895:
1760:
883:
1959:. The procedure begins actually with redox chemistry at the protected phosphorus atom. A tricoordinate phosphorus, used on account of the high reactivity, is tagged with a cyanoethyl protecting group on a free oxygen. After the coupling step follows an oxidation to phosphate, whereby the protecting group stays attached. Free OH-groups, which did not react in the coupling step, are acetylated in an intermediate step. These additionally-introduced protecting groups then inhibit, that these OH-groups might couple in the next cycle.
447:
1652:
1805:
1723:
1819:
407:
1433:
540:
247:
1868:
33:
168:
266:
1925:
1284:
1708:
400:
1276:
513:
869:
1963:
1906:
235:
regarding the reaction conditions such as reaction time, temperature and reagents can be standardized so that they are carried out by a machine, while yields of well over 99% can be achieved. Otherwise, the separation of the resulting mixture of reaction products is virtually impossible (see also
1488:
Many groups can suffice for the alcoholic component, and the specific cleaving conditions are contrariwise generally quite similar: each ester can be hydrolyzed in a basic water-alcohol solution. Instead, most ester protecting groups vary in how mildly they can be formed from the original acid.
3039:
András Lipták, János Imre, János
Harangi, Pál Nánási, András Neszmélyi: "Chemo-, stereo- and regioselective hydrogenolysis of carbohydrate benzylidene acetals. Synthesis of benzyl ethers of benzyl α-D-, methyl β-D-mannopyranosides and benzyl α-D-rhamnopyranoside by ring cleavage of benzylidene
1647:: the transformation of an alkene with a diene leads to a cyclic alkene, which is nevertheless similarly endangered by electrophilic attack as the original alkene. The cleavage of a protecting diene proceeds thermically, for the Diels-Alder reaction is a reversible (equilibrium) reaction.
3535:
John N. Haseltine, Maria Paz Cabal, Nathan B. Mantlo, Nobuharu
Iwasawa, Dennis S. Yamashita, Robert S. Coleman, Samuel J. Danishefsky, Gayle K. Schulte: "Total synthesis of calicheamicinone: new arrangements for actuation of the reductive cycloaromatization of aglycon congeners", in:
2292:
Morris J. Robins, Vicente Samano, Mark D. Johnson: "Nucleic acid-related compounds. 58. Periodinane oxidation, selective primary deprotection, and remarkably stereoselective reduction of tert-butyldimethylsilyl-protected ribonucleosides. Synthesis of 9-(β-D-xylofuranosyl)adenine or
1793:
does not employ protective groups. As an alternative, Baran presented a novel protective-group free synthesis of the compound hapalindole U. The previously published synthesis according to Baran, contained 20 steps with multiple protective group manipulations (two confirmed):
1321:
Cyclic acetals are very much more stable against acid hydrolysis than acyclic acetals. Consequently acyclic acetals are used practically only when a very mild cleavage is required or when two different protected carbonyl groups must be differentiated in their liberation.
158:
As a rule, the introduction of a protecting group is straightforward. The difficulties honestly lie in their stability and in selective removal. Apparent problems in synthesis strategies with protecting groups are rarely documented in the academic literature.
2768:
Karel F. Bernady, M. Brawner Floyd, John F. Poletto, Martin J. Weiss: "Prostaglandins and congeners. 20. Synthesis of prostaglandins via conjugate addition of lithium trans-1-alkenyltrialkylalanate reagents. A novel reagent for conjugate 1,4-additions", in:
1955:‑butyl group (acidic cleavage) and diverse protecting groups for functional groups on the amino acid side-chains are used. Up to four different protecting groups per nucleobase are used for the automated synthesis of DNA and RNA sequences in the
118:
Protecting groups are more common in small-scale laboratory work and initial development than in industrial production because they add additional steps and material costs. However, compounds with repetitive functional groups – generally,
1898:'s research group. Here 42 functional groups (39 hydroxyls, one diol, an amine group, and a carboxylic acid) required protection. These proceeded through 8 different protecting groups (a methyl ester, five acetals, 20 TBDMS esters, nine
3630:
Yves Rubin, Carolyn B. Knobler, Francois
Diederich: "Precursors to the cyclocarbons: from 3,4-dialkynyl-3-cyclobutene-1,2-diones and 3,4-dialkynyl-3-cyclobutene-1,2-diols to cyclobutenodehydroannulenes and higher oxides of carbon", in:
1770:
for deprotection of the right-side ester group is reduced and it stays intact. Significantly by placing the deuterium atoms next to the left-side ester group or by changing the wavelength to 254 nm the other monoarene is obtained.
2374:
Leo A. Paquette, Annette M. Doherty, Christopher M. Rayner: "Total synthesis of furanocembranolides. 1. Stereocontrolled preparation of key heterocyclic building blocks and assembly of a complete seco-pseudopterane framework", in:
4191:
Martin
Banwell, David Hockless, Bevyn Jarrott, Brian Kelly, Andrew Knill, Robert Longmore, Gregory Simpson: "Chemoenzymatic approaches to the decahydro-as-indacene cores associated with the spinosyn class of insecticide", in:
1700:) and subsequently reaction with chlorotrimethylsilane to a terminally TMS-protected alkyne. Cleavage follows hydrolytically – with potassium carbonate in methanol – or with fluoride ions like for example with
3011:
R.E. Ireland, D.W. Norbeck: "Convergent synthesis of polyether ionophore antibiotics: the synthesis of the monensin bis(tetrahydrofuran) via the
Claisen rearrangement of an ester enolate with a β-leaving group", in:
1255:
The most common protecting groups for carbonyls are acetals and typically cyclic acetals with diols. The runners-up used are also cyclic acetals with 1,2‑hydroxythiols or dithioglycols – the so-called
1687:
For alkynes there are in any case two types of protecting groups. For terminal alkynes it is sometimes important to mask the acidic hydrogen atom. This normally proceeds from deprotonation (via a strong base like
1780:
The use of protective groups is pervasive but not without criticism. In practical terms their use adds two steps (protection-deprotection sequence) to a synthesis, either or both of which can dramatically lower
352:; thus silicon protecting groups are almost invariably removed by fluoride ions. Each type of counterion, i.e. cleavage reagent, can also selectively cleave different silicon protecting groups depending on
535:
Aliphatic methyl ethers cleave with difficulty and only under drastic conditions, so that these are in general only used with quinonic phenols. However, hemiacetals and acetals are much easier to cleave.
102:
groups, and cannot be discouraged by any means. When an ester must be reduced in the presence of a carbonyl, hydride attack on the carbonyl must be prevented. One way to do so converts the carbonyl into an
4163:
Hideyuki Tanaka, Takashi
Kamikubo, Naoyuki Yoshida, Hideki Sakagami, Takahiko Taniguchi, Kunio Ogasawara: "Enantio- and Diastereocontrolled Synthesis of (−)-Iridolactone and (+)-Pedicularis-lactone", in:
709: — Comparable stability to MOM, MEM und SEM, but also admits reductive removal: sodium in liquid ammonia, catalytic hydrogenation (palladium hydroxide on activated carbon), or Raney nickel in ethanol
2149:
Tod K Jones, Robert A. Reamer, Richard
Desmond, Sander G. Mills: "Chemistry of tricarbonyl hemiketals and application of Evans technology to the total synthesis of the immunosuppressant (−)-FK-506", in:
2931:
Toshiyuki Kan, Masaru
Hashimoto, Mitsutoshi Yanagiya, Haruhisa Shirahama: "Effective deprotection of 2-(trimethylsilylethoxy)methylated alcohols (SEM ethers). Synthesis of thyrsiferyl-23 acetate", in:
4136:
Antonius J. H. Klunder, Jie Zhu, Binne
Zwanenburg: "The Concept of Transient Chirality in the Stereoselective Synthesis of Functionalized Cycloalkenes Applying the Retro-Diels-Alder Methodology", in:
2699:
Serge David, Annie
Thieffry, Alain Veyrières: "A mild procedure for the regiospecific benzylation and allylation of polyhydroxy-compounds via their stannylene derivatives in non-polar solvents", in:
3170:
Robert M. Williams, Peter J. Sinclair, Dongguan Zhai, Daimo Chen: "Practical asymmetric syntheses of α-amino acids through carbon-carbon bond constructions on electrophilic glycine templates", in:
1913:
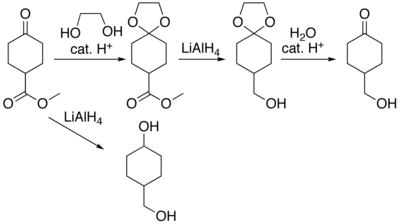
The introduction or modification of a protecting group occasionally influences the reactivity of the whole molecule. For example, diagrammed below is an excerpt of the synthesis of an analogue of
1396:-acetals with acid catalysis from a dithiol and the carbonyl compound. Because of the greater stability of thioacetals, the equilibirum lies on the side of the acetal. In contradistinction to the
772: — More labile than MEM and MOM to acid hydrolysis: 0.1 M hydrochloric acid in methanol, concentrated hydrofluoric acid in acetonitrile, boron trifluoride etherate in dichloromethane, or
3873:
Tainejiro Hiyama, Akihiro Kanakura, Hajime Yamamoto, Hitosi Nozaki: "General Route to α,β-unsaturated Aldehydes of Homoterpenoid and terpenoid Structure. Sythesis of JH-II and β-Sinensal", in:
1422:-acetals. Their formation follows analogously from the thioalcohol. Also their cleavage proceeds under similar conditions and predominantly through mercury(II) compounds in wet acetonitrile.
3388:
Juji Yoshimura, Shigeomi Horito, Hiroriobu Hashimoto: "Facile Synthesis of 2,3,4,6-Tetra-O-benzyl-D-glucopyranosylidene Acetals Using Trimethylsilyl Trifluoromethanesulfonate Catalyst", in:
2741:
Paul A. Wender, Carlos R. D. Correia: "Intramolecular photoinduced diene-diene cyaloadditions: a selective method for the synthesis of complex eight-membered rings and polyquinanes", in:
3508:
Samuel J. Danishefsky, Nathan B. Mantlo, Dennis S. Yamashita, Gayle. Schulte: "Concise route to the calichemicin-esperamicin series: the crystal structure of an aglycone prototype", in:
3576:
Peter Mohr, Nada Waespe-Šarčević, Christoph Tamm, Krystyna Gawronska, Jacek K. Gawronski: "A Study of Stereoselective Hydrolysis of Symmetrical Diesters with Pig Liver Esterase", in:
875:
An exceptional case appears with the benzylideneprotecting group,which also admits reductive cleavage. This proceeds either through catalytic hydrogenation or with the hydride donor
4020:
Timothy S. Butcher, Feng Zhou, Michael R. Detty: "Debrominations of vic-Dibromides with Diorganotellurides. 1. Stereoselectivity, Relative Rates, and Mechanistic Implications", in:
2478:
M.L. García, J. Pascual, L. Borràs, J.A. Andreu, E. Fos, D. Mauleón, G. Carganico, F. Arcamone: "Synthesis of new ether glycerophospholipids structurally related to modulator", in:
1759:
2958:
Joseph P. Marino, Scott L. Dax: "An efficient desilylation method for the generation of o-quinone methides: application to the synthesis of (+)- and (−)-hexahydrocannabinol", in:
3966:
Ahmed M. Tafesh, Jens Weiguny: "A Review of the Selective Catalytic Reduction of Aromatic Nitro Compounds into Aromatic Amines, Isocyanates, Carbamates, and Ureas Using CO", in:
620:-Butyldimethylsilyl (TBDMS or TBS) — Cleaved with acetic acid in tetrahydrofuran/water, Pyridinium tosylate in methanol, trifluoroacetic acid in water, hydrofluoric acid in
4533:
Synthetic studies of marine alkaloids hapalindoles. Part 2. Lithium aluminum hydride reduction of the electron-rich carbon-carbon double bond conjugated with the indole nucleus
3685:
G. Bauduin, D. Bondon, Y. Pietrasanta, B. Pucci: "Reactions de transcetalisation – II: Influence des facteurs steriques et electroniques sur les energies de cetalisation", in:
231:
A common example for this application, the Fmoc peptide synthesis, in which peptides are grown in solution and on solid phase, is very important. The protecting groups in
224:-butyl ether on the phenol group. The benzyl ester can be removed by hydrogenolysis, the fluorenylmethylenoxy group (Fmoc) by bases (such as piperidine), and the phenolic
246:
1291:
Overall, trans-acetalation plays a lesser role in forming protective acetals; they are formed as a rule from glycols through dehydration. Normally a simple glycol like
2558:
H. Nagaoka, W. Rutsch, G. Schmidt, H. Ito, M.R. Johnson, Y. Kishi: "Total synthesis of rifamycins. 1. Stereocontrolled synthesis of the aliphatic building block", in:
1943:
Protecting group chemistry finds itself an important application in the automated synthesis of peptides and nucleosides. The technique was introduced in the field of
3739:
M.P. Bosch, M. Pilar Bosch, Francisco Camps, Jose Coll, Angel Guerrero, Toshio Tatsuoka, Jerrold Meinwald: "A stereoselective total synthesis of (±)-muzigadial", in:
3442:
Kwan Soo Kim, Yang Heon Song, Bong Ho Lee, Chi Sun Hahn: "Efficient and selective cleavage of acetals and ketals using ferric chloride adsorbed on silica gel", in:
4360:
Rosa F. Lockwood, Kenneth M. Nicholas: "Transition metal-stabilized carbenium ions as synthetic intermediates. I. α- carbenium ions as propargylating agents", in:
879:(DIBAL). The cleavage with DIBAL deprotects one alcohol group, for the benzyl moiety stays as a benzyl ether on the second, sterically hindered hydroxy group.
