445:
332:
146:
831:
155:
592:
133:
29:
866:, when all the ascorbic acid in the solution has been used up, there will not be any electrons available to reduce the DCPIPH and the solution remains pink due to the DCPIPH. The end point is a pink color that persists for 10 seconds or more, if there is not enough ascorbic acid to reduce all of the DCPIPH. Pharmacological experiments suggest that DCPIP may serve as a
732:
840:. If vitamin C, which is a good reducing agent, is present, the blue dye, which turns pink in acid conditions, is reduced to a colorless compound by ascorbic acid. This reaction is a redox reaction: vitamin C (ascorbic acid) is oxidized to
600:
572:
678:
745:
494:
815:
46:
674:
241:
93:
818:, that is normally the final electron carrier in photosynthesis. As DCPIP is reduced and becomes colorless, the resultant increase in
65:
459:
112:
72:
666:
830:
50:
17:
806:
family. When exposed to light in a photosynthetic system, the dye is decolorised by chemical reduction. DCPIP has a higher
662:
79:
752:
640:
402:
423:
706:
61:
591:
39:
1038:
339:
1033:
698:
694:
682:
327:
289:
1043:
614:
584:
928:"Ascorbate in plasma as measured by liquid chromatography and by dichlorophenolindophenol colorimetry"
86:
841:
173:
980:"Antimelanoma activity of the redox dye DCPIP (2,6-dichlorophenolindophenol) is antagonized by NQO1"
927:
440:
145:
819:
654:
207:
154:
646:
658:
1009:
939:
906:
823:
807:
780:
309:
959:
Design an investigation to compare the amount of vitamin C in different fruits and vegetables
999:
991:
879:
517:
686:
411:
784:
670:
217:
444:
331:
1004:
979:
901:
799:
723:
269:
1027:
814:
and the photosynthetic electron transport chain can reduce DCPIP as a substitute for
320:
714:
896:
803:
650:
391:
875:
867:
632:
132:
28:
702:
995:
957:
891:
811:
554:
300:
628:
468:
InChI=1S/C12H7Cl2NO2/c13-10-5-8(6-11(14)12(10)17)15-7-1-3-9(16)4-2-7/h1-6,16H
863:
837:
478:
InChI=1/C12H7Cl2NO2/c13-10-5-8(6-11(14)12(10)17)15-7-1-3-9(16)4-2-7/h1-6,16H
1013:
943:
870:
chemotherapeutic targeting human cancer cells in an animal model of human
871:
788:
874:; DCPIP-induced cancer cell death occurs by depletion of intracellular
378:
340:
624:
280:
722:
Except where otherwise noted, data are given for materials in their
620:
366:
16:"DCIP" redirects here. For the children's rights organization, see
792:
260:
240:
230:
710:
357:
791:, DCPIP is blue with a maximal absorption at 600 nm; when
22:
178:
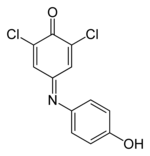
4-(3,5-dichloro-4-hydroxyphenyl)iminocyclohexa-2,5-dien-1-one
690:
428:
153:
144:
978:
Cabello CM, Bair WB, Bause AS, Wondrak GT (August 2009).
844:, and DCPIP is reduced to the colorless compound DCPIPH
740:
53:. Unsourced material may be challenged and removed.
390:
216:
926:VanderJagt DJ, Garry PJ, Hunt WC (June 1986).
441:DTXSID501174455 DTXSID7061352, DTXSID501174455
8:
836:DCPIP can also be used as an indicator for
443:
330:
308:
124:
1003:
798:DCPIP can be used to measure the rate of
410:
113:Learn how and when to remove this message
918:
499:
464:
439:
195:2,6-Dichloro-4--2,5-cyclohexadien-1-one
851:DCPIP (blue) + H → DCPIPH (pink)
321:
502:Cl\C2=CC(=N/c1ccc(O)cc1)/C=C(/Cl)C2=O
471:Key: CCBICDLNWJRFPO-UHFFFAOYSA-N
288:
268:
7:
51:adding citations to reliable sources
854:DCPIPH (pink) + vitamin C → DCPIPH
481:Key: CCBICDLNWJRFPO-UHFFFAOYAL
381:
365:
14:
829:
730:
590:
541:
535:
529:
131:
27:
726:(at 25 °C , 100 kPa).
