752:
111:
308:
403:
ca. 0.3 nm, the
Helmholtz model predicts a differential capacitance value of about 18 μF/cm. This value can be used to calculate capacitance values using the standard formula for conventional plate capacitors if only the surface of the electrodes is known. This capacitance can be calculated
160:
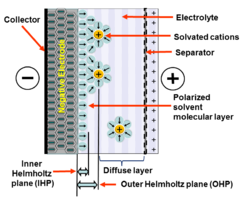
The amount of charge in the electrode is matched by the magnitude of counter-charges in the outer
Helmholtz plane (OHP). This is the area close to the IHP, in which the polarized electrolyte ions are collected. This separation of two layers of polarized ions through the double-layer stores electrical
476:
Because an electrochemical capacitor is composed out of two electrodes, electric charge in the
Helmholtz layer at one electrode is mirrored (with opposite polarity) in the second Helmholtz layer at the second electrode. Therefore, the total capacitance value of a double-layer capacitor is the result
171:
The "thickness" of a charged layer in the metallic electrode, i.e., the average extension perpendicular to the surface, is about 0.1 nm, and mainly depends on the electron density because the atoms in solid electrodes are stationary. In the electrolyte, the thickness depends on the size of the
136:
When a voltage is applied to the capacitor, two layers of polarized ions are generated at the electrode interfaces. One layer is within the solid electrode (at the surfaces of crystal grains from which it is made that are in contact with the electrolyte). The other layer, with opposite polarity,
285:, the dielectric layer of aluminum electrolytic capacitors, is approximately 1.4 nm/V. For a 6.3 V capacitor therefore the layer is 8.8 nm. The electric field is 6.3 V/8.8 nm = 716 kV/mm, around 7 times lower than in the double-layer. The
289:
of some 5000 kV/mm is unrealizable in conventional capacitors. No conventional dielectric material could prevent charge carrier breakthrough. In a double-layer capacitor the chemical stability of the solvent's molecular bonds prevents breakthrough.
132:
such as water. Where the liquid electrolyte contacts the electrode's conductive metallic surface, an interface is formed which represents a common boundary between the two phases of matter. It is at this interface that the double layer effect occurs.
311:
Structure and function of an ideal double-layer capacitor. Applying a voltage to the capacitor at both electrodes a
Helmholtz double-layer will be formed separating the adhered ions in the electrolyte in a mirror charge distribution of opposite
468:
In real produced supercapacitors with a high amount of double-layer capacitance the capacitance value depends first on electrode surface and DL distance. Parameters such as electrode material and structure, electrolyte mixture, and amount of
272:
316:
The double-layer is like the dielectric layer in a conventional capacitor, but with the thickness of a single molecule. Using the early
Helmholtz model to calculate the capacitance the model predicts a constant
699:
387:
477:
of two capacitors connected in series. If both electrodes have approximately the same capacitance value, as in symmetrical supercapacitors, the total value is roughly half that of one electrode.
293:
The forces that cause the adhesion of solvent molecules in the IHP are physical forces rather than chemical bonds. Chemical bonds exist within the adsorbed molecules, but they are polarized.
443:
128:
Every capacitor has two electrodes, mechanically separated by a separator. These are electrically connected via the electrolyte, a mixture of positive and negative ions dissolved in a
461:
and a small distance d between plates. Because activated carbon electrodes have a very high surface area and an extremely thin double-layer distance which is on the order of a few
296:
The magnitude of the electric charge that can accumulate in the layers corresponds to the concentration of the adsorbed ions and the electrodes surface. Up to the electrolyte's
114:
Simplified view of a double-layer of negative ions in the electrode and solvated positive ions in the liquid electrolyte, separated by a layer of polarized solvent molecules.
197:
145:
ions distributed in the electrolyte that have moved towards the polarized electrode. These two layers of polarized ions are separated by a monolayer of solvent
703:
791:
465:(0.3-0.8 nm), it is understandable why supercapacitors have the highest capacitance values among the capacitors (in the range of 10 to 40 μF/cm).