3792:
Ulrich Schmidt, Thomas Beuttler, Albrecht Lieberknecht, Helmut Griesser: "Aminosäuren und peptide – XXXXII. Synthese von Chlamydocin + epi-Chlamydocin", in:
1429:
ions is shown below. Here it is applied, that aldehydes are very much more activated carbonyls than ketones and that many addition reactions are reversible.
3993:
Evan L. Allred, Boyd R. Beck, Kent J. Voorhees: "Formation of carbon-carbon double bonds by the reaction of vicinal dihalides with sodium in ammonia", in:
641:‑Butyldiphenylsilyl (TBDPS) — Similar conditions to TBS but even longer reaction times (100–250× slower than TBS and 5–10× slower than TIPS)
4074:
Corrado Malanga, Serena Mannucci, Luciano Lardicci: "Carbon-halogen bond activation by nickel catalyst: Synthesis of alkenes, from 1,2-dihalides", in:
3603:
Théophile Tschamber, Nada Waespe-Šarčević, Christoph Tamm: "Stereocontrolled Synthesis of an Epimer of the C(19)-to-C(27) Segment of Rifamycin S", in:
4454:
Baran, Phil S.; Maimone, Thomas J.; Richter, Jeremy M. (22 March 2007). "Total synthesis of marine natural products without using protecting groups".
672:
4815:
2796:
Elias J. Corey, Haruki Niwa, Jochen Knolle: "Total synthesis of (S)-12-hydroxy-5,8,14-cis,-10-trans-eicosatetraenoic acid (Samuelsson's HETE)", in:
284:
exhibit very similar reactivities, a transformation that protects or deprotects a single hydroxy group must be possible for a successful synthesis.
292:
Many reaction conditions have been established that will cleave protecting groups. One can roughly distinguish between the following environments:
265:
1141:
1034:
2204:
David A. Evans, Stephen W. Kaldor, Todd K. Jones, Jon Clardy, Thomas J. Stout: "Total synthesis of the macrolide antibiotic cytovaricin", in:
4237:
2049:
1993:
3285:
Festphasensynthese eines tumorassoziierten Sialyl-Tn-Antigen-Glycopeptids mit einer Partialsequenz aus dem "Tandem Repeat" des MUC-1-Mucins
894:
3415:
Bruce H. Lipshutz, Daniel Pollart, Joseph Monforte, Hiyoshizo Kotsuki: "Pd(II)-catalyzed acetal/ketal hydrolysis/exchange reactions", in:
3075:
James A. Marshall, Joseph D. Trometer, Bruce E. Blough, Thomas D. Crute: "Stereochemistry of SN2' additions to acyclic vinyloxiranes", in
818:
Methyl ethers – Cleavage is by TMSI in dichloromethane or acetonitrile or chloroform. An alternative method to cleave methyl ethers is BBr
4826:
A user site excerpting the classic Greene and Wuts text regarding stability of a few key groups, from this reference's extensive tables.
1353:, which one begins with to form these acetals, have a very unpleasant stench and are poisonous, which severely limit their applications.
82:, specific parts of the molecules cannot survive the required reagents or chemical environments. These parts (functional groups) must be
2722:
Kaoru Fuji, Shigetoshi Nakano, Eiichi Fujita: "An Improved Method for Methoxymethylation of Alcohols under Mild Acidic Conditions", in:
216:
is a strategy allowing the specific deprotection of one protective group in a multiply-protected structure. For example, the amino acid
2320:
R. Roger F. Newton, Derek P. Reynolds, Colin F. Webb, Stanley M. Roberts: "A short and efficient total synthesis of (±) prostaglandin D
4840:
4665:
Merrifield, R. B.; Barany, G.; Cosand, W. L.; Engelhard, M.; Mojsov, S. (1977). "Proceedings of the 5th American Peptide Symposium".
2428:
Michel Bessodes, Dimitri Komiotis, Kostas Antonakis: "Rapid and selective detritylation of primary alcohols using formic acid", in:
841:) present for protecting-group chemistry a special class of alcohols. One can exploit the adjacency of two hydroxy groups, e.g. in
3197:
Glenn L. Stahl, Roderich Walter, Clarck W. Smith: "General procedure for the synthesis of mono-N-acylated 1,6-diaminohexanes", in:
2639:
W. Clark Still, Shizuaki Murata, Gilbert Revial, Kazuo Yoshihara: "Synthesis of the cytotoxic germacranolide eucannabinolide", in:
111:
for the carbonyl. After the hydride step is complete, aqueous acid removes the acetal, restoring the carbonyl. This step is called
3658:
Sunggak Kim, Yong Gil Kim, Deog-il Kim: "A novel method for selective dioxolanation of ketones in the presence of aldehydes", in:
2231:
James A. Marshall, Richard Sedrani: "A convergent, highly stereoselective synthesis of a C-11-C-21 subunit of the macbecins", in:
696:, (DMT) — Removed by weak acid. DMT group is widely used for protection of 5'-hydroxy group in nucleosides, particularly in
1838:
Although the use of protecting groups is not preferred in industrial syntheses, they are still used in industrial contexts, e.g.
1299:
is used for acetalation.Modern variants also use glycols, but with the hydroxyl hydrogens replaced with a trimethylsilyl group.
4836:
4704:
Serge L. Beaucage, Radhakrishman P. Iyer: "Advances in the Synthesis of Oligonucleotides by the Phosphoramidite Approach", in:
356:. The advantage of fluoride-labile protecting groups is that no other protecting group is attacked by the cleavage conditions.
4047:
C. J. Li, David N. Harpp: "Bis(triphenylstanyl)telluride a mild and selective reagent for telluration and debromination", in:
2181:-Di-O-methylelaiophylidene – preparation from elaiophylin and total synthesis from (R)-3-hydroxybutyrate and (S)-malate", in:
1632:‑1,2‑dibromoalkane: the regeneration of the alkene then follows with preservation of conformation via elemental
4783:
4279:
Barry J. Teobald: "The Nicholas reaction: the use of dicobalt hexacarbonyl-stabilised propargylic cations in synthesis", in:
4194:
2701:
2326:
718:
4101:
Byung Woo Yoo, Seo Hee Kim, Jun Ho Kim: "A Mild, Efficient, and Selective Debromination of vic-Dibromides to Alkenes with Cp
370:(30–40 °C). Because enzymes have very high substrate specificity, the method is quite rare, but extremely attractive.
1373:-acetals, very much stabler against acid hydrolysis. This enables the selective cleavage of the latter in the presence of
1735:
1079:
1045:
777:
2904:
Steven D. Burke, Gregory J. Pacofsky: "The ester enolate claisen rearrangement. Total synthesis of (±)-ethisolide", in:
769:
220:
could be protected as a benzyl ester on the carboxyl group, a fluorenylmethylenoxy carbamate on the amine group, and a
4858:
1846:
1701:
1593:– Removed by strong hot acid (pH < 1, T > 100 °C) or alkali (pH > 12, T > 100 °C), but not e.g.
1571:
Allyl esters — As with allyl ethers, also removed by diverse platinum complexes – connected with acid workup
1565:
1243:
876:
773:
625:
393:
2505:
Yuji Oikawa, Tadao Yoshioka, Osamu Yonemitsu: "Specific removal of o-methoxybenzyl protection by DDQ oxidation", in:
2177:
Dieter Seebach, Hak-Fun Chow, Richard F.W. Jackson, Marius A. Sutter, Suvit Thaisrivongs, Jürg Zimmermann: "(+)-11,11
574:(Piv) – Removed by acid, base or reductant agents. It is substantially more stable than other acyl protecting groups.
4637:
J.M. McClure, Samuel J. Danishefsky: "A novel Heck arylation reaction: rapid access to congeners of FR 900482", in:
981:
or Birch reduction, but have a decided drawback relative to the carbamates or amides: they retain a basic nitrogen.
4863:
2877:
Robert C. Gadwood, Renee M. Lett, Jane E. Wissinger: "Total synthesis of (±)-poitediol and (±)4-epipoitediol", in:
1750:
1616:
Alkenes rarely need protection or are protected. They are as a rule only involved in undesired side reactions with
1404:‑acetal case, it is not needed to remove water from the reaction mixture in order to shift the equilibrium.
525:
504:, for these exhibit differential removal. Sterically hindered esters are less susceptible to nucleophilic attack:
144:
3224:
Naomi Sakai, Yasufumi Ohfune: "Total synthesis of galantin I. Acid-catalyzed cyclization of galantinic acid", in:
3900:
F. Huet, A. Lechevallier, M. Pellet, J.M. Conia: "Wet Silica Gel; A Convenient Reagent for Deacetalization", in:
3819:
Elias J. Corey, Plato A. Magriotis: "Total synthesis and absolute configuration of 7,20-diisocyanoadociane", in:
1956:
1951:
in 1977. For peptide synthesis via automated machine, the orthogonality of the Fmoc group (basic cleavage), the
1887:
1665:
1594:
1119:
763:
697:
629:
87:
2457:
1302:
Acetals can be removed in acidic aqueous conditions. For those ends, the mineral acids are appropriate acids.
4333:
Richard E. Connor, Kenneth M. Nicholas: "Isolation, characterization, and stability of α- carbonium ions", in:
439:, which is activated through radiation with an appropriate wavelength and so can be removed. For examples the
1928:
Part of the synthesis of an analogue of Mitomycin C with modified reactivity through protecting-group exchange
882:
4816:
A further set of study notes in tutorial form, with guidance and comments, from Profs. Grossman and Cammers.
4111:
4225:
2041:
1948:
1689:
1557:
1084:
978:
706:
1876:
1754:
1307:
1064:
744:
389:
1843:
1222:
603:
4561:
4535:
4509:
3301:
1902:‑methoxybenzyl ethers, four benzoates, a methyl hemiacetal, an acetone acetal and an SEM ester).
1716:
1707:
1637:
1601:
521:
453:
The rare double-layer protecting group is a protected protecting group, which exemplify high stability.
232:
4831:
3361:
T. Tsunoda, M. Suzuki, R. Noyori: "A facile procedure for acetalization under aprotic conditions", in:
862:
815:
in methanol, palladium on activated carbon, or diverse platinum complexes – conjoined with acid workup.
