
2298:
2292:
2291:
2289:
2288:
2276:
2263:
2260:
2259:
2256:
2255:
2253:
2252:
2247:
2242:
2241:
2240:
2235:
2230:
2219:
2217:
2210:
2209:
2204:
2199:
2194:
2188:
2186:
2180:
2179:
2177:
2176:
2171:
2166:
2160:
2158:
2152:
2151:
2149:
2148:
2143:
2138:
2133:
2128:
2123:
2118:
2112:
2110:
2104:
2103:
2101:
2100:
2095:
2090:
2085:
2080:
2075:
2070:
2064:
2062:
2056:
2055:
2053:
2052:
2047:
2042:
2037:
2032:
2027:
2022:
2017:
2011:
2009:
2003:
2002:
2000:
1999:
1994:
1993:
1992:
1982:
1977:
1972:
1967:
1962:
1960:Direct methods
1956:
1954:
1950:
1949:
1947:
1946:
1945:
1944:
1939:
1929:
1924:
1923:
1922:
1917:
1907:
1906:
1905:
1900:
1889:
1887:
1881:
1880:
1872:
1870:
1868:
1867:
1862:
1857:
1852:
1847:
1845:Ewald's sphere
1842:
1837:
1831:
1828:
1827:
1825:
1824:
1819:
1814:
1813:
1812:
1807:
1797:
1796:
1795:
1790:
1788:Frenkel defect
1785:
1783:Bjerrum defect
1775:
1774:
1773:
1763:
1762:
1761:
1756:
1751:
1749:Peierls stress
1746:
1741:
1736:
1731:
1726:
1721:
1719:Burgers vector
1711:
1709:Stacking fault
1706:
1700:
1697:
1696:
1694:
1693:
1688:
1683:
1678:
1672:
1670:
1668:Grain boundary
1661:
1655:
1654:
1652:
1651:
1646:
1641:
1636:
1631:
1626:
1621:
1616:
1615:
1614:
1612:Liquid crystal
1609:
1604:
1599:
1588:
1586:
1578:
1577:
1575:
1574:
1573:
1572:
1562:
1561:
1560:
1550:
1549:
1548:
1543:
1532:
1529:
1528:
1526:
1525:
1520:
1515:
1510:
1505:
1500:
1494:
1492:
1483:
1482:
1477:
1475:Periodic table
1472:
1471:
1470:
1465:
1460:
1455:
1450:
1439:
1437:
1428:
1427:
1422:
1417:
1416:
1415:
1404:
1402:
1398:
1397:
1392:
1390:
1389:
1382:
1375:
1367:
1361:
1360:
1342:
1324:Hagen Kleinert
1319:
1316:
1314:
1313:
1270:
1231:
1196:
1157:
1110:
1067:
1048:(3): 591–648.
1032:
989:
971:
964:
946:
891:
856:
829:(4): 625–631.
823:Europhys. Lett
810:
764:
718:
691:(5): 305–331.