745:
517:
172:
solvent molecules and of the movement and concentration of ions in the solvent. It ranges from 0.1 to 10 nm as described by the
121:
laid the theoretical foundations for understanding the double layer phenomenon. The formation of double layers is exploited in every
341:
486:
684:
87:
23:
410:
800:
493:
46:
with opposing polarity form, one at the surface of the electrode, and one in the electrolyte. These two layers,
318:
300:, this arrangement behaves like a capacitor in which the stored electrical charge is linearly dependent on the
138:
751:
Download CHAPTER 2, ELECTRODE/ELECTROLYTE INTERFACES: STRUCTURE AND KINETICS OF CHARGE TRANSFER (pdf, 769 kB)
749:
168:
in the molecular IHP layer of the solvent molecules that corresponds to the strength of the applied voltage.
278:
153:
on the electrode surface and separates the oppositely polarized ions from each other, forming a molecular
118:
534:
297:
73:
The double-layer capacitance is the physical principle behind the electrostatic double-layer type of
829:
533:. Vol. 274. Graduate School of Arts and Sciences, University of Pennsylvania. pp. 55–79.
593:
Leitner, K. W.; Winter, M.; Besenhard, J. O. (2003-12-01). "Composite supercapacitor electrodes".
281:, the capacitors with the thinnest dielectric among conventional capacitors. The voltage proof of
618:
562:
550:
162:
267:{\displaystyle E={\frac {U}{d}}={\frac {2\ {\text{V}}}{0.4\ {\text{nm}}}}=5000\ {\text{kV/mm}}}
741:
647:
610:
513:
470:
639:
602:
583:
542:
505:
392:
If the electrolyte solvent is water then the influence of the high field strength creates a
764:
110:
50:
on the electrode and ions in the electrolyte, are typically separated by a single layer of
574:
307:
43:
277:
To compare this figure with values from other capacitor types requires an estimation for
538:
161:
charges in the same way as in a conventional capacitor. The double-layer charge forms a
149:. The molecular monolayer forms the inner Helmholtz plane (IHP). It adheres by physical
728:
585:
Electrochemical
Supercapacitors: Scientific Fundamentals and Technological Applications
462:
286:
282:
165:
122:
105:
93:
74:
823:
740:
S. Srinivasan, Fuel Cells, From
Fundamentals to Applications, Springer eBooks, 2006,
622:
554:
393:
173:
183:
over the separating solvent molecules. At a potential difference of, for example,
528:
497:
399:
of 6 (instead of 80 without an applied electric field) and the layer separation
66:. The amount of charge stored in double-layer capacitor depends on the applied
39:
327:
independent from the charge density, even depending on the dielectric constant
643:
606:
154:
150:
59:
651:
614:
509:
146:
142:
63:
35:
630:
Yu., M.; Volfkovich, T. M. (September 2002). "Electrochemical
Capacitors".
546:
55:
47:
453:
is greatest in components made from materials with a high permittivity
301:
176:. The sum of the thicknesses is the total thickness of a double layer.
129:
67:
51:
27:
660:
Electrochemical
Technologies for Energy Storage and Conversion, Band 1
727:
306:
109:
31:
502:
Carbons for Electrochemical Energy Storage and Conversion Systems
86:
Development of the double layer and pseudocapacitance model see
16:
Capacitance present in the interface between a surface and fluid
498:"8 Electrical Double-Layer Capacitors and Pseudocapacitors"
179:
The IHP's small thickness creates a strong electric field
382:{\displaystyle \ C_{d}={\frac {\epsilon }{4\pi \delta }}}
685:
Z. Stojek, The Electrical Double Layer and Its Structure
793:
A SURVEY OF ELECTROCHEMICAL SUPERCAPACITOR TECHNOLOGY
413:
344:
200:
763:Marin S. Halper, James C. Ellenbogen (March 2006).