384:
Only a few protecting groups can be detached oxidatively: the methoxybenzyl ethers, which oxidize to a
4753:
4559:
Synthetic studies of marine alkaloids hapalindoles. Part 3 Total synthesis of (±)-hapalindoles H and U
4507:
Synthetic studies of marine alkaloids hapalindoles. Part I Total synthesis of (±)-hapalindoles J and M
2823:
Elias J. Corey, Mark G. Bock: "Protection of primary hydroxyl groups as methylthiomethyl ethers", in:
1932:
The exchange of a protecting group from a methyl ether to a MOM-ether inhibits here the opening of an
1804:
858:
4719:
4465:
4383:
K.M. Nicholas, R. Pettit: "On the stability of α-(alkynyl)dicobalt hexacarbonyl carbonium ions", in:
4371:
4348:
4321:
4294:
4089:
4062:
3888:
3807:
3673:
3430:
3376:
3132:
2946:
2919:
2838:
2520:
2493:
2443:
1790:
1644:
1529:
1236:
1209:
1167:
752:
4810:
501:
497:
4398:
4385:
4335:
4124:
3902:
3700:
3063:
2850:
Elias J. Corey, Duy H. Hua, Bai Chuan Pan, Steven P. Seitz: "Total synthesis of aplasmomycin", in:
2724:
2347:
Kyriacos C. Nicolaou, R. A. Daines, T. K. Chakraborty: "Total synthesis of amphoteronolide B", in:
2106:
1746:
1296:
1190:
1182:
1021:
928:. These characteristics imply that new protecting groups for amines are always under development.
728:
594:
363:
95:
72:
528:
byproduct. The trimethylsilyl ethers are also extremely sensitive to acid hydrolysis (for example
4489:
4252:
Wenzel E. Davidsohn, Malcolm C. Henry: "Organometallic Acetylenes of the Main Groups III–V", in:
4229:
4218:
2183:
1918:
1861:
1505:
1311:
586:
3329:
Moussa, Ziad; D. Romo (2006). "Mild deprotection of primary N-(p-toluenesufonyl) amides with SmI
2077:
Michael Schelhaas, Herbert Waldmann: "Schutzgruppenstrategien in der organischen Synthese", in:
2008:
Michael Schelhaas, Herbert Waldmann: "Schutzgruppenstrategien in der organischen Synthese", in:
957:, which admit reductive cleavage, and the trifluoroacetamides, which hydrolyze easily in base.
950:-butoxycarbonyl, benzoxycarbonyl, fluorenylmethylenoxycarbonyl, and allyloxycarbonyl compounds.
4628:, 4th Ed., John Wiley & Sons Inc., Hoboken, New Jersey, pp. 10–13; ISBN 0-471-69754-0.
1856:
An important example of industrial applications of protecting group theory is the synthesis of
1178:
4758:
4639:
4481:
4436:
4362:
4308:
4233:
4049:
3926:
3875:
3848:
3846:
Elias J. Corey, Kyriacos C. Nicolaou, Takeshi Toru: "Total synthesis of (±)-vermiculine", in:
3821:
3794:
3714:
3660:
3633:
3538:
3510:
3417:
3363:
3226:
3172:
3119:
3014:
2933:
2906:
2879:
2852:
2825:
2798:
2743:
2641:
2614:
2560:
2507:
2430:
2377:
2349:
2267:
2206:
2152:
2079:
2045:
2010:
1989:
1944:
1818:
1697:
1651:
1542:
1509:
1452:– Removed by acid. Normally, the cleavage of acyclic acetals is easier than of cyclic acetals.
1144:(trichloroethyl chloroformate ) group – Removed by Zn insertion in the presence of acetic acid
1060:
917:
734:
713:
685:‑Dimethoxybenzyl ether — Removed via oxidation with DDQ or ceric ammonium chloride
611:
532:
suffices as a proton donator) and are consequently rarely used nowadays as protecting groups.
406:
2192:
1715:
In order to protect the triple bond itself, sometimes a transition metal-alkyne complex with
4735:
4674:
4586:
4569:
4543:
4517:
4473:
4456:
4428:
3934:
3605:
3578:
3344:
2680:
2324:
methyl ester involving a new method for the cleavage of a dimethyl-t-butylsilyl ether", in:
1739:
1722:
1605:
1306:
is a common cosolvent, used to promote dissolution. For a non-acidic cleavage technique, a
1015:
798:
635:
Triisopropylsilyl (TIPS) ethers) — Similar conditions to TBS but longer reaction times.
367:
353:
320:
136:
79:
68:
64:
4771:
4599:
4584:
T. Reichstein, A. Grüssner: "Eine ergiebige Synthese der L-Ascorbinsäure (C-Vitamin)", in:
3618:
3591:
3265:
2092:
2023:
1481:
are the esters of various alcohols. Occasionally, esters are protected as ortho-esters or
32:
1478:
1292:
854:
825:
693:
607:
128:
36:
4469:
1867:
345:
Various groups are cleaved in acid or base conditions, but the others are more unusual.
2685:
2668:
2104:
V.N. Rajasekharan Pillai: "Photoremovable Protecting Groups in Organic Synthesis", in:
1786:
1782:
1578:
1425:
For aldehydes, a temporary protection of the carbonyl group the presence of ketones as
1226:
1216:
1204:
1028:
1003:
943:
756:
653:
582:
475:
335:
281:
252:
Schematic diagram of a solid-state peptide synthesis with orthogonal protecting groups
206:
194:
167:
4573:
4547:
4521:
4412:
1883:
and then deprotected after the oxidation of the primary alcohols to carboxylic acids.
606: — 10–100× stabler than a TMS group. Cleaved with trifluoroacetic acid in water/
4852:
4825:
4678:
4022:
3995:
3741:
3444:
3199:
2960:
2771:
2587:
2295:
2233:
1895:
1857:
1767:
1743:
1621:
1075:
999:
559:
493:
417:
374:
148:
2669:"Metabolism of 3,4-dimethoxycinnamyl alcohol and derivatives by Coriolus versicolor"
1349:-acetals also have an application, albeit scant, as carbonyl protecting groups too.
762:
Tris(isopropyl)silyloxymethyl (TOM) — Commonly protects 2'-hydroxy function in
446:
4615:, VCH Verlagsgesellschaft mbH, Weinheim 1996, pp. 711–729, ISBN 3-527-29284-5.
4493:
2710:
2335:
1693:
1617:
1432:
850:
722:
621:
385:
378:
277:
4805:
4652:
4267:
4008:
3939:
3921:
3861:
3834:
3754:
3727:
3646:
3551:
3523:
3457:
3403:
3239:
3212:
3185:
3088:
3027:
2973:
2892:
2865:
2811:
2784:
2756:
2654:
2627:
2600:
2573:
2390:
2362:
2308:
2280:
2246:
2219:
2165:
4706:
4281:
4076:
3687:
3390:
3050:
2480:
2262:
1924:
1914:
1850:
1584:
1549:
1135:
954:
931:
925:
921:
804:
794:
748:
671:(PMB) — Removed by acid, hydrogenolysis, or oxidation – commonly with
590:
478:
471:
436:
421:
413:
302:
186:
152:
120:
4749:, 4th Ed., John Wiley & Sons Inc., Hoboken, New Jersey, ISBN 0-471-69754-0.
1624:
or catalytic hydration. For alkenes two protecting groups are basically known:
1414:-Acetals are hydrolyzed a factor of 10,000 times faster than the corresponding
1275:
516:
Trimethylsilyl chloride, activated with imidazole, protects a secondary alcohol
399:
4420:
4254:
4166:
4138:
3968:
1822:
1785:. Crucially, added complexity impedes the use of synthetic total synthesis in
1535:
1459:
1426:
1354:
1315:
1283:
1186:
1071:
1056:
529:
520:
Triorganosilyl sources have quite variable prices, and the most economical is
425:
132:
4820:
4778:
4179:
4151:
4035:
3981:
1891:
1839:
1590:
1520:‑dimethylformamide, or methanol and catalytic trimethylsilyl chloride
1482:
1011:
993:
966:
939:
935:
665:
512:
326:
17:
4792:
4485:
4440:
4413:"Isotope Effects in Photochemistry: Application to Chromatic Orthogonality"
4203:
2612:
Masahiro Hirama, Mitsuko Uei: "A chiral total synthesis of compactin", in:
1798:
Protected and unprotected syntheses of the marine alkaloid, hapalindole U.
731:(MOM) — Removed by 6 M hydrochloric acid in tetrahydrofuran/water
712:
Ethoxyethyl ethers (EE) – Cleavage more trivial than simple ethers e.g. 1N
4568:, Pages 6351–6360 Hideaki Muratake, Harumi Kumagami and Mitsutaka Natsume
3348:
1962:
4216:
Clayden, Jonathan; Greeves, Nick; Warren, Stuart; Wothers, Peter (2000).
3712:
John E. McMurry, Stephen J. Isser: "Total synthesis of longifolene", in:
1937:
1553:
1465:
1123:
1040:(Boc) group — Removed by concentrated strong acid (such as HCl or CF
1007:
598:
571:
308:
217:
99:
27:
Group of atoms introduced into a compound to prevent subsequent reactions
4477:
973:‑sulfonylamides,which are far too stable with aliphatic amines.
3335:
1933:
1880:
1541:
esters – Also removed by acid and some reductants. Can be formed from
1303:
1163:
1131:
1127:
1109:
962:
899:
868:
565:
429:
349:
124:
4432:
1905:
1758:
538:
445:
405:
398:
276:
A further important example of orthogonal protecting groups occurs in
135:– may require protecting groups to order their assembly. Also, cheap
3920:
Romanski, J.; Nowak, P.; Kosinski, K.; Jurczak, J. (September 2012).
1671:
1523:
1501:
1498:
1455:
1445:
1374:
1200:
1159:
1099:
1088:
958:
846:
842:
838:
659:
649:
555:
482:
359:
314:
171:
Orthogonal protection of L-Tyrosine (Protecting groups are marked in
104:
44:
40:
4811:
Senior undergraduate study notes on this subject, from Prof. Rizzo.
271:
Fmoc solid state peptide synthesis with orthogonal protecting groups
4742:, 1st ed., Georg Thieme Verlag, Stuttgart 1994, ISBN 3-13-135601-4.
1087:
group — Removed with complexes of metals like palladium(0) or
539:
492:
The most important esters with common protecting-group use are the
4306:
Kenneth M. Nicholas, R. Pettit: "An alkyne protection group", in:
3270:(PDF; 663 kB) In: Michael W. Pennington, Ben M. Dunn (eds.):
1961:
1923:
1904:
1866:
1817:
1809:
1803:
1574:
1449:
1350:
1282:
1274:
1174:
1147:
812:
511:
486:
467:
332:
Protecting groups cleaved by heavy metal salts or their complexes.
228:-butyl ether cleaved with acids (e.g. with trifluoroacetic acid).
166:
107:, which does not react with hydrides. The acetal is then called a
91:
48:
31:
3922:"High-pressure transesterification of sterically hindered esters"
1886:
A very spectacular example application of protecting groups from
1664:
2-cyanoethyl – removed by mild base. The group is widely used in
71:
in a subsequent chemical reaction. It plays an important role in
2585:
W. Clark Still, Dominick Mobilio: "Synthesis of asperdiol", in:
1719:
is used. The release of the cobalt then follows from oxidation.
1633:
1052:
296:
845:, in that one protects both hydroxy groups codependently as an
721:(MEM) — Removed by hydrobromic acid in tetrahydrofuran or
662:(triphenylmethyl, Tr) — Removed by acid and hydrogenolysis
4806:
Introduction of protecting group and mechanism of deprotection
969: — verily any aza-heterocycle — admit protection as
737:(THP) — Removed by acetic acid in tetrahydrofuran/water,
4756:: "Schutzgruppenstrategien in der organischen Synthese", in:
1875:
In order to prevent oxidation of the secondary alcohols with
4695:. Reprint 2004, Oxford University Press, ISBN 0-19-963724-5.
1721:
1706:
1650:
1431:
881:
867:
656:. Bn group is widely used in sugar and nucleoside chemistry.
63:
is introduced into a molecule by chemical modification of a
3770:, Ferdinand Enke Verlag, Stuttgart 1965, pp. 127–133.
1568:-catalyzed high-pressure methanolysis at room temperature.
703:
Methoxytrityl (MMT) – Removed by acid and hydrogenolysis.
