31:
135:
In outer-sphere ET reactions, the participating redox centers are not linked via any bridge during the ET event. Instead, the electron "hops" through space from the reducing center to the acceptor. Outer sphere electron transfer can occur between different chemical species or between identical
116:
In inner-sphere ET, the two redox centers are covalently linked during the ET. This bridge can be permanent, in which case the electron transfer event is termed intramolecular electron transfer. More commonly, however, the covalent linkage is transitory, forming just prior to the ET and then
117:
disconnecting following the ET event. In such cases, the electron transfer is termed intermolecular electron transfer. A famous example of an inner sphere ET process that proceeds via a transitory bridged intermediate is the reduction of by . In this case, the chloride
166:
is that the rates of such self-exchange reactions are mathematically related to the rates of "cross reactions". Cross reactions entail partners that differ by more than their oxidation states. One example (of many thousands) is the reduction of permanganate by
446:
Susan B. Piepho, Elmars R. Krausz, P. N. Schatz; J. Am. Chem. Soc., 1978, 100 (10), pp 2996–3005; Vibronic coupling model for calculation of mixed-valence absorption profiles;
233:
Especially in proteins, electron transfer often involves hopping of an electron from one redox-active center to another. The hopping pathway, which is viewed as a
502:
301:. In proteins, ET rates are governed by the bond structures: the electrons, in effect, tunnel through the bonds comprising the chain structure of the proteins.
273:, has guided most discussions of electron transfer ever since. Both theories are, however, semiclassical in nature, although they have been extended to fully
136:
chemical species that differ only in their oxidation state. The latter process is termed self-exchange. As an example, self-exchange describes the
714:
591:
548:
348:
315:
855:
495:
467:
31 May 1991: Vol. 252 no. 5010 pp. 1285–1288; Protein electron transfer rates set by the bridging secondary and tertiary structure;
434:
413:
362:
Piechota, Eric J.; Meyer, Gerald J. (2019). "Introduction to
Electron Transfer: Theoretical Foundations and Pedagogical Examples".
184:
Reactants diffuse together, forming an "encounter complex", out of their solvent shells => precursor complex (requires work =w
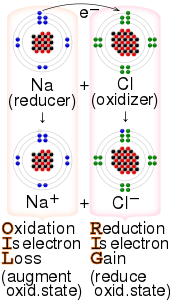
156:
In general, if electron transfer is faster than ligand substitution, the reaction will follow the outer-sphere electron transfer.
763:
758:
568:
262:
254:
130:
111:
923:
928:
241:, e.g. the 4Fe-4S ferredoxins. These site are often separated by 7-10 Å, a distance compatible with fast outer-sphere ET.
954:
488:
949:
298:
893:
583:
234:
620:
520:
850:
290:
86:
898:
699:
282:
258:
137:
653:
286:
883:
815:
673:
663:
878:
606:
371:
94:
82:
888:
820:
805:
748:
310:
238:
213:
In heterogeneous electron transfer, an electron moves between a chemical species and a solid-state
913:
683:
512:
387:
274:
908:
903:
865:
810:
709:
645:
430:
409:
320:
294:
250:
90:
840:
789:
743:
468:
447:
379:
218:
74:
340:
159:
Often occurs when one/both reactants are inert or if there is no suitable bridging ligand.
918:
830:
779:
375:
625:
614:
278:
78:
943:
873:
845:
753:
704:
678:
391:
270:
163:
67:
to another such chemical entity. ET is a mechanistic description of certain kinds of
825:
631:
538:
528:
293:. Furthermore, theories have been put forward to take into account the effects of
237:, guides and facilitates ET within an insulating matrix. Typical redox centers are
141:
17:
784:
719:
383:
93:
ET is a step in some commercial polymerization reactions. It is foundational to
261:
approach. The Marcus theory of electron transfer was then extended to include
222:
835:
480:
472:
266:
217:. Theories addressing heterogeneous electron transfer have applications in
214:
145:
30:
64:
56:
451:
197:
Relaxation of bond lengths, solvent molecules => successor complex
172:
168:
118:
121:
is the bridging ligand that covalently connects the redox partners.
738:
68:
35:
29:
191:
Changing bond lengths, reorganize solvent => activated complex
60:
484:
558:
27:
Relocation of an electron from an atom or molecule to another
249:
The first generally accepted theory of ET was developed by
864:
798:
772:
728:
692:
644:
605:
582:
519:
77:are ET reactions. ET reactions are relevant to
38:reaction between sodium and chlorine, with the
496:
8:
71:reactions involving transfer of electrons.
503:
489:
481:
408:(2nd ed.). Oxford: Butterworth-Heinemann.