725:
Supercap, Grundlagen - Eigenschaften – Anwendungen.
437:
381:
266:
92:Development of the electrochemical components see
58:to the surface of the electrode and act like a
191:= 0.4 nm, the electric field strength is
8:
438:{\displaystyle C={\frac {\varepsilon A}{d}}}
772:(Technical report). MITRE Nanosystems Group
504:. Taylor & Francis. pp. 329–375.
420:
412:
361:
352:
343:
259:
242:
229:
220:
207:
199:
26:which appears at the interface between a
680:
678:
676:
187:= 2 V and a molecular thickness of
672:
595:Journal of Solid State Electrochemistry
22:is the important characteristic of the
570:
560:
530:On the Structure of Charged Interfaces
473:also contribute to capacitance value.
457:, large electrode plate surface areas
694:
692:
7:
34:(for example, between a conductive
632:Russian Journal of Electrochemistry
799:(Technical report). Archived from
42:). At this boundary two layers of
14:
766:Supercapacitors: A Brief Overview
723:Daniel Gräser, Christoph Schmid:
331:and the charge layer separation
487:Double layer (surface science)
1:
700:"The electrical double layer"
588:(in German), Berlin: Springer
125:to store electrical energy.
846:
103:
88:Double layer (interfacial)
607:10.1007/s10008-003-0412-x
123:electrochemical capacitor
510:10.1201/9781420055405-c8
319:differential capacitance
20:Double-layer capacitance
644:10.1023/A:1020220425954
279:electrolytic capacitors
38:and an adjacent liquid
24:electrical double layer
790:Adam Marcus Namisnyk.
702:. 2011. Archived from
547:10.1098/rspa.1963.0114
527:Müller, Klaus (1963).
439:
383:
313:
268:
115:
662:(in German), Weinheim
582:B. E. Conway (1999),
440:
384:
310:
298:decomposition voltage
269:
113:
496:(18 November 2009).
494:Frackowiak, Elzbieta
411:
342:
198:
539:1963RSPSA.274...55B
492:Béguin, Francois;
435:
379:
314:
264:
116:
62:in a conventional
746:978-0-387-35402-6
519:978-1-4200-5307-4
471:pseudocapacitance
433:
377:
347:
262:
258:
248:
245:
241:
232:
228:
215:
837:
815:
814:
812:
811:
805:
798:
787:
781:
780:
778:
777:
771:
760:
754:
738:
732:
721:
715:
714:
712:
711:
696:
687:
682:
663:
655:
626:
589:
578:
572:
568:
566:
558:
523:
460:
456:
452:
449:The capacitance
444:
442:
441:
436:
434:
429:
421:
402:
398:
388:
386:
385:
380:
378:
376:
362:
357:
356:
345:
334:
330:
326:
273:
271:
270:
265:
263:
260:
256:
249:
247:
246:
243:
239:
234:
233:
230:
226:
221:
216:
208:
190:
186:
182:
845:
844:
840:
839:
838:
836:
835:
834:
820:
819:
818:
809:
807:
803:
796:
789:
788:
784:
775:
773:
769:
762:
761:
757:
739:
735:
722:
718:
709:
707:
698:
697:
690:
683:
674:
670:
658:
629:
592:
581:
569:
559:
526:
520:
491:
483:
458:
454:
450:
422:
409:
408:
400:
396:
366:
348:
340:
339:
332:
328:
325:
321:
235:
222:
196:
195:
188:
184:
180:
163:static electric
108:
102:
94:Supercapacitors
83:
75:supercapacitors
54:molecules that
44:electric charge
17:
12:
11:
5:
843:
841:
833:
832:
822:
821:
817:
816:
782:
755:
733:
716:
688:
671:
669:
666:
665:
664:
656:
638:(9): 935–959.