568:(Bz) – Removed by acid or base, more stable than Ac group.
4613:
Classics in Total Synthesis: Targets, Strategies, Methods
4542:, Pages 6343–6350 Hideaki Muratake and Mitsutaka Natsume
4516:, Pages 6331–6342 Hideaki Muratake and Mitsutaka Natsume
2261:
James D. White, Motoji Kawasaki: "Total synthesis of (+)-
3274:
volume 35, 1995, ISBN 978-0-89603-273-6, pp. 17–27.
485:, deprotected by weak acids. In rarer cases, a carbon
1138:. Too stable to readily remove from aliphatic amides.
1825:'s protecting-group free synthesis, reported in 2007.
1508:. Can be formed from diazomethane in diethyl ether,
1055:) group — Removed by base, such as 20–50 %
797:(tBu) – Removed with anhydrous trifluoroacetic acid,
1763:
Orthogonal protection Application in Photochemistry
1044:COOH), or by heating to >80 °C. Common in
508:
Chloroacetyl > acetyl > benzoyl > pivaloyl
90:is a highly reactive reagent that usefully reduces
4217:
3115:An Exceptionally Mild Deprotection of Phthalimides
2257:
2255:
1587:– Converted to standard ester by mild aqueous acid
1545:: isobutene in dioxane and catalytic sulfuric acid
1532:esters — Same as benzyl, but easier to cleave
791:-Methoxyphenyl ether (PMP) – Removed by oxidation.
628:in THF. Commonly protects 2'-hydroxy function in
1150:(Ts) group – Removed by concentrated acid (HBr, H
977:‑benzylated amines can be removed through
466:The classical protecting groups for alcohols are
4842:Schutzgruppen in der organischen Synthesechemie
3291:volume 109, 1997, pp. 629–631 (in German).
4747:Green's Protective Groups in Organic Synthesis
4626:Green's Protective Groups in Organic Synthesis
4411:Blanc, Aurélien; Bochet, Christian G. (2007).
3113:John O. Osby, Michael G. Martin, Bruce Ganem:
481:, deprotected by acids and fluoride ions; and
381:: ethers, esters, urethanes, carbonates, etc.
51:reduction, vs. unprotected reduction to a diol
1984:Theodora W. Greene; Peter G. M. Wuts (1999).
1742:orthogonal deprotection is demonstrated in a
1526:esters — Also removed by hydrogenolysis.
1468:– Removed by metal salts or oxidizing agents.
1388:-acetals normally follows analogously to the
1130:. Removed by base, often aqueous or gaseous
801:in acetic acid, or 4 N hydrochloric acid
8:
1318:can be performed with workup in chloroform.
953:Other, more exotic amine protectors are the
237:
1871:The Reichstein synthesis (of ascorbic acid)
942:. When a carbamate deprotects, it evolves
610:, acetic acid in water/tetrahydrofuran, or
2293:3'-deuterioadenosine from adenosine", in:
1879:, they are protected via acetalation with
624:, pyridinium fluoride in tetrahydrofuran,
3938:
2684:
1628:Temporary halogenation with bromine to a
1477:The most important protecting groups for
1018:, or lithium or sodium in liquid ammonia.
996:group – Removed by acid and mild heating.
946:. The commonest-used carbamates are the
4777:Krzysztof Jarowicki, Philip Kocieński: "
1808:Hideaki Muratake's 1990 synthesis using
893:
741:‑toluenesulfonic acid in methanol
443:-nitrobenzylgroup ought be listed here.
432:(resp. amine) hydrolyzes in light acid.
348:Fluoride ions form very strong bonds to
2036:Chan, Weng C.; White, Peter D. (2004).
1976:
242:
1986:Protecting Groups in Organic Synthesis
1674:(Me) – removed by strong nucleophiles
4745:Peter G.M. Wuts, Theodora W. Greene:
4624:Peter G.M. Wuts, Theodora W. Greene:
1189:or other soft thiol nucleophiles, or
770:β‑(Trimethylsilyl)ethoxymethyl
435:Photolabile protecting groups bear a
7:
3267:Methods for Removing the Fmoc Group.
916:Amines have a special importance in
558:(Ac) – Removed by acid or base (see
420:to a vinyl group in the presence of
3303:Fmoc Solid Phase Peptide Synthesis.
2667:Kamaya, Yasushi; T Higuchi (2006).
1966:Automatic oligonucleotide synthesis
1606:Grignard (organomagnesium) reagents
1310:acetonitrile complex in acetone or
849:. Common in this situation are the
778:Hexamethyl phosphoric acid triamide
362:and other enzymes cleave ethers at
4693:Fmoc Solid Phase Peptide Synthesis
3253:Fmoc Solid Phase Peptide Synthesis
2686:10.1111/j.1574-6968.1984.tb01309.x
2066:Fmoc Solid Phase Peptide Synthesis
2038:Fmoc Solid Phase Peptide Synthesis
1812:protecting groups (shown in blue).
1577:esters – Also removed by base and
906:-butyloxycarbonyl group is marked
25:
4720:doi:10.1016/S0040-4020(01)88752-4
4372:doi:10.1016/S0040-4039(01)83455-9
4349:doi:10.1016/S0022-328X(00)89454-1
4322:doi:10.1016/S0040-4039(01)97209-0
4295:doi:10.1016/S0040-4020(02)00315-0
4090:doi:10.1016/S0040-4020(97)10203-4
4063:doi:10.1016/S0040-4039(00)97045-X
3889:doi:10.1016/S0040-4039(01)94936-6
3808:doi:10.1016/S0040-4039(00)88171-X
3674:doi:10.1016/S0040-4039(00)92243-3
3431:doi:10.1016/S0040-4039(00)89114-5
3377:doi:10.1016/S0040-4039(00)74575-8
3333:following trifluoroacetylation".
3133:doi:10.1016/S0040-4039(01)81169-2
2947:doi:10.1016/S0040-4039(00)82883-X
2920:doi:10.1016/S0040-4039(00)85501-X
2839:doi:10.1016/S0040-4039(00)91422-9
2521:doi:10.1016/S0040-4039(00)86974-9
2494:doi:10.1016/S0040-4020(01)96051-X
2444:doi:10.1016/S0040-4039(00)84045-9
1683:Terminal alkyne protecting groups
1473:Carboxylic acid protecting groups
1027:(Moz or MeOZ) group – Removed by
78:In many preparations of delicate
4399:doi:10.1016/0022-328X(72)80037-8
4125:doi:10.5012/bkcs.2010.31.10.2757
3701:doi:10.1016/0040-4020(78)80243-9
3064:doi:10.1016/0040-4020(82)80083-5
1548:2,6‑Dialkylphenols (e.g.
1504: — Also removed by acid or
1158:) & strong reducing agents (
1118:) groups — common in
934:are primarily protected through
341:Double-layered protecting groups
280:chemistry. As carbohydrates or
264:
245:
205:-butyl ether protected phenolic
1890:is the 1994 total synthesis of
807: — Removed with potassium
4784:J. Chem. Soc., Perkin Trans. 1
4691:Weng C. Chan, Peter D. White:
4611:K.C. Nicolaou, E.J. Sorensen:
4195:J. Chem. Soc., Perkin Trans. 1
3251:Weng C. Chan, Peter D. White:
2702:J. Chem. Soc., Perkin Trans. 1
2327:J. Chem. Soc., Perkin Trans. 1
2064:Weng C. Chan, Peter D. White:
1365:-acetals are, unlike the pure
1051:9-Fluorenylmethyloxycarbonyl (
1002:(Cbz) group — Removed by
751:metal oxidants: base-buffered
428:(from a protected alcohol) or
238:§ Industrial applications
1:
4821:A review by Prof. Kocienski.
4574:10.1016/S0040-4020(01)96007-7
4548:10.1016/S0040-4020(01)96006-5
4522:10.1016/S0040-4020(01)96005-3
2193:doi:10.1002/jlac.198619860714
1853:(Tamiflu, an antiviral drug)
1736:Photolabile protecting groups
1080:solid phase peptide synthesis
1046:solid phase peptide synthesis
177:, the amino acid is shown in
4772:doi:10.1002/ange.19961081805
4679:10.1016/0307-4412(79)90078-5
4600:doi:10.1002/hlca.19340170136
3940:10.1016/j.tetlet.2012.07.094
3619:doi:10.1002/hlca.19860690311
3592:doi:10.1002/hlca.19830660815
2093:doi:10.1002/ange.19961081805
2024:doi:10.1002/ange.19961081805
1377:-protected carbonyl groups.
388:. They can be removed with
3306:retrieved 16 November 2013.
3272:Peptide Synthesis Protocols
1702:tetrabutylammonium fluoride
1659:Phosphate protecting groups
1244:ammonium cerium(IV) nitrate
1181:) groups — Removed by
924:and also relatively strong
877:diisobutyl aluminum hydride
774:tetrabutylammonium fluoride
747: — Removed by acid or
626:tetrabutylammonium fluoride
394:dichlorodicyanobenzoquinone
73:multistep organic synthesis
4880:
3040:derivatives with the LiAlH
2458:Carbonhydr. Chem. Biochem.
1816:
1751:trimethylsilyldiazomethane
1251:Carbonyl protecting groups
377:removes a wide variety of
145:enantioselective synthesis
2673:FEMS Microbiology Letters
1957:oligonucleotide synthesis
1888:natural product synthesis
1666:oligonucleotide synthesis
1564:) — Also removed in
1242:(PMP) group – Removed by
1219:, more labile than benzyl
1120:oligonucleotide synthesis
1025:-Methoxybenzyloxycarbonyl
920:, but are a quite potent
764:oligonucleotide synthesis
719:Methoxyethoxymethyl ether
698:oligonucleotide synthesis
630:oligonucleotide synthesis
462:Alcohol protecting groups
329:-labile protecting groups
323:-labile protecting groups
317:-labile protecting groups
311:-labile protecting groups
305:-labile protecting groups
299:-labile protecting groups
193:) benzyl ester protected
88:lithium aluminium hydride
2711:doi:10.1039/P19810001796
2492:, pp. 10023–10034;
2336:doi:10.1039/P19810002055
1988:(3 ed.). J. Wiley.
1203:(Bn) group – Removed by
828:(THF) – Removed by acid.
457:Common protecting groups
137:chiral protecting groups
98:. It always reacts with
4653:doi:10.1021/ja00067a026
4564:, Volume 46, Issue 18,
4538:, Volume 46, Issue 18,
4512:, Volume 46, Issue 18,
4268:doi:10.1021/cr60245a003
4226:Oxford University Press
4112:Bull. Korean Chem. Soc.