675:
632:
585:
565:
550:
533:
527:2013-02-03 at
513:
455:
453:
450:
449:
448:
443:
438:
433:
431:Bjerrum defect
426:
423:
402:
399:
386:
383:
382:
381:
374:
371:
366:
363:
362:
361:
356:On planes of
354:
343:
333:
323:
307:
306:Planar defects
304:
256:Burgers vector
214:
211:
210:
209:
205:
182:
181:
170:
163:
148:
147:
144:Frenkel defect
131:
130:
112:
79:
76:
26:
24:
18:Crystal defect
14:
13:
10:
9:
6:
4:
3:
2:
2319:
2308:
2305:
2304:
2302:
2287:
2286:
2277:
2275:
2274:
2265:
2264:
2261:
2251:
2248:
2246:
2243:
2239:
2236:
2234:
2231:
2229:
2226:
2225:
2224:
2221:
2220:
2218:
2214:
2208:
2205:
2203:
2200:
2198:
2195:
2193:
2190:
2189:
2187:
2185:
2181:
2175:
2172:
2170:
2167:
2165:
2162:
2161:
2159:
2157:
2153:
2147:
2144:
2142:
2139:
2137:
2134:
2132:
2129:
2127:
2124:
2122:
2119:
2117:
2114:
2113:
2111:
2109:
2105:
2099:
2096:
2094:
2091:
2089:
2086:
2084:
2081:
2079:
2076:
2074:
2071:
2069:
2066:
2065:
2063:
2061:
2057:
2051:
2048:
2046:
2043:
2041:
2038:
2036:
2033:
2031:
2028:
2026:
2023:
2021:
2018:
2016:
2013:
2012:
2010:
2008:
2004:
1998:
1995:
1991:
1988:
1987:
1986:
1983:
1981:
1980:Patterson map
1978:
1976:
1973:
1971:
1968:
1966:
1963:
1961:
1958:
1957:
1955:
1951:
1943:
1940:
1938:
1935:
1934:
1933:
1930:
1928:
1925:
1921:
1918:
1916:
1913:
1912:
1911:
1908:
1904:
1901:
1899:
1896:
1895:
1894:
1891:
1890:
1888:
1886:
1882:
1876:
1866:
1863:
1861:
1858:
1856:
1853:
1851:
1850:Friedel's law
1848:
1846:
1843:
1841:
1838:
1836:
1833:
1832:
1823:
1820:
1818:
1815:
1811:
1808:
1806:
1803:
1802:
1801:
1798:
1794:
1793:Wigner effect
1791:
1789:
1786:
1784:
1781:
1780:
1779:
1778:Interstitials
1776:
1772:
1769:
1768:
1767:
1764:
1760:
1757:
1755:
1752:
1750:
1747:
1745:
1742:
1740:
1737:
1735:
1732:
1730:
1727:
1725:
1722:
1720:
1717:
1716:
1715:
1712:
1710:
1707:
1705:
1702:
1701:
1692:
1689:
1687:
1684:
1682:
1679:
1677:
1674:
1673:
1671:
1669:
1665:
1662:
1660:
1656:
1650:
1647:
1645:
1642:
1640:
1637:
1635:
1632:
1630:
1627:
1625:
1624:Precipitation
1622:
1620:
1617:
1613:
1610:
1608:
1605:
1603:
1600:
1598:
1595:
1594:
1593:
1592:Phase diagram
1590:
1589:
1587:
1585:
1579:
1571:
1568:
1567:
1566:
1563:
1559:
1556:
1555:
1554:
1551:
1547:
1544:
1542:
1539:
1538:
1537:
1534:
1533:
1524:
1521:
1519:
1516:
1514:
1511:
1509:
1506:
1504:
1501:
1499:
1496:
1495:
1493:
1491:
1487:
1481:
1478:
1476:
1473:
1469:
1466:
1464:
1461:
1459:
1456:
1454:
1451:
1449:
1446:
1445:
1444:
1441:
1440:
1438:
1436:
1432:
1426:
1423:
1421:
1418:
1414:
1411:
1410:
1409:
1406:
1405:
1403:
1399:
1395:
1388:
1383:
1381:
1376:
1374:
1369:
1368:
1365:
1358:
1357:3-527-25872-8
1354:
1350:
1346:
1343:
1341:
1340:9971-5-0210-0
1337:
1333:
1329:
1325:
1322:
1321:
1317:
1309:
1305:
1301:
1297:
1293:
1289:
1285:
1281:
1274:
1271:
1266:
1262:
1258:
1254:
1250:
1246:
1242:
1235:
1232:
1227:
1223:
1219:
1215:
1211:
1207:
1200:
1197:
1192:
1188:
1184:
1180:
1176:
1172:
1168:
1161:
1158:
1153:
1149:
1145:
1141:
1137:
1133:
1129:
1125:
1121:
1114:
1111:
1106:
1102:
1098:
1094:
1090:
1086:
1082:
1078:
1071:
1068:
1063:
1059:
1055:
1051:
1047:
1043:
1036:
1033:
1028:
1024:
1020:
1016:
1012:
1008:
1004:
1000:
993:
990:
985:
983:
975:
972:
967:
961:
957:
950:
947:
942:
936:
927:
922:
918:
914:
911:(1): 012008.