297:on electron transfer; in particular, the
269:and Marcus. The resultant theory called
404:Greenwood, N. N.; Earnshaw, A. (1997).
332:
529:Unimolecular nucleophilic substitution
200:Diffusion of products (requires work=w
179:Five steps of an outer sphere reaction
144:and its one-electron reduced relative
539:Bimolecular nucleophilic substitution
7:
425:Holleman, A. F.; Wiberg, E. (2001).
592:Electrophilic aromatic substitution
559:Nucleophilic internal substitution
549:Nucleophilic aromatic substitution
463:Beratan DN, Betts JN, Onuchic JN,
316:Electrochemical reaction mechanism
25:
351:from the original on 2022-11-03.
715:Lindemann–Hinshelwood mechanism
299:PKS theory of electron transfer
209:Heterogeneous electron transfer
764:Outer sphere electron transfer
759:Inner sphere electron transfer
569:Nucleophilic acyl substitution
289:and following earlier work in
263:inner-sphere electron transfer
255:outer-sphere electron transfer
131:Outer-sphere electron transfer
125:Outer-sphere electron transfer
112:Inner-sphere electron transfer
106:Inner-sphere electron transfer
1:
929:Diffusion-controlled reaction
429:. San Diego: Academic Press.
364:Journal of Chemical Education
285:, and others proceeding from
454:; Publication Date: May 1978
101:Classes of electron transfer
584:Electrophilic substitutions
384:10.1021/acs.jchemed.9b00489
971:
894:Energy profile (chemistry)
856:More O'Ferrall–Jencks plot
521:Nucleophilic substitutions
229:Vectoral electron transfer
128:
109:
87:transition metal complexes
924:Michaelis–Menten kinetics
406:Chemistry of the Elements
291:non-radiative transitions
75:Electrochemical processes
851:Potential energy surface
730:Electron/Proton transfer
615:Unimolecular elimination
899:Transition state theory
700:Intramolecular reaction
626:Bimolecular elimination
473:10.1126/science.1656523
259:transition-state theory
175:and, again, manganate.
693:Unimolecular reactions
654:Electrophilic addition
283:Alexander M. Kuznetsov
45:
884:Rate-determining step
816:Reactive intermediate
674:Free-radical addition
664:Nucleophilic addition
607:Elimination reactions
85:and commonly involve
33:
879:Equilibrium constant
239:iron-sulfur clusters
95:photoredox catalysis
955:Reaction mechanisms
889:Reaction coordinate
821:Radical (chemistry)
806:Elementary reaction
749:Grotthuss mechanism
513:reaction mechanisms
452:10.1021/ja00478a011
427:Inorganic Chemistry
376:2019JChEd..96.2450P
311:Electron equivalent
287:Fermi's golden rule
257:and was based on a
36:reduction–oxidation
950:Physical chemistry
914:Arrhenius equation
684:Oxidative addition
646:Addition reactions
275:quantum mechanical
271:Marcus-Hush theory
221:and the design of
59:relocates from an
46:
937:
936:
909:Activated complex
904:Activation energy
866:Chemical kinetics
811:Reaction dynamics
710:Photodissociation
370:(11): 2450–2466.
321:Solvated electron
295:vibronic coupling
251:Rudolph A. Marcus
194:Electron transfer
162:A key concept of
140:reaction between
91:organic chemistry
55:) occurs when an
49:Electron transfer
18:Electron-transfer
16:(Redirected from
962:
841:Collision theory
790:Matrix isolation
744:Harpoon reaction
621:E1cB-elimination
505:
498:
491:
482:
475:
461:
455:
444:
438:
423:
417:
402:
396:
395:
359:
353:
352:
337:
219:electrochemistry
43:
21:
970:
969:
965:
964:
963:
961:
960:
959:
940:
939:
938:
933:
919:Eyring equation
860:
831:Stereochemistry
794:
780:Solvent effects
768:
724:
688:
669:
659:
640:
635:
601:
597:
578:
574:
564:
554:
544:
534:
515:
509:
479:
478:
462:
458:
445:
441:
424:
420:
403:
399:
361:
360:
356:
339:
338:
334:
329:
307:
247:
231:
211:
203:
187:
181:
133:
127:
114:
108:
103:
39:
28:
23:
22:
15:
12:
11:
5:
968:
966:
958:
957:
952:
942:
941:
935:
934:
932:
931:
926:
921:
916:
911:
906:
901:
896:
891:
886:
881:
876:
870:
868:
862:
861:
859:
858:
853:
848:
843:
838:
833:
828:
823:
818:
813:
808:
802:
800:
799:Related topics
796:
795:
793:
792:
787:
782:
776:
774:
773:Medium effects
770:
769:
767:
766:
761:
756:
751:
746:
741:
735:
733:
726:
725:
723:
722:
717:
712:
707:
702:
696:
694:
690:
689:
687:
686:
681:
676:
671:
667:
661:
657:
650:
648:
642:
641:
639:
638:
633:
629:
623:
618:
611:
609:
603:
602:
600:
599:
595:
588:
586:
580:
579:
577:
576:
572:
566:
562:
556:
552:
546:
542:
536:
532:
525:
523:
517:
516:
510:
508:
507:
500:
493:
485:
477:
476:
456:
439:
418:
397:
354:
331:
330:
328:
325:
324:
323:
318:
313:
306:
303:
279:Joshua Jortner
277:treatments by
246:
243:
230:
227:
210:
207:
206:
205:
201:
198:
195:
192:
189:
185:
180:
177:
154:
153:
129:Main article:
126:
123:
110:Main article:
107:
104:
102:
99:
79:photosynthesis
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
967:
956:
953:
951:
948:
947:
945:
930:
927:
925:
922:
920:
917:
915:
912:
910:
907:
905:
902:
900:
897:
895:
892:
890:
887:
885:
882:
880:
877:
875:
874:Rate equation
872:
871:
869:
867:
863:
857:
854:
852:
849:
847:
846:Arrow pushing
844:
842:
839:
837:
834:
832:
829:
827:
824:
822:
819:
817:
814:
812:
809:
807:
804:
803:
801:
797:
791:
788:
786:
783:
781:
778:
777:
775:
771:
765:
762:
760:
757:
755:
754:Marcus theory
752:
750:
747:
745:
742:
740:
737:
736:
734:
731:
727:
721:
718:
716:
713:
711:
708:
706:
705:Isomerization
703:
701:
698:
697:
695:
691:
685:
682:
680:
679:Cycloaddition
677:
675:
672:
665:
662:
655:
652:
651:
649:
647:
643:
637:
630:
627:
624:
622:
619:
616:
613:
612:
610:
608:
604:
593:
590:
589:
587:
585:
581:
570:
567:
560:
557:
550:
547:
540:
537:
530:
527:
526:
524:
522:
518:
514:
506:
501:
499:
494:
492:
487:
486:
483:
474:
470:
466:
460:
457:
453:
449:
443:
440:
436:
435:0-12-352651-5
432:
428:
422:
419:
415:
414:0-7506-3365-4
411:
407:
401:
398:
393:
389:
385:
381:
377:
373:
369:
365:
358:
355:
350:
346:
342:
336:
333:
326:
322:
319:
317:
314:
312:
309:
308:
304:
302:
300:
296:
292:
288:
284:
280:
276:
272:
268:
264:
260:
256:
252:
244:
242:
240:
236:
228:
226:
224:
220:
216:
208:
199:
196:
193:
190:
183:
182:
178:
176:
174:
170:
165:
164:Marcus theory
160:
157:
151:
150:
149:
147:
143:
139:
132:
124:
122:
120:
113:
105:
100:
98:
96:
92:
88:
84:
80:
76:
72:
70:
66:
62:
58:
54:
50:
42:
37:
34:Example of a
32:
19:
826:Molecularity
729:
464:
459:
442:
426:
421:
405:
400:
367:
363:
357:
344:
335:
248:
232:
212:
161:
158:
155:
142:permanganate
134:
115:
73:
52:
48:
47:
40:
785:Cage effect
720:RRKM theory
636:elimination
253:to address
223:solar cells
83:respiration
944:Categories
327:References
138:degenerate
836:Catalysis
732:reactions
392:208754569
267:Noel Hush
215:electrode
146:manganate
349:Archived
345:Bitesize
341:"Metals"
305:See also
171:to form
152:+ → +
65:molecule
57:electron
44:mnemonic
465:Science
372:Bibcode
347:. BBC.
41:OIL RIG
511:Basic
433:
412:
390:
245:Theory
235:vector
173:iodine
169:iodide
119:ligand
739:Redox
575:Acyl)
388:S2CID
89:. In
69:redox
628:(E2)
617:(E1)
431:ISBN
410:ISBN
81:and
61:atom
598:Ar)
555:Ar)
469:doi
448:doi
380:doi
265:by
63:or
946::
666:(A
656:(A
594:(S
571:(S
565:i)
561:(S
551:(S
545:2)
541:(S
535:1)
531:(S
386:.
378:.
368:96
366:.
343:.
281:,
225:.
148::
97:.
53:ET
670:)
668:N
660:)
658:E
634:i
632:E
596:E
573:N
563:N
553:N
543:N
533:N
504:e
497:t
490:v
471::
450::
437:.
416:.
394:.
382::
374::
204:)
202:p
188:)
186:r
51:(
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.