4009:doi:10.1021/jo00926a024
3862:doi:10.1021/ja00841a058
3835:doi:10.1021/ja00235a052
3768:Katalytische Hydrierung
3755:doi:10.1021/jo00356a002
3728:doi:10.1021/ja00775a044
3647:doi:10.1021/ja00160a047
3552:doi:10.1021/ja00010a030
3524:doi:10.1021/ja00228a051
3458:doi:10.1021/jo00353a027
3404:doi:10.1246/cl.1981.375
3240:doi:10.1021/ja00029a031
3213:doi:10.1021/jo00405a045
3186:doi:10.1021/ja00213a031
3089:doi:10.1021/jo00253a020
3028:doi:10.1021/ja00297a038
2974:doi:10.1021/jo00193a051
2893:doi:10.1021/ja00325a032
2866:doi:10.1021/ja00388a074
2812:doi:10.1021/ja00474a058
2785:doi:10.1021/jo01323a017
2757:doi:10.1021/ja00242a053
2655:doi:10.1021/ja00341a055
2628:doi:10.1021/ja00379a037
2601:doi:10.1021/jo00172a070
2574:doi:10.1021/ja00547a037
2391:doi:10.1021/ja00036a045
2363:doi:10.1021/ja00241a063
2309:doi:10.1021/jo00289a004
2281:doi:10.1021/ja00168a071
2247:doi:10.1021/jo00019a004
2220:doi:10.1021/ja00175a038
2166:doi:10.1021/ja00164a023
2042:Oxford University Press
1949:Robert Bruce Merrifield
1834:Industrial applications
1766:Due to this effect the
1690:methylmagnesium bromide
1122:for protection of N in
979:catalytic hydrogenation
890:Amine protecting groups
780:) or in tetrahydrofuran
755:in wet acetonitrile or
652:(Bn) — Removed by
288:Cleavage categorization
4830:Organic-Reaction.com:
4791:, pp. 4005–4037;
4770:, pp. 2192–2219;
4718:, pp. 2223–2311;
4651:, pp. 6094–6100;
4370:, pp. 4163–4166;
4320:, pp. 3475–3478;
4293:, pp. 4133–4170;
4202:, pp. 3555–3558;
4150:, pp. 1163–1190;
4123:, pp. 2757–2758;
4088:, pp. 1021–1028;
4061:, pp. 6291–6293;
4007:, pp. 1426–1427;
3980:, pp. 2035–2052;
3887:, pp. 3051–3054;
3860:, pp. 2287–2288;
3806:, pp. 3573–3576;
3726:, pp. 7132–7137;
3699:, pp. 3269–3274;
3672:, pp. 2565–2566;
3645:, pp. 1607–1617;
3590:, pp. 2501–2511;
3550:, pp. 3850–3866;
3522:, pp. 6890–6891;
3375:, pp. 1357–1358;
3211:, pp. 2285–2286;
3131:, pp. 2093–2096;
3062:, pp. 3721–3727;
3026:, pp. 3279–3285;
2972:, pp. 3671–3672;
2945:, pp. 5417–5418;
2891:, pp. 3869–3870;
2864:, pp. 6818–6820;
2837:, pp. 3269–3270;
2810:, pp. 1942–1943;
2783:, pp. 1438–1447;
2755:, pp. 2523–2525;
2709:, pp. 1796–1801;
2626:, pp. 4251–4253;
2599:, pp. 4785–4786;
2572:, pp. 7962–7965;
2536:‑methoxybenzyl.
2389:, pp. 3910–3926;
2361:, pp. 2208–2210;
2334:, pp. 2055–2058;
2279:, pp. 4991–4993;
2245:, pp. 5496–5498;
2218:, pp. 7001–7031;
2191:, pp. 1281–1308;
2164:, pp. 2998–3017;
2091:, pp. 2195–2200;
1967:
1929:
1910:
1877:potassium permanganate
1872:
1826:
1813:
1764:
1755:kinetic isotope effect
1726:
1712:
1655:
1602:organolithium reagents
1436:
1308:palladium(II) chloride
1288:
1280:
1031:, more labile than Cbz
913:
886:
872:
837:The 1,2‑diols (
759:in wet tetrahydrofuran
745:Methylthiomethyl ether
543:
517:
450:
410:
403:
390:ceric ammonium nitrate
210:
52:
4667:Biochemical Education
4180:doi:10.1021/ol0070029
4152:doi:10.1021/cr9803840
4036:doi:10.1021/jo9713363
3982:doi:10.1021/cr950083f
3349:10.1055/s-2006-951530
3238:, pp. 998–1010;
3184:, p. 1547–1557;
3087:, pp. 4274–4282
1965:
1927:
1908:
1870:
1821:
1807:
1762:
1725:
1717:dicobalt octacarbonyl
1711:Alkyne TMS protection
1710:
1654:
1643:Protection through a
1638:titanocene dichloride
1554:2,6-diisopropylphenol
1512:and methyl iodide in
1435:
1286:
1278:
1074:in DMF for sensitive
1068:-Methyl-2-pyrrolidone
897:
885:
871:
542:
522:chlorotrimethylsilane
515:
449:
409:
402:
233:solid-phase synthesis
214:Orthogonal protection
170:
163:Orthogonal protection
35:
4793:doi:10.1039/A803688H
4598:, pp. 311–328;
4204:doi:10.1039/b006759h
4178:, pp. 679–681;
4034:, pp. 169–176;
3833:, pp. 287–289;
3753:, pp. 773–784;
3617:, pp. 621–625;
3456:, pp. 404–407;
3429:, pp. 705–708;
3402:, pp. 375–376;
2918:, pp. 445–448;
2653:, pp. 625–627;
2519:, pp. 885–888;
2442:, pp. 579–580;
2307:, pp. 410–412;
1791:biomimetic synthesis
1645:Diels-Alder reaction
1440:Types of protectants
1287:1,3‑Propadiol
1225:(DMPM) – Removed by
1168:sodium naphthalenide
669:-Methoxybenzyl ether
614:in water or pyridine
4837:Universität Marburg
4752:Michael Schelhaas,
4736:Philip J. Kocieński
4478:10.1038/nature05569
4470:2007Natur.446..404B
4386:J. Organomet. Chem.
4336:J. Organomet. Chem.
4266:, pp. 73–106;
3957:, pp. 139–142.
3499:, pp. 178–180.
3319:, pp. 199–201.
3283:B. Liebe, H. Kunz:
3148:, pp. 220–227.
2732:, pp. 276–277.
2532:See literature for
1842:(sweetener) or the
1799:
1747:transesterification
1696:in tetrahydrofuran/
1229:, more labile than
1223:3,4-Dimethoxybenzyl
1215:(PMB) – Removed by
1191:tributyltin hydride
1173:Other sulfonamide (
729:Methoxymethyl ether
595:potassium carbonate
4859:Chemical synthesis
3766:F. Zymalkokowski:
3473:, S. 167–170.
2184:Liebigs Ann. Chem.
1968:
1930:
1911:
1873:
1827:
1814:
1797:
1765:
1727:
1713:
1678:. thiophenole/TEA.
1656:
1550:2,6-dimethylphenol
1506:pig liver esterase
1437:
1312:iron(III) chloride
1289:
1281:
914:
887:
873:
725:in dichloromethane
587:Potassium fluoride
544:
518:
451:
411:
404:
211:
53:
4864:Protecting groups
4779:Protecting groups
4759:Angewandte Chemie
4740:Protecting Groups
4640:J. Am. Chem. Soc.
4464:(7134): 404–408.
4433:10.1021/ol070820h
4427:(14): 2649–2651.
4363:Tetrahedron Lett.
4309:Tetrahedron Lett.
4239:978-0-19-850346-0
4220:Organic Chemistry
4109:/Ga System", in:
4050:Tetrahedron Lett.
3955:Protecting Groups
3933:(39): 5287–5289.
3927:Tetrahedron Lett.
3910:, pp. 63–64.
3876:Tetrahedron Lett.
3849:J. Am. Chem. Soc.
3822:J. Am. Chem. Soc.
3795:Tetrahedron Lett.
3781:Protecting Groups
3715:J. Am. Chem. Soc.
3661:Tetrahedron Lett.
3634:J. Am. Chem. Soc.
3565:Protecting Groups
3539:J. Am. Chem. Soc.
3511:J. Am. Chem. Soc.
3497:Protecting Groups
3484:Protecting Groups
3471:Protecting Groups
3418:Tetrahedron Lett.
3364:Tetrahedron Lett.
3343:(19): 3294–3298.
3317:Protecting Groups
3264:Gregg B. Fields:
3255:, pp. 27–30.
3227:J. Am. Chem. Soc.
3173:J. Am. Chem. Soc.
3159:Protecting Groups
3146:Protecting Groups
3120:Tetrahedron Lett.
3102:Protecting Groups
3015:J. Am. Chem. Soc.
3000:Protecting Groups
2989:, pp. 59–60.
2987:Protecting Groups
2934:Tetrahedron Lett.
2907:Tetrahedron Lett.
2880:J. Am. Chem. Soc.
2853:J. Am. Chem. Soc.
2826:Tetrahedron Lett.
2799:J. Am. Chem. Soc.
2744:J. Am. Chem. Soc.
2642:J. Am. Chem. Soc.
2615:J. Am. Chem. Soc.
2561:J. Am. Chem. Soc.
2547:Protecting Groups
2508:Tetrahedron Lett.
2431:Tetrahedron Lett.
2419:, pp. 46–49.
2417:Protecting Groups
2404:Protecting Groups
2378:J. Am. Chem. Soc.
2350:J. Am. Chem. Soc.
2268:J. Am. Chem. Soc.
2207:J. Am. Chem. Soc.
2153:J. Am. Chem. Soc.
2138:Protecting Groups
2125:Protecting Groups
2080:Angewandte Chemie
2051:978-0-19-963724-9
2022:, pp. 2194;
2011:Angewandte Chemie
1995:978-0-471-16019-9
1945:peptide synthesis
1860:(Vitamin C) à la
1831:
1830:
1698:dimethylsulfoxide
1543:carboalkoxylation
1510:caesium carbonate
1493:Protecting groups
1380:The formation of
1085:Allyloxycarbamate
1061:dimethylformamide
1038:-Butyloxycarbonyl
938:, typically as a
918:peptide synthesis
811:‑butoxide
795:Tert-butyl ethers
753:mercuric chloride
735:Tetrahydropyranyl
714:hydrochloric acid
612:hydrogen fluoride
470:, deprotected by
338:protecting groups
185:) Fmoc-protected
80:organic compounds
16:(Redirected from
4871:
4832:Protecting Group
4754:Herbert Waldmann
4723:
4702:
4696:
4689:
4683:
4682:
4662:
4656:
4635:
4629:
4622:
4616:
4609:
4603:
4587:Helv. Chim. Acta
4582:
4576:
4556:
4550:
4530:
4524:
4504:
4498:
4497:
4451:
4445:
4444:
4417:
4408:
4402:
4381:
4375:
4358:
4352:
4331:
4325:
4304:
4298:
4277:
4271:
4250:
4244:
4243:
4223:
4213:
4207:
4189:
4183:
4161:
4155:
4134:
4128:
4099:
4093:
4072:
4066:
4045:
4039:
4018:
4012:
3991:
3985:
3964:
3958:
3953:P.J. Kocieński:
3951:
3945:
3944:
3942:
3917:
3911:
3898:
3892:
3871:
3865:
3844:
3838:
3817:
3811:
3790:
3784:
3779:P.J. Kocieński:
3777:
3771:
3764:
3758:
3737:
3731:
3710:
3704:
3683:
3677:
3656:
3650:
3628:
3622:
3606:Helv. Chim. Acta
3601:
3595:
3579:Helv. Chim. Acta
3574:
3568:
3563:P.J. Kocieński:
3561:
3555:
3533:
3527:
3506:
3500:
3495:P.J. Kocieński:
3493:
3487:
3482:P.J. Kocieński:
3480:
3474:
3469:P.J. Kocieński:
3467:
3461:
3440:
3434:
3413:
3407:
3386:
3380:
3359:
3353:
3352:
3326:
3320:
3315:P.J. Kocieński:
3313:
3307:
3298:
3292:
3281:
3275:
3262:
3256:
3249:
3243:
3222:
3216:
3195:
3189:
3168:
3162:
3157:P.J. Kocieński:
3155:
3149:
3144:P.J. Kocieński:
3142:
3136:
3111:
3105:
3100:P.J. Kocieński:
3098:
3092:
3073:
3067:
3037:
3031:
3009:
3003:
2998:P.J. Kocieński:
2996:
2990:
2985:P.J. Kocieński:
2983:
2977:
2956:
2950:
2929:
2923:
2902:
2896:
2875:
2869:
2848:
2842:
2821:
2815:
2794:
2788:
2766:
2760:
2739:
2733:
2720:
2714:
2697:
2691:
2690:
2688:
2679:(2–3): 225–229.
2664:
2658:
2637:
2631:
2610:
2604:
2583:
2577:
2556:
2550:
2545:P.J. Kocieński:
2543:
2537:
2530:
2524:
2503:
2497:
2476:
2470:
2453:
2447:
2426:
2420:
2415:P.J. Kocieński:
2413:
2407:
2402:P.J. Kocieński:
2400:
2394:
2372:
2366:
2345:
2339:
2318:
2312:
2290:
2284:
2259:
2250:
2229:
2223:
2202:
2196:
2180:
2175:
2169:
2147:
2141:
2136:P.J. Kocieński:
2134:
2128:
2123:P.J. Kocieński:
2121:
2115:
2114:, pp. 1–26.