910:
906:
902:
895:
892:
887:
883:
879:
875:
872:(7): 073104.
871:
867:
860:
857:
852:
848:
844:
840:
836:
832:
828:
824:
817:
815:
811:
806:
802:
798:
794:
790:
786:
783:(5): 055505.
782:
778:
771:
769:
765:
760:
756:
752:
748:
744:
740:
737:(8): 4084–9.
736:
732:
725:
723:
719:
714:
710:
706:
702:
698:
694:
690:
686:
679:
676:
671:
667:
663:
659:
655:
651:
647:
643:
636:
633:
628:
624:
620:
616:
612:
608:
604:
600:
596:
589:
586:
583:
582:1-55899-373-8
579:
575:
569:
566:
561:
554:
551:
547:
543:
537:
534:
530:
529:archive.today
526:
523:
517:
514:
509:
505:
500:
495:
491:
487:
483:
479:
475:
471:
467:
460:
457:
451:
447:
444:
442:
439:
437:
434:
432:
429:
428:
424:
422:
420:
415:
411:
407:
400:
398:
396:
392:
391:disclinations
384:
379:
375:
372:
369:
368:
364:
359:
355:
352:
348:
347:twin boundary
344:
341:
337:
334:
331:
327:
324:
321:
318:
317:
312:
305:
303:
301:
296:
295:Disclinations
292:
290:
286:
282:
278:
274:
269:
267:
262:
259:
257:
252:
248:
241:
236:
232:
230:
226:
222:
218:
212:
206:
203:
202:dangling bond
199:
195:
192:
191:
186:
179:
175:
171:
167:
164:
161:
157:
152:
151:
150:
145:
141:
140:
135:
128:
124:
120:
116:
113:
110:
105:
102:
101:
100:
98:
94:
90:
86:
78:Point defects
77:
75:
73:
69:
65:
61:
57:
53:
49:
41:
37:
32:
19:
2283:
2271:
2216:Associations
2184:Organisation
1676:Disclination
1658:
1607:Polymorphism
1570:Quasicrystal
1513:Orthorhombic
1453:Miller index
1401:Key concepts
1348:
1327:
1283:
1279:
1273:
1248:
1244:
1234:
1209:
1205:
1199:
1174:
1171:Phys. Rev. B
1170:
1160:
1127:
1124:Phys. Rev. B
1123:
1113:
1080:
1076:
1070:
1045:
1041:
1035:
1002:
998:
992:
981:
974:
955:
949:
935:cite journal
908:
904:
894:
869:
865:
859:
826:
822:
780:
776:
734:
731:Nano Letters
730:
688:
684:
678:
645:
641:
635:
602:
598:
588:
573:
568:
559:
553:
545:
536:
516:
473:
469:
459:
408:, classical
404:
388:
378:precipitates
365:Bulk defects
340:close-packed
293:
283:techniques.
270:
266:malleability
263:
260:
253:
249:
245:
239:
228:
224:
221:Dislocations
219:
216:
213:Line defects
165:
155:
149:
88:
81:
47:
45:
2169:Ewald Prize
1937:Diffraction
1915:Diffraction
1898:Diffraction
1840:Bragg plane
1835:Bragg's law
1714:Dislocation
1629:Segregation
1541:Crystallite
1458:Point group
1330:, Vol. II,
85:dislocation
1953:Algorithms
1942:Scattering
1920:Scattering
1903:Scattering
1771:Slip bands
1734:Cross slip
1584:transition
1518:Tetragonal
1508:Monoclinic
1420:Metallurgy
452:References
281:atom probe
2060:Databases
1523:Triclinic
1503:Hexagonal
1443:Unit cell
1435:Structure
851:250805987
713:119348046
194:Amorphous
127:palladium
64:unit cell
56:molecules
36:monolayer
2301:Category
2273:Category
2108:Journals
2040:OctaDist
2035:JANA2020
2007:Software
1893:Electron
1810:F-center
1597:Eutectic
1558:Fiveling
1553:Twinning
1546:Equiaxed
1308:55943037
1105:11019086
1027:22751288
805:12633371
759:22775230
627:10058001
525:Archived
508:25695374
476:: 6293.