38:needs additional citations for
544:
523:
18:Defence for Children Palestine
1:
190:2,6-Dichlorophenolindophenol;
765:2,6-Dichlorophenolindophenol
140:
1060:
62:"Dichlorophenolindophenol"
15:
996:10.1016/j.bcp.2009.04.016
720:
571:
566:
510:
490:
455:
200:
184:
172:
167:
139:
130:
126:Dichlorophenolindophenol
822:can be measured using a
641:Precautionary statements
192:2,6-Dichloroindophenol;
795:, DCPIP is colorless.
187:Dichloroindophenol ();
158:
149:
157:
148:
878:and upregulation of
842:dehydroascorbic acid
802:. It is part of the
174:Preferred IUPAC name
47:improve this article
820:light transmittance
810:for electrons than
562: g·mol
127:
984:Biochem. Pharmacol
753:Infobox references
255:DCPIP, DCIP, DPIP
159:
150:
125:
824:spectrophotometer
781:chemical compound
761:Chemical compound
759:
758:
615:Hazard statements
424:CompTox Dashboard
242:Interactive image
163:
162:
123:
122:
115:
97:
1051:
1039:Redox indicators
1018:
1017:
1007:
975:
969:
968:
967:
966:
954:
948:
947:
923:
880:oxidative stress
833:
743:
737:
734:
733:
716:
712:
708:
704:
700:
696:
692:
688:
684:
680:
676:
672:
668:
664:
660:
656:
652:
648:
634:
630:
626:
622:
594:
561:
546:
543:
537:
531:
525:
518:Chemical formula
448:
447:
432:
430:
414:
394:
383:
369:
342:
334:
323:
312:
292:
272:
244:
220:
141:
135:
128:
118:
111:
107:
104:
98:
96:
55:
31:
23:
1059:
1058:
1054:
1053:
1052:
1050:
1049:
1048:
1034:Indophenol dyes
1024:
1023:
1022:
1021:
977:
976:
972:
964:
962:
956:
955:
951:
925:
924:
920:
915:
888:
857:
847:
762:
755:
750:
749:
748: ?)
739:
735:
731:
727:
643:
617:
603:
587:
559:
549:
540:
534:
528:
520:
506:
503:
498:
497:
486:
483:
482:
479:
473:
472:
469:
463:
462:
451:
433:
426:
417:
397:
384:
372:
352:
315:
295:
275:
247:
234:
223:
210:
196:
193:
191:
188:
180:
179:
119:
108:
102:
99:
56:
54:
44:
32:
21:
12:
11:
5:
1057:
1055:
1047:
1046:
1041:
1036:
1026:
1025:
1020:
1019:
970:
949:
917:
916:
914:
911:
910:
909:
907:Wurster's blue
904:
902:Methylene blue
899:
894:
887:
884:
860:
859:
855:
852:
845:
800:photosynthesis
760:
757:
756:
751:
729:
728:
724:standard state
721:
718:
717:
679:P305+P351+P338
644:
639:
636:
635:
618:
613:
610:
609:
604:
599:
596:
595:
588:
583:
580:
579:
569:
568:
564:
563:
557:
551:
550:
547:
538:
532:
526:
521:
516:
513:
512:
508:
507:
505:
504:
501:
493:
492:
491:
488:
487:
485:
484:
480:
477:
476:
474:
470:
467:
466:
458:
457:
456:
453:
452:
450:
449:
436:
434:
422:
419:
418:
416:
415:
407:
405:
399:
398:
396:
395:
387:
385:
377:
374:
373:
371:
370:
362:
360:
354:
353:
351:
350:
346:
344:
336:
335:
325:
317:
316:
314:
313:
305:
303:
297:
296:
294:
293:
285:
283:
277:
276:
274:
273:
265:
263:
257:
256:
253:
252:Abbreviations
249:
248:
246:
245:
237:
235:
228:
225:
224:
222:
221:
213:
211:
206:
203:
202:
198:
197:
186:
182:
181:
177:
176:
170:
169:
165:
164:
161:
160:
151:
137:
136:
121:
120:
35:
33:
26:
13:
10:
9:
6:
4:
3:
2:
1056:
1045:
1042:
1040:
1037:
1035:
1032:
1031:
1029:
1015:
1011:
1006:
1001:
997:
993:
990:(4): 344–54.
989:
985:
981:
974:
971:
961:
960:
953:
950:
945:
941:
938:(6): 1004–6.