2102:
2096:
2075:
2069:
2068:, S. 10–12.
2062:
2056:
2055:
2033:
2027:
2006:
2000:
1999:
1981:
1800:
1796:
1740:proof of concept
1479:carboxylic acids
1117:
1116:
1107:
1106:
1016:activated carbon
911:
863:cyclopentylidene
799:hydrogen bromide
424:. The residual
354:steric hindrance
268:
249:
176:
129:oligosaccharides
109:protecting group
69:chemoselectivity
65:functional group
61:protective group
57:protecting group
21:
4879:
4878:
4874:
4873:
4872:
4870:
4869:
4868:
4849:
4848:
4802:
4732:
4730:Further reading
4727:
4726:
4703:
4699:
4690:
4686:
4664:
4663:
4659:
4636:
4632:
4623:
4619:
4610:
4606:
4583:
4579:
4557:
4553:
4531:
4527:
4505:
4501:
4453:
4452:
4448:
4415:
4410:
4409:
4405:
4382:
4378:
4359:
4355:
4332:
4328:
4305:
4301:
4278:
4274:
4251:
4247:
4240:
4215:
4214:
4210:
4190:
4186:
4162:
4158:
4135:
4131:
4108:
4104:
4100:
4096:
4073:
4069:
4046:
4042:
4019:
4015:
3992:
3988:
3965:
3961:
3952:
3948:
3919:
3918:
3914:
3899:
3895:
3872:
3868:
3845:
3841:
3818:
3814:
3791:
3787:
3783:, pp. 136.
3778:
3774:
3765:
3761:
3738:
3734:
3711:
3707:
3684:
3680:
3657:
3653:
3629:
3625:
3602:
3598:
3575:
3571:
3567:, pp. 119.
3562:
3558:
3534:
3530:
3507:
3503:
3494:
3490:
3486:, pp. 176.
3481:
3477:
3468:
3464:
3441:
3437:
3414:
3410:
3387:
3383:
3360:
3356:
3332:
3328:
3327:
3323:
3314:
3310:
3299:
3295:
3282:
3278:
3263:
3259:
3250:
3246:
3223:
3219:
3196:
3192:
3169:
3165:
3156:
3152:
3143:
3139:
3112:
3108:
3099:
3095:
3074:
3070:
3047:
3043:
3038:
3034:
3010:
3006:
2997:
2993:
2984:
2980:
2957:
2953:
2930:
2926:
2903:
2899:
2876:
2872:
2849:
2845:
2822:
2818:
2795:
2791:
2767:
2763:
2740:
2736:
2721:
2717:
2698:
2694:
2666:
2665:
2661:
2638:
2634:
2611:
2607:
2584:
2580:
2557:
2553:
2544:
2540:
2531:
2527:
2504:
2500:
2477:
2473:
2454:
2450:
2427:
2423:
2414:
2410:
2401:
2397:
2373:
2369:
2346:
2342:
2323:
2319:
2315:
2291:
2287:
2260:
2253:
2230:
2226:
2203:
2199:
2178:
2176:
2172:
2148:
2144:
2135:
2131:
2122:
2118:
2103:
2099:
2076:
2072:
2063:
2059:
2052:
2035:
2034:
2030:
2007:
2003:
1996:
1983:
1982:
1978:
1973:
1836:
1778:
1732:
1685:
1661:
1614:
1598:
1495:
1475:
1442:
1293:ethylene glycol
1279:Ethylene glycol
1253:
1183:samarium iodide
1157:
1153:
1114:
1113:
1104:
1103:
1043:
987:
907:
892:
859:cyclohexylidene
835:
826:Tetrahydrofuran
821:
707:Benzyloxymethyl
694:Dimethoxytrityl
645:Benzyl ethers:
608:tetrahydrofuran
549:
502:pivalate esters
489:might be used.
464:
459:
414:Allyl compounds
290:
282:hydroxyl groups
272:
269:
260:
250:
172:
165:
86:. For example,
37:Ethylene glycol
28:
23:
22:
15:
12:
11:
5:
4877:
4875:
4867:
4866:
4861:
4851:
4850:
4847:
4846:
4834:
4828:
4823:
4818:
4813:
4808:
4801:
4800:External links
4798:
4797:
4796:
4775:
4750:
4743:
4731:
4728:
4725:
4724:
4697:
4684:
4657:
4630:
4617:
4604:
4577:
4551:
4525:
4499:
4446:
4403:
4376:
4353:
4326:
4299:
4272:
4245:
4238:
4208:
4184:
4156:
4129:
4106:
4102:
4094:
4067:
4040:
4013:
3986:
3959:
3946:
3912:
3893:
3866:
3839:
3812:
3785:
3772:
3759:
3732:
3705:
3678:
3651:
3623:
3596:
3569:
3556:
3528:
3501:
3488:
3475:
3462:
3435:
3408:
3381:
3354:
3330:
3321:
3308:
3300:ChemPep Inc.:
3293:
3276:
3257:
3244:
3217:
3190:
3163:
3161:, p. 195.
3150:
3137:
3106:
3104:, p. 186.
3093:
3068:
3048:reagent", in:
3045:
3041:
3032:
3004:
2991:
2978:
2951:
2924:
2897:
2870:
2843:
2816:
2789:
2761:
2734:
2715:
2692:
2659:
2632:
2605:
2578:
2551:
2538:
2525:
2498:
2471:
2469:, pp. 79.
2455:B. Helferich:
2448:
2421:
2408:
2395:
2367:
2340:
2321:
2313:
2285:
2251:
2224:
2197:
2170:
2142:
2129:
2116:
2097:
2070:
2057:
2050:
2028:
2001:
1994:
1975:
1974:
1972:
1969:
1835:
1832:
1829:
1828:
1815:
1789:. In contrast
1787:drug discovery
1783:chemical yield
1777:
1774:
1773:
1772:
1753:utilizing the
1731:
1728:
1684:
1681:
1680:
1679:
1669:
1660:
1657:
1649:
1648:
1641:
1613:
1610:
1609:
1608:
1596:
1588:
1582:
1579:organometallic
1572:
1569:
1546:
1533:
1527:
1521:
1494:
1491:
1474:
1471:
1470:
1469:
1463:
1453:
1441:
1438:
1357:and the mixed
1333:-acetals, the
1252:
1249:
1248:
1247:
1240:-Methoxyphenyl
1234:
1233:-methoxybenzyl
1227:hydrogenolysis
1220:
1217:hydrogenolysis
1213:-Methoxybenzyl
1207:
1205:hydrogenolysis
1196:Benzylamines:
1194:
1193:
1171:
1155:
1151:
1145:
1139:
1095:Other amides:
1093:
1092:
1082:
1049:
1041:
1032:
1029:hydrogenolysis
1019:
1004:hydrogenolysis
1000:Carbobenzyloxy
997:
986:
983:
944:carbon dioxide
891:
888:
855:isopropylidene
834:
831:
830:
829:
823:
819:
816:
802:
792:
784:Other ethers:
782:
781:
767:
760:
757:silver nitrate
742:
732:
726:
716:
710:
704:
701:
687:
686:
676:
663:
657:
654:hydrogenolysis
643:
642:
636:
633:
615:
601:
583:Trimethylsilyl
578:Silyl ethers:
576:
575:
569:
563:
548:
545:
526:Direct Process
510:
509:
476:triorganosilyl
463:
460:
458:
455:
343:
342:
339:
333:
330:
324:
318:
312:
306:
300:
289:
286:
274:
273:
270:
263:
261:
251:
244:
207:hydroxyl group
195:carboxyl group
164:
161:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
4876:
4865:
4862:
4860:
4857:
4856:
4854:
4844:
4843:
4838:
4835:
4833:
4829:
4827:
4824:
4822:
4819:
4817:
4814:
4812:
4809:
4807:
4804:
4803:
4799:
4794:
4790:
4786:
4785:
4780:
4776:
4773:
4769:
4765:
4761:
4760:
4755:
4751:
4748:
4744:
4741:
4737:
4734:
4733:
4729:
4721:
4717:
4713:
4709:
4708:
4701:
4698:
4694:
4688:
4685:
4680:
4676:
4672:
4668:
4661:
4658:
4654:
4650:
4646:
4642:
4641:
4634:
4631:
4627:
4621:
4618:
4614:
4608:
4605:
4601:
4597:
4593:
4589:
4588:
4581:
4578:
4575:
4571:
4567:
4563:
4560:
4555:
4552:
4549:
4545:
4541:
4537:
4534:
4529:
4526:
4523:
4519:
4515:
4511:
4508:
4503:
4500:
4495:
4491:
4487:
4483:
4479:
4475:
4471:
4467:
4463:
4459:
4458:
4450:
4447:
4442:
4438:
4434:
4430:
4426:
4423:
4422:
4414:
4407:
4404:
4400:
4396:
4392:
4388:
4387:
4380:
4377:
4373:
4369:
4365:
4364:
4357:
4354:
4350:
4346:
4342:
4338:
4337:
4330:
4327:
4323:
4319:
4315:
4311:
4310:
4303:
4300:
4296:
4292:
4288:
4284:
4283:
4276:
4273:
4269:
4265:
4261:
4257:
4256:
4249:
4246:
4241:
4235:
4231:
4227:
4222:
4221:
4212:
4209:
4205:
4201:
4197:
4196:
4188:
4185:
4181:
4177:
4173:
4169:
4168:
4160:
4157:
4153:
4149:
4145:
4141:
4140:
4133:
4130:
4126:
4122:
4118:
4114:
4113:
4098:
4095:
4091:
4087:
4083:
4079:
4078:
4071:
4068:
4064:
4060:
4056:
4052:
4051:
4044:
4041:
4037:
4033:
4029:
4025:
4024:
4023:J. Org. Chem.
4017:
4014:
4010:
4006:
4002:
3998:
3997:
3996:J. Org. Chem.
3990:
3987:
3983:
3979:
3975:
3971:
3970:
3963:
3960:
3956:
3950:
3947:
3941:
3936:
3932:
3929:
3928:
3923:
3916:
3913:
3909:
3905:
3904:
3897:
3894:
3890:
3886:
3882:
3878:
3877:
3870:
3867:
3863:
3859:
3855:
3851:
3850:
3843:
3840:
3836:
3832:
3828:
3824:
3823:
3816:
3813:
3809:
3805:
3801:
3797:
3796:
3789:
3786:
3782:
3776:
3773:
3769:
3763:
3760:
3756:
3752:
3748:
3744:
3743:
3742:J. Org. Chem.
3736:
3733:
3729:
3725:
3721:
3717:
3716:
3709:
3706:
3702:
3698:
3694:
3690:
3689:
3682:
3679:
3675:
3671:
3667:
3663:
3662:
3655:
3652:
3648:
3644:
3640:
3636:
3635:
3627:
3624:
3620:
3616:
3612:
3608:
3607:
3600:
3597:
3593:
3589:
3585:
3581:
3580:
3573:
3570:
3566:
3560:
3557:
3553:
3549:
3545:
3541:
3540:
3532:
3529:
3525:
3521:
3517:
3513:
3512:
3505:
3502:
3498:
3492:
3489:
3485:
3479:
3476:
3472:
3466:
3463:
3459:
3455:
3451:
3447:
3446:
3445:J. Org. Chem.
3439:
3436:
3432:
3428:
3424:
3420:
3419:
3412:
3409:
3405:
3401:
3397:
3393:
3392:
3385:
3382:
3378:
3374:
3370:
3366:
3365:
3358:
3355:
3350:
3346:
3342:
3338:
3337:
3325:
3322:
3318:
3312:
3309:
3305:
3304:
3297:
3294:
3290:
3286:
3280:
3277:
3273:
3269:
3268:
3261:
3258:
3254:
3248:
3245:
3241:
3237:
3233:
3229:
3228:
3221:
3218:
3214:
3210:
3206:
3202:
3201:
3200:J. Org. Chem.
3194:
3191:
3187:
3183:
3179:
3175:
3174:
3167:
3164:
3160:
3154:
3151:
3147:
3141:
3138:
3134:
3130:
3126:
3122:
3121:
3116:
3110:
3107:
3103:
3097:
3094:
3090:
3086:
3082:
3078:
3077:J. Org. Chem.