446:F-center
425:See also
397:theory.
395:homotopy
174:graphene
123:hydrogen
93:F-center
72:homotopy
2285:Commons
2233:Germany
1910:Neutron
1800:Vacancy
1659:Defects
1644:GP-zone
1490:Systems
1288:Bibcode
1253:Bibcode
1214:Bibcode
1179:Bibcode
1152:9977614
1132:Bibcode
1085:Bibcode
1050:Bibcode
1007:Bibcode
913:Bibcode
874:Bibcode
831:Bibcode
785:Bibcode
739:Bibcode
693:Bibcode
670:9984528
650:Bibcode
607:Bibcode
499:4346634
478:Bibcode
289:silicon
89:centers
2228:France
2223:Europe
2156:Awards
1686:Growth
1536:Growth
1355:
1338:
1306:
1150:
1103:
1025:
962:
849:
803:
757:
711:
668:
625:
580:
506:
496:
300:cracks
198:silica
2250:Japan
2197:IOBCr
2050:SHELX
2045:Olex2
1932:X-ray
1582:Phase
1498:Cubic
1304:S2CID
847:S2CID
709:S2CID
544:, in
229:screw
52:atoms
2192:IUCr
2093:ICDD
2088:ICSD
2073:CCDC
2020:Coot
2015:CCP4
1766:Slip
1729:Kink
1353:ISBN
1336:ISBN
1148:PMID
1101:PMID
1023:PMID
960:ISBN
941:link
801:PMID
755:PMID
666:PMID
623:PMID
578:ISBN
504:PMID
412:and
279:and
225:edge
2207:DMG
2202:RAS
2098:PDB
2083:COD
2078:CIF
2030:DSR
1754:GND
1681:CSL
1296:doi
1261:doi
1222:doi
1187:doi
1140:doi
1093:doi
1058:doi
1015:doi
921:doi
909:115
882:doi
839:doi
793:doi
747:doi
701:doi
658:doi
615:doi
494:PMC
486:doi
238:An
125:in
58:in
54:or
38:of
2303::
2245:US
2238:UK
1347::
1326:,
1302:.
1294:.
1284:78
1282:.
1259:.
1249:83
1247:.
1243:.
1220:.
1210:80
1208:.
1185:.
1175:58
1173:.
1169:.
1146:.
1138:.
1128:51
1126:.
1122:.
1099:.
1091:.
1081:84
1079:.
1056:.
1046:51
1044:.
1021:.
1013:.
1003:14
1001:.
937:}}
933:{{
919:.
907:.
903:.
880:.
870:82
868:.
845:.
837:.
827:71
825:.
813:^
799:.
791:.
781:90
779:.
767:^
753:.
745:.
735:12
733:.
721:^
707:.
699:.
689:47
687:.
664:.
656:.
646:54
644:.
621:.
613:.
603:74
601:.
597:.
502:.
492:.
484:.
472:.
468:.
345:A
302:.
291:.
275:,
99:.
46:A
1386:e
1379:t
1372:v
1359:.
1310:.
1298::
1290::
1267:.
1263::
1255::
1228:.
1224::
1216::
1193:.
1189::
1181::
1154:.
1142::
1134::
1107:.
1095::
1087::
1064:.
1060::
1052::
1029:.
1017::
1009::
986:.
968:.
943:)
929:.
923::
915::
888:.
884::
876::
853:.
841::
833::
807:.
795::
787::
761:.
749::
741::
715:.
703::
695::
672:.
660::
652::
629:.
617::
609::
510:.
488::
480::
474:6
380:.
129:.
111:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.