937:
933:
929:
922:
919:
912:
908:
905:
903:
900:
898:
895:
893:
890:
889:
885:
883:
881:
877:
873:
869:
865:
853:
850:
849:
848:
843:
839:
834:
832:
827:
825:
821:
817:
813:
809:
805:
804:Hill reagents
801:
796:
794:
790:
786:
782:
778:
774:
770:
766:
754:
747:
742:
725:
719:
645:
642:
638:
637:
619:
616:
612:
611:
608:
605:
602:
598:
597:
593:
589:
586:
582:
581:
577:
575:
570:
565:
558:
556:
553:
552:
522:
519:
515:
514:
509:
500:
496:
489:
475:
465:
461:
454:
446:
442:
438:
437:
435:
425:
421:
420:
413:
409:
408:
406:
404:
401:
400:
393:
389:
388:
386:
380:
376:
375:
368:
364:
363:
361:
359:
356:
355:
348:
347:
345:
343:
338:
337:
333:
329:
326:
324:
322:ECHA InfoCard
319:
318:
311:
307:
306:
304:
302:
299:
298:
291:
287:
286:
284:
282:
279:
278:
271:
267:
266:
264:
262:
259:
258:
254:
251:
250:
243:
239:
238:
236:
232:
227:
226:
219:
215:
214:
212:
209:
205:
204:
199:
194:
183:
175:
171:
166:
156:
152:
147:
143:
142:
138:
134:
129:
117:
114:
106:
95:
92:
88:
85:
81:
78:
74:
71:
67:
64: –
63:
59:
58:Find sources:
52:
48:
42:
41:
36:This article
34:
30:
25:
24:
19:
1044:Chloroarenes
987:
983:
973:
963:, retrieved
958:
952:
935:
931:
921:
897:Metachromasy
861:
835:
828:
797:
776:
772:
768:
764:
763:
606:
573:
290:ChEMBL500871
201:Identifiers
189:
185:Other names
109:
100:
90:
83:
76:
69:
57:
45:Please help
40:verification
37:
876:glutathione
868:pro-oxidant
858:(colorless)
601:Signal word
511:Properties
328:100.012.254
1028:Categories
965:2023-11-18
932:Clin. Chem
913:References
892:Indophenol
812:ferredoxin
783:used as a
585:Pictograms
555:Molar mass
412:C35QN2Z58B
301:ChemSpider
229:3D model (
208:CAS Number
73:newspapers
864:titration
838:vitamin C
785:redox dye
707:P403+P233
699:P337+P313
695:P332+P313
675:P304+P340
671:P302+P352
667:P301+P312
576:labelling
349:213-479-8
341:EC Number
270:CHEBI:945
103:June 2015
1014:19394313
886:See also
872:melanoma
862:In this
808:affinity
789:oxidized
787:. When
567:Hazards
310:10661857
218:956-48-9
1005:2742658
944:3708799
793:reduced
779:) is a
746:what is
744: (
607:Warning
379:PubChem
87:scholar
1012:
1002:
942:
741:verify
738:
560:268.09
495:SMILES
367:C00102
281:ChEMBL
168:Names
89:
82:
75:
68:
60:
769:DCPIP
460:InChI
392:13726
261:ChEBI
231:JSmol
94:JSTOR
80:books
1010:PMID
940:PMID
816:NADP
777:DPIP
773:DCIP
715:P501
711:P405
703:P362
691:P330
687:P321
683:P312
663:P280
659:P271
655:P270
651:P264
647:P261
633:H335
629:H319
625:H315
621:H302
403:UNII
358:KEGG
66:news
1000:PMC
992:doi
775:or
574:GHS
429:EPA
382:CID
49:by
1030::
1008:.
998:.
988:78
986:.
982:.
936:32
934:.
930:.
882:.
826:.
771:,
713:,
709:,
705:,
701:,
697:,
693:,
689:,
685:,
681:,
677:,
673:,
669:,
665:,
661:,
657:,
653:,
649:,
631:,
627:,
623:,
578::
536:Cl
527:12
1016:.
994::
946:.
856:2
846:2
767:(
736:N
548:2
545:O
542:N
539:2
533:7
530:H
524:C
431:)
427:(
233:)
116:)
110:(
105:)
101:(
91:·
84:·
77:·
70:·
43:.
20:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.