3072:
3069:
3065:
3061:
3057:
3053:
3052:
3036:
3033:
3029:
3025:
3021:
3017:
3016:
3008:
3005:
3002:, p. 62.
3001:
2995:
2992:
2988:
2982:
2979:
2975:
2971:
2967:
2963:
2962:
2961:J. Org. Chem.
2955:
2952:
2948:
2944:
2940:
2936:
2935:
2928:
2925:
2921:
2917:
2913:
2909:
2908:
2901:
2898:
2894:
2890:
2886:
2882:
2881:
2874:
2871:
2867:
2863:
2859:
2855:
2854:
2847:
2844:
2840:
2836:
2832:
2828:
2827:
2820:
2817:
2813:
2809:
2805:
2801:
2800:
2793:
2790:
2786:
2782:
2778:
2774:
2773:
2772:J. Org. Chem.
2765:
2762:
2758:
2754:
2750:
2746:
2745:
2738:
2735:
2731:
2727:
2726:
2719:
2716:
2712:
2708:
2704:
2703:
2696:
2693:
2687:
2682:
2678:
2674:
2670:
2663:
2660:
2656:
2652:
2648:
2644:
2643:
2636:
2633:
2629:
2625:
2621:
2617:
2616:
2609:
2606:
2602:
2598:
2594:
2590:
2589:
2588:J. Org. Chem.
2582:
2579:
2575:
2571:
2567:
2563:
2562:
2555:
2552:
2549:, p. 77.
2548:
2542:
2539:
2535:
2529:
2526:
2522:
2518:
2514:
2510:
2509:
2502:
2499:
2495:
2491:
2487:
2483:
2482:
2475:
2472:
2468:
2464:
2460:
2459:
2452:
2449:
2445:
2441:
2437:
2433:
2432:
2425:
2422:
2418:
2412:
2409:
2406:, p. 40.
2405:
2399:
2396:
2392:
2388:
2384:
2380:
2379:
2371:
2368:
2364:
2360:
2356:
2352:
2351:
2344:
2341:
2337:
2333:
2329:
2328:
2317:
2314:
2310:
2306:
2302:
2298:
2297:
2296:J. Org. Chem.
2289:
2286:
2282:
2278:
2274:
2270:
2269:
2264:
2258:
2256:
2252:
2248:
2244:
2240:
2236:
2235:
2234:J. Org. Chem.
2228:
2225:
2221:
2217:
2213:
2209:
2208:
2201:
2198:
2194:
2190:
2186:
2185:
2174:
2171:
2167:
2163:
2159:
2155:
2154:
2146:
2143:
2140:, p. 31.
2139:
2133:
2130:
2127:, p. 29.
2126:
2120:
2117:
2113:
2109:
2108:
2101:
2098:
2094:
2090:
2086:
2082:
2081:
2074:
2071:
2067:
2061:
2058:
2053:
2047:
2043:
2039:
2032:
2029:
2025:
2021:
2017:
2013:
2012:
2005:
2002:
1997:
1991:
1987:
1980:
1977:
1970:
1964:
1960:
1958:
1954:
1950:
1946:
1941:
1939:
1935:
1926:
1922:
1920:
1916:
1907:
1903:
1901:
1897:
1896:Yoshito Kishi
1893:
1889:
1884:
1882:
1878:
1869:
1865:
1863:
1859:
1858:ascorbic acid
1854:
1852:
1848:
1845:
1841:
1833:
1824:
1820:
1811:
1806:
1802:
1801:
1795:
1792:
1788:
1784:
1775:
1769:
1768:quantum yield
1761:
1756:
1752:
1748:
1745:
1744:photochemical
1741:
1737:
1734:
1733:
1729:
1724:
1720:
1718:
1709:
1705:
1703:
1699:
1695:
1691:
1682:
1677:
1673:
1670:
1667:
1663:
1662:
1658:
1653:
1646:
1642:
1639:
1635:
1631:
1627:
1626:
1625:
1623:
1622:isomerization
1619:
1618:electrophilic
1611:
1607:
1603:
1599:
1592:
1589:
1586:
1583:
1580:
1576:
1573:
1570:
1567:
1563:
1561:
1555:
1551:
1547:
1544:
1540:
1538:
1534:
1531:
1528:
1525:
1522:
1519:
1515:
1511:
1507:
1503:
1500:
1497:
1496:
1492:
1490:
1486:
1484:
1480:
1472:
1467:
1464:
1461:
1458:– Removed by
1457:
1454:
1451:
1447:
1444:
1443:
1439:
1434:
1430:
1428:
1423:
1421:
1417:
1413:
1409:
1405:
1403:
1399:
1395:
1391:
1387:
1383:
1378:
1376:
1372:
1368:
1364:
1360:
1356:
1352:
1348:
1344:
1340:
1336:
1332:
1328:
1323:
1319:
1317:
1313:
1309:
1305:
1300:
1298:
1297:1,3-propadiol
1294:
1285:
1277:
1273:
1271:
1267:
1263:
1259:
1250:
1245:
1241:
1239:
1235:
1232:
1228:
1224:
1221:
1218:
1214:
1212:
1208:
1206:
1202:
1199:
1198:
1197:
1192:
1188:
1184:
1180:
1176:
1172:
1169:
1165:
1161:
1149:
1146:
1143:
1140:
1137:
1133:
1129:
1125:
1121:
1111:
1101:
1098:
1097:
1096:
1090:
1086:
1083:
1081:
1078:. Common in
1077:
1076:glycopeptides
1073:
1069:
1067:
1062:
1058:
1054:
1050:
1047:
1039:
1037:
1033:
1030:
1026:
1024:
1020:
1017:
1013:
1009:
1005:
1001:
998:
995:
992:
991:
990:
984:
982:
980:
976:
972:
968:
964:
960:
956:
951:
949:
945:
941:
937:
933:
929:
927:
923:
919:
910:
905:
901:
896:
889:
884:
880:
878:
870:
866:
864:
860:
856:
852:
848:
844:
840:
832:
827:
824:
817:
814:
810:
806:
803:
800:
796:
793:
790:
787:
786:
785:
779:
775:
771:
768:
765:
761:
758:
754:
750:
746:
743:
740:
736:
733:
730:
727:
724:
720:
717:
715:
711:
708:
705:
702:
699:
695:
692:
691:
690:
684:
680:
677:
674:
670:
668:
664:
661:
658:
655:
651:
648:
647:
646:
640:
637:
634:
631:
627:
623:
619:
616:
613:
609:
605:
604:Triethylsilyl
602:
600:
596:
592:
588:
585:(TMS) —
584:
581:
580:
579:
573:
570:
567:
564:
561:
560:Acetoxy group
557:
554:
553:
552:
546:
541:
537:
533:
531:
527:
523:
514:
507:
506:
505:
503:
499:
495:
490:
488:
484:
483:(hemi)acetals
480:
477:
473:
469:
461:
456:
454:
448:
444:
442:
438:
433:
431:
427:
423:
419:
415:
408:
401:
397:
395:
391:
387:
382:
380:
379:benzyl groups
376:
375:hydrogenation
371:
369:
365:
364:biological pH
361:
357:
355:
351:
346:
340:
337:
334:
331:
328:
325:
322:
319:
316:
313:
310:
307:
304:
301:
298:
295:
294:
293:
287:
285:
283:
279:
267:
262:
259:
255:
248:
243:
241:
239:
234:
229:
227:
223:
219:
215:
208:
204:
200:
196:
192:
188:
184:
180:
175:
169:
162:
160:
156:
154:
150:
149:shikimic acid
146:
142:
138:
134:
130:
126:
122:
116:
114:
110:
106:
101:
97:
93:
89:
85:
81:
76:
74:
70:
66:
62:
58:
50:
46:
42:
38:
34:
30:
19:
4841:
4788:
4782:
4774:(in German).
4767:
4763:
4757:
4746:
4739:
4715:
4711:
4705:
4700:
4692:
4687:
4673:(4): 93–94.
4670:
4666:
4660:
4648:
4644:
4638:
4633:
4625:
4620:
4612:
4607:
4595:
4591:
4585:
4580:
4565:
4558:
4554:
4539:
4532:
4528:
4513:
4506:
4502:
4461:
4455:
4449:
4424:
4419:
4406:
4394:
4390:
4384:
4379:
4367:
4361:
4356:
4344:
4340:
4334:
4329:
4317:
4313:
4307:
4302:
4290:
4286:
4280:
4275:
4263:
4259:
4253:
4248:
4219:
4211:
4199:
4193:
4187:
4175:
4171:
4165:
4159:
4147:
4143:
4137:
4132:
4120:
4116:
4110:
4097:
4085:
4081:
4075:
4070:
4058:
4054:
4048:
4043:
4031:
4027:
4021:
4016:
4004:
4000:
3994:
3989:
3977:
3973:
3967:
3962:
3954:
3949:
3930:
3925:
3915:
3907:
3901:
3896:
3884:
3880:
3874:
3869:
3857:
3853:
3847:
3842:
3830:
3826:
3820:
3815:
3810:(in German).
3803:
3799:
3793:
3788:
3780:
3775:
3767:
3762:
3750:
3746:
3740:
3735:
3723:
3719:
3713:
3708:
3696:
3692:
3686:
3681:
3669:
3665:
3659:
3654:
3642:
3638:
3632:
3626:
3614:
3610:
3604:
3599:
3587:
3583:
3577:
3572:
3564:
3559:
3547:
3543:
3537:
3531:
3519:
3515:
3509:
3504:
3496:
3491:
3483:
3478:
3470:
3465:
3453:
3449:
3443:
3438:
3426:
3422:
3416:
3411:
3399:
3395:
3389:
3384:
3372:
3368:
3362:
3357:
3340:
3334:
3324:
3316:
3311:
3302:
3296:
3289:Angew. Chem.
3288:
3284:
3279:
3271:
3266:
3260:
3252:
3247:
3235:
3231:
3225:
3220:
3208:
3204:
3198:
3193:
3181:
3177:
3171:
3166:
3158:
3153:
3145:
3140:
3128:
3124:
3118:
3114:
3109:
3101:
3096:
3084:
3080:
3076:
3071:
3059:
3055:
3049:
3035:
3023:
3019:
3013:
3007:
2999:
2994:
2986:
2981:
2969:
2965:
2959:
2954:
2942:
2938:
2932:
2927:
2915:
2911:
2905:
2900:
2888:
2884:
2878:
2873:
2861:
2857:
2851:
2846:
2834:
2830:
2824:
2819:
2807:
2803:
2797:
2792:
2780:
2776:
2770:
2764:
2752:
2748:
2742:
2737:
2729:
2723:
2718:
2706:
2700:
2695:
2676:
2672:
2662:
2650:
2646:
2640:
2635:
2623:
2619:
2613:
2608:
2596:
2592:
2586:
2581:
2569:
2565:
2559:
2554:
2546:
2541:
2533:
2528:
2516:
2512:
2506:
2501:
2489:
2485:
2479:
2474:
2466:
2462:
2456:
2451:
2439:
2435:
2429:
2424:
2416:
2411:
2403:
2398:
2386:
2382:
2376:
2370:
2358:
2354:
2348:
2343:
2331:
2325:
2316:
2304:
2300:
2294:
2288:
2276:
2272:
2266:
2242:
2238:
2232:
2227:
2215:
2211:
2205:
2200:
2188:
2182:
2173:
2161:
2157:
2151:
2145:
2137:
2132:
2124:
2119:
2111:
2105:
2100:
2095:(in German).
2088:
2084:
2078:
2073:
2065:
2060:
2037:
2031:
2026:(in German).