627:
590:
579:
524:
518:
489:
482:
479:
447:
446:
432:
428:
425:
419:
416:
390:
389:
375:
372:
369:
365:
360:
355:
351:
323:
287:field strength
283:aluminum oxide
275:
274:
255:
252:
238:
225:
219:
214:
211:
206:
203:
106:Supercapacitor
101:
98:
97:
96:
90:
82:
79:
15:
13:
10:
9:
6:
4:
3:
2:
842:
831:
828:
827:
825:
806:on 2014-12-22
802:
795:
794:
786:
783:
768:
767:
759:
756:
753:
750:
747:
743:
737:
734:
730:
726:
720:
717:
706:on 2011-05-31
705:
701:
695:
693:
689:
686:
681:
679:
677:
673:
667:
661:
657:
653:
649:
645:
641:
637:
633:
628:
624:
620:
616:
612:
608:
604:
600:
596:
591:
587:
586:
580:
576:
564:
556:
552:
548:
544:
540:
536:
532:
531:
525:
521:
515:
511:
507:
503:
499:
495:
490:
488:
485:
484:
480:
478:
474:
472:
466:
464:
430:
426:
423:
417:
414:
407:
406:
405:
395:
373:
370:
367:
363:
358:
353:
349:
338:
337:
336:
320:
309:
305:
303:
299:
294:
291:
288:
284:
280:
253:
250:
236:
223:
217:
212:
209:
204:
201:
194:
193:
192:
177:
175:
169:
167:
164:
158:
156:
152:
148:
144:
140:
134:
131:
126:
124:
120:
112:
107:
99:
95:
91:
89:
85:
84:
80:
78:
76:
71:
69:
65:
61:
57:
53:
49:
45:
41:
37:
33:
29:
25:
21:
808:. Retrieved
801:the original
792:
785:
774:. Retrieved
765:
758:
736:
724:
719:
708:. Retrieved
704:the original
659:
635:
631:
601:(1): 15–16.
598:
594:
584:
529:
501:
475:
467:
448:
394:permittivity
391:
315:
295:
292:
276:
178:
174:Debye length
170:
159:
135:
127:
117:
72:
19:
18:
571:|work=
137:forms from
100:Capacitance
40:electrolyte
830:Capacitors
810:2014-01-20
776:2014-01-20
710:2014-01-20
668:References
481:Literature
155:dielectric
151:adsorption
104:See also:
60:dielectric
652:1608-3342
615:1433-0768
573:ignored (
563:cite book
463:ångströms
424:ε
374:δ
371:π
364:ϵ
147:molecules
139:dissolved
119:Helmholtz
64:capacitor
48:electrons
36:electrode
824:Category
623:95416761
555:94958336
312:polarity
143:solvated
535:Bibcode
302:voltage
130:solvent
81:History
68:voltage
52:solvent
28:surface
744:
650:
621:
613:
553:
516:
404:with:
346:
257:
240:
227:
56:adhere
30:and a
804:(PDF)
797:(PDF)
770:(PDF)
619:S2CID
551:S2CID
261:kV/mm
166:field
32:fluid
742:ISBN
648:ISSN
611:ISSN
575:help
514:ISBN
254:5000
141:and
729:PDF
640:doi
603:doi
543:doi
506:doi
237:0.4
826::
731:).
691:^
675:^
646:.
636:38
634:.
617:.
609:.
597:.
567::
565:}}
561:{{
549:.
541:.
512:.
500:.
335:.
304:.
244:nm
157:.
77:.
70:.
813:.
779:.
748:,
713:.
654:.
642::
625:.
605::
599:8
577:)
557:.
545::
537::
522:.
508::
459:A
455:ε
451:C
445:.
431:d
427:A
418:=
415:C
401:δ
397:ε
368:4
359:=
354:d
350:C
333:δ
329:ε
324:d
322:C
251:=
231:V
224:2
218:=
213:d
210:U
205:=
202:E
189:d
185:U
181:E
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.