2019:
2015:
2009:
2004:
1985:
1979:
1952:
1942:
1931:
1912:
1899:
1885:
1874:
1855:
1837:
1779:
1714:
1694:butyllithium
1686:
1675:
1629:
1615:
1562:-butylphenol
1559:
1536:
1517:
1513:
1487:
1476:
1424:
1419:
1415:
1411:
1407:
1406:
1401:
1397:
1393:
1389:
1385:
1381:
1379:
1370:
1366:
1362:
1358:
1346:
1342:
1338:
1334:
1330:
1326:
1325:Besides the
1324:
1320:
1301:
1290:
1269:
1265:
1261:
1257:
1254:
1237:
1230:
1210:
1195:
1094:
1065:
1035:
1022:
989:Carbamates:
988:
974:
970:
955:phthalimides
952:
947:
932:Amine groups
930:
915:
908:
903:
874:
836:
808:
788:
783:
738:
723:zinc bromide
688:
682:
678:
666:
644:
638:
622:acetonitrile
617:
577:
550:
534:
524:(TMS-Cl), a
519:
491:
472:nucleophiles
465:
452:
440:
434:
422:noble metals
412:
386:quinomethide
383:
372:
368:temperatures
358:
347:
344:
291:
278:carbohydrate
275:
257:
253:
230:
225:
221:
213:
212:
209:of Tyrosine.
202:
198:
190:
182:
178:
173:
157:
140:
121:biomolecules
117:
113:deprotection
112:
108:
83:
77:
60:
56:
54:
47:) during an
29:
18:Deprotection
4845:(in German)
4707:Tetrahedron
4562:Tetrahedron
4536:Tetrahedron
4510:Tetrahedron
4397:, C21–C24;
4347:, C45–C48;
4282:Tetrahedron
4228:. pp.
4077:Tetrahedron
3688:Tetrahedron
3391:Chem. Lett.
3051:Tetrahedron
2481:Tetrahedron
2263:latrunculin
1919:Danishefsky
1915:Mitomycin C
1851:oseltamivir
1585:Orthoesters
1460:Lewis acids
1355:Thioacetals
1136:methylamine
922:nucleophile
851:benzylidene
591:acetic acid
437:chromophore
336:Photolabile
187:amino group
153:oseltamivir
133:nucleotides
39:protects a
4853:Categories
4421:Org. Lett.
4255:Chem. Rev.
4167:Org. Lett.
4139:Chem. Rev.
3969:Chem. Rev.
1971:References
1862:Reichstein
1823:Phil Baran
1530:Benzhydryl
1483:oxazolines
1427:hemiaminal
1316:silica gel
1187:thiophenol
1162:in liquid
1072:morpholine
1057:piperidine
967:imidazoles
530:silica gel
426:enol ether
373:Catalytic
366:(5-9) and
139:may often
67:to obtain
3903:Synthesis
2725:Synthesis
2107:Synthesis
1909:Palytoxin
1892:palytoxin
1847:synthesis
1840:sucralose
1776:Criticism
1591:Oxazoline
1581:reagents.
1466:Dithianes
1272:-acetals.
1126:and N in
1070:, or 50%
1063:(DMF) or
1012:palladium
994:Carbamate
985:Selection
940:carbamate
936:acylation
865:acetals.
833:1,2-Diols
776:in HMPT (
689:Acetals:
418:isomerize
392:(CAN) or
327:Oxidation
321:Reduction
84:protected
4486:17377577
4441:17555322
2265:A", in:
1938:aldehyde
1894:acid by
1636:or with
1620:attack,
1124:cytosine
1008:hydrogen
963:pyrroles
599:methanol
572:Pivaloyl
551:Esters:
498:benzoate
309:Fluoride
218:tyrosine
125:peptides
100:carbonyl
96:alcohols
4781:", in:
4494:4357378
4466:Bibcode
3336:Synlett
1934:epoxide
1881:acetone
1558:2,6-di-
1456:Acylals
1446:Acetals
1304:Acetone
1164:ammonia
1132:ammonia
1128:adenine
1110:Benzoyl
959:Indoles
900:glycine
839:glycols
566:Benzoyl
494:acetate
430:enamine
396:(DDQ).
360:Lipases
350:silicon
141:shorten
43:(as an
4492:
4484:
4457:Nature
4439:
4236:
3117:, in:
2048:
1992:
1936:to an
1672:Methyl
1612:Alkene
1539:-Butyl
1524:Benzyl
1502:esters
1499:Methyl
1450:Ketals
1375:sulfur
1351:Thiols
1341:- and
1201:Benzyl
1177:&
1160:sodium
1100:Acetyl
1089:nickel
902:. The
847:acetal
843:sugars
822:in DCM
660:Trityl
650:Benzyl
556:Acetyl
500:, and
479:ethers
468:esters
315:Enzyme
147:(e.g.
105:acetal
92:esters
45:acetal
41:ketone
4490:S2CID
4416:(PDF)
3044:-AlCl
1844:Roche
1810:Tosyl
1738:As a
1730:Other
1630:trans
1595:LiAlH
1575:Silyl
1264:– or
1246:(CAN)
1175:Nosyl
1148:Tosyl
926:bases
813:DABCO
805:Allyl
487:ether
416:will
240:).
197:and (
179:black
123:like
49:ester
4789:1998
4764:1996
4712:1992
4645:1993
4592:1934
4566:1990
4540:1990
4514:1990
4482:PMID
4437:PMID
4391:1972
4368:1977
4341:1977
4314:1971
4287:2002
4260:1967
4234:ISBN
4230:1291
4200:2000
4172:2001
4144:1999
4117:2010
4105:TiCl
4082:1998
4055:1990
4028:1998
4001:1974
3974:1996
3908:1978
3881:1978
3854:1975
3827:1987
3800:1983
3747:1986
3720:1972
3693:1978
3666:1992
3639:1990
3611:1986
3584:1983
3544:1991
3516:1988
3450:1986
3423:1985
3396:1981
3369:1980
3341:2006
3287:In:
3232:1992
3205:1978
3178:1988
3125:1984
3081:1988
3056:1982
3020:1985
2966:1984
2939:1988
2912:1986
2885:1984
2858:1982
2831:1975
2804:1978
2777:1979
2749:1987
2730:1975
2707:1981
2647:1983
2620:1982
2593:1983
2566:1980
2513:1982
2486:1991
2463:1948
2436:1986
2383:1991
2355:1987
2332:1981
2301:1990
2273:1990
2239:1991
2212:1990
2189:1986
2158:1990
2112:1980
2085:1996
2046:ISBN
2016:1996
1990:ISBN
1953:tert
1634:zinc
1560:tert
1537:tert
1448:and
1142:Troc
1091:(0).
1053:Fmoc
1036:tert
1010:and
965:und
948:tert
909:blue
904:tert
898:BOC
857:and
809:tert
749:soft
639:tert
618:tert
547:List
303:Base
297:Acid
256:and
226:tert
222:tert
203:tert
181:). (
174:blue
151:for
4768:103
4675:doi
4649:115
4570:doi
4544:doi
4518:doi
4474:doi
4462:446
4429:doi
4345:125
3935:doi
3831:109
3643:112
3548:113
3520:110
3345:doi
3236:114
3182:110
3024:107
2889:106
2862:104
2808:100
2753:109
2681:doi
2651:105
2624:104
2570:102
2387:109
2359:109
2277:112
2216:112
2162:112
2089:103
2020:103
1947:by
1917:by
1849:of
1757::
1749:by
1692:or
1676:e.c
1604:or
1566:DBU
1314:on
1295:or
1179:Nps
1166:or
1134:or
1108:),
1059:in
1014:on
861:or
673:DDQ
597:in
593:or
189:, (
155:).
143:an
131:or
94:to
59:or
4855::
4839::
4787:,
4766:,
4762:,
4738::
4716:48
4714:,
4710:,
4669:.
4647:,
4643:,
4596:17
4594:,
4590:,
4488:.
4480:.
4472:.
4460:.
4435:.
4418:.
4395:44
4393:,
4389:,
4366:,
4343:,
4339:,
4318:37
4316:,
4312:,
4291:58
4289:,
4285:,
4264:67
4262:,
4258:,
4232:.
4224:.
4198:,
4174:,
4170:,
4148:99
4146:,
4142:,
4121:31
4119:,
4115:,
4086:54
4084:,
4080:,
4059:31
4057:,
4053:,
4032:63
4030:,
4026:,
4005:39
4003:,
3999:,
3978:96
3976:,
3972:,
3931:53
3924:.
3906:,
3885:19
3883:,
3879:,
3858:97
3856:,
3852:,
3829:,
3825:,
3804:24
3802:,
3798:,
3751:51
3749:,
3745:,
3724:94
3722:,
3718:,
3697:34
3695:,
3691:,
3670:33
3668:,
3664:,
3641:,
3637:,
3615:69
3613:,
3609:,
3588:66
3586:,
3582:,
3546:,
3542:,
3518:,
3514:,
3454:51
3452:,
3448:,
3427:26
3425:,
3421:,
3400:10
3398:,
3394:,
3373:21
3371:,
3367:,
3339:.
3234:,
3230:,
3209:43
3207:,
3203:,
3180:,
3176:,
3129:25
3127:,
3123:,
3085:53
3083:,
3079:,
3060:38
3058:,
3054:,
3022:,
3018:,
2970:49
2968:,
2964:,
2943:29
2941:,
2937:,
2916:27
2914:,
2910:,
2887:,
2883:,
2860:,
2856:,
2835:16
2833:,
2829:,
2806:,
2802:,
2781:44
2779:,
2775:,
2751:,
2747:,
2728:,
2705:,
2677:24
2675:.
2671:.
2649:,
2645:,
2622:,
2618:,
2597:48
2595:,
2591:,
2568:,
2564:,
2517:23
2515:,
2511:,
2490:47
2488:,
2484:,
2465:,
2461:,
2440:27
2438:,
2434:,
2385:,
2381:,
2357:,
2353:,
2330:,
2305:55
2303:,
2299:,
2275:,
2271:,
2254:^
2243:56
2241:,
2237:,
2214:,
2210:,
2187:,
2160:,
2156:,
2110:,
2087:,
2083:,
2044:.
2040:.
2018:,
2014:,
1940:.
1921:.
1864:.
1704:.
1600:,
1556:,
1552:,
1485:.
1185:,
1154:SO
1115:Bz
1105:Ac
1006::
961:,
853:,
589:,
562:).
496:,
474:;
201:)
127:,
115:.
75:.
55:A
4795:.
4722:.
4681:.
4677::
4671:7
4655:.
4602:.
4572::
4546::
4520::
4496:.
4476::
4468::
4443:.
4431::
4425:9
4401:.
4374:.
4351:.
4324:.
4297:.
4270:.
4242:.
4206:.
4182:.
4176:3
4154:.
4127:.
4107:2
4103:2
4092:.
4065:.
4038:.
4011:.
3984:.
3943:.
3937::
3891:.
3864:.
3837:.
3757:.
3730:.
3703:.
3676:.
3649:.
3621:.
3594:.
3554:.
3526:.
3460:.
3433:.
3406:.
3379:.
3351:.
3347::
3331:2
3242:.
3215:.
3188:.
3135:.
3091:.
3066:.
3046:3
3042:4
3030:.
2976:.
2949:.
2922:.
2895:.
2868:.
2841:.
2814:.
2787:.
2759:.
2713:.
2689:.
2683::
2657:.
2630:.
2603:.
2576:.
2534:p
2523:.
2496:.
2467:3
2446:.
2393:.
2365:.
2338:.
2322:2
2311:.
2283:.
2249:.
2222:.
2195:.
2179:′
2168:.
2054:.
1998:.
1900:p
1668:.
1640:.
1597:4
1518:N
1516:,
1514:N
1462:.
1420:S
1418:,
1416:S
1412:O
1410:,
1408:S
1402:O
1400:,
1398:O
1394:O
1392:,
1390:O
1386:S
1384:,
1382:S
1371:O
1369:,
1367:O
1363:O
1361:,
1359:S
1347:S
1345:,
1343:S
1339:O
1337:,
1335:S
1331:O
1329:,
1327:O
1270:S
1268:,
1266:S
1262:S
1260:,
1258:O
1238:p
1231:p
1211:p
1170:)
1156:4
1152:2
1112:(
1102:(
1066:N
1048:.
1042:3
1023:p
975:N
971:N
912:.
820:3
789:p
766:.
739:p
700:.
683:m
681:,
679:p
675:.
667:p
632:.
441:o
258:Y
254:X
199:3
191:2
183:1
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.