166:
less relevant in this field as a fuel, the demonstrated method of using a series of enzymes to completely oxidize the cell's fuel gave researchers a way forward, and much work is now devoted to using similar methods to achieve complete oxidation of more complicated compounds, such as glucose. In addition, and perhaps what is more important, 1998 was the year in which enzyme “immobilization” was successfully demonstrated, which increased the usable life of the methanol fuel cell from just eight hours to over a week. Immobilization also provided researchers with the ability to put earlier discoveries into practice, in particular the discovery of enzymes that can be used to directly transfer electrons from the enzyme to the electrode. This process had been understood since the 1980s but depended heavily on placing the enzyme as close to the electrode as possible, which meant that it was unusable until after immobilization techniques were devised. In addition, developers of enzymatic biofuel cells have applied some of the advances in
158:, there was difficulty in transporting the electrons liberated from the glucose fuel to the fuel cell's electrode and further difficulties in keeping the system stable enough to produce electricity at all due to the enzymes’ tendency to move away from where they needed to be in order for the fuel cell to function. These difficulties led to an abandonment by biofuel cell researchers of the enzyme-catalyst model for nearly three decades in favor of the more conventional metal catalysts (principally platinum), which are used in most fuel cells. Research on the subject did not begin again until the 1980s after it was realized that the metallic-catalyst method was not going to be able to deliver the qualities desired in a biofuel cell, and since then work on enzymatic biofuel cells has revolved around the resolution of the various problems that plagued earlier efforts at producing a successful enzymatic biofuel cell.
105:. Enzymes are also specifically designed to process organic compounds such as sugars and alcohols, which are extremely common in nature. Most organic compounds cannot be used as fuel by fuel cells with metal catalysts because the carbon monoxide formed by the interaction of the carbon molecules with oxygen during the fuel cell's functioning will quickly “poison” the precious metals that the cell relies on, rendering it useless. Because sugars and other biofuels can be grown and harvested on a massive scale, the fuel for enzymatic biofuel cells is extremely cheap and can be found in nearly any part of the world, thus making it an extraordinarily attractive option from a logistics standpoint, and even more so for those concerned with the adoption of
501:
drawback to this method is that the ratio of hydrogenase covering the surface of the nanotube network leaves hydrogenase to cover only the scarce defective spots in the network. It is also found that some adsorption procedures tend to damage the enzymes whereas covalently coupling them stabilized the enzyme and allows it to remain stable for longer. The catalytic activity of hydrogenase-MWCNT electrodes provided stability for over a month whereas the hydrogenase-graphite electrodes only lasted about a week.
65:
154:, specifically as a power source that could be put directly into the human body. These two applications – use of animal or vegetable products as fuel and development of a power source that can be directly implanted into the human body without external refueling – remain the primary goals for developing these biofuel cells. Initial results, however, were disappointing. While the early cells did successfully produce
261:
A drawback with the use of enzymes is size; given the large size of enzymes, they yield a low current density per unit electrode area due to the limited space. Since it is not possible to reduce enzyme size, it has been argued that these types of cells will be lower in activity. One solution has been
165:
using a series (or “cascade”) of enzymes in a biofuel cell. Previous to this time, the enzyme catalysts had failed to completely oxidize the cell's fuel, delivering far lower amounts of energy than what was expected given what was known about the energy capacity of the fuel. While methanol is now far
112:
Enzymatic biofuel cells also have operating requirements not shared by traditional fuel cells. What is most significant is that the enzymes that allow the fuel cell to operate must be “immobilized” near the anode and cathode in order to work properly; if not immobilized, the enzymes will diffuse into
397:
Possible solutions for greater efficiency of electron delivery include the immobilization of hydrogenase with the most exposed FeS cluster close enough to the electrode or the use of a redox mediator to carry out the electron transfer. Direct electron transfer is also possible through the adsorption
233:
of its own "bio-batteries" in the following year. In explaining their pursuit of the technology, both organizations emphasized the extraordinary abundance (and extraordinarily low expense) of fuel for these cells, a key advantage of the technology that is likely to become even more attractive if the
476:
There are many ways to adsorb hydrogenases onto carbon electrodes that have been modified with polymers. An example is a study done by
Morozov et al. where they inserted NiFe hydrogenase into polypyrrole films and to provide proper contact to the electrode, there were redox mediators entrapped into
447:
is the fuel at the cathode and therefore must be physically separated or else the hydrogenase enzymes at the anode would be inactivated. Secondly, there is a positive potential imposed on hydrogenase at the anode by the enzyme on the cathode. This further enhances the inactivation of hydrogenase by
500:
Another way of coupling hydrogenase to the nanotubes was to covalently bind them to avoid a time delay. Hydrogenase isolated from D. gigas (jumbo squid) was coupled to multiwalled carbon nanotube (MWCNT) networks and produced a current ~30 times higher than the graphite-hydrogenase anode. A slight
406:
Immediate comparison of the size of hydrogenase with standard inorganic molecular catalysts reveal that hydrogenase is very bulky. It is approximately 5 nm in diameter compared to 1-5 nm for Pt catalysts. This limits the possible electrode coverage by capping the maximum current density.
535:
The beginning concept of applying enzymatic biofuel cells for self-powered biosensing applications has been introduced since 2001. With continued efforts, several types of self-powered enzyme-based biosensors have been demonstrated. In 2016, the first example of stretchable textile-based biofuel
509:
A fully enzymatic hydrogen fuel cell was constructed by the
Armstrong group who used the cell to power a watch. The fuel cell consisted of a graphite anode with hydrogenase isolated from R. metallidurans and a graphite cathode modified with fungal laccase. The electrodes were placed in a single
410:
Since altering the size of hydrogenase is not a possibility, to increase the density of enzyme present on the electrode to maintain fuel cell activity, a porous electrode can be used instead of one that is planar. This increases the electroactive area allowing more enzyme to be loaded onto the
125:
risks for fuel cells intended to be used inside the human body. Finally, completely processing the complex fuels used in enzymatic biofuel cells requires a series of different enzymes for each step of the ‘metabolism’ process; producing some of the required enzymes and maintaining them at the
117:. This can be done either directly from the enzyme to the electrode (“direct electron transfer”) or with the aid of other chemicals that transfer electrons from the enzyme to the electrode (“mediated electron transfer”). The former technique is possible only with certain types of enzymes whose
393:
reaction, hydrogenase must be immobilized on the electrode in such a way that it can exchange electrons directly with the electrode to facilitate the transfer of electrons. This proves to be a challenge in that the active site of hydrogenase is buried in the center of the enzyme where the FeS
84:
Enzymatic biofuel cells work on the same general principles as all fuel cells: use a catalyst to separate electrons from a parent molecule and force it to go around an electrolyte barrier through a wire to generate an electric current. What makes the enzymatic biofuel cell distinct from more
986:
Chenevier, P.; Mugherli, L.; Darbe, S.; Darchy, L.; DiManno, S.; Tran, P.D.; Valentino, F.; Iannello, M.; Volbeda, A.; Cavazza, C.; Artero, V. (2013). "Hydrogenase enzymes: application in biofuel cells and inspiration for the design of noble-metal free catalysts for H2 oxidation".
514:
gas in air and there was no membrane due to the tolerance of the hydrogenase to oxygen. The fuel cell produced a voltage of 950mV and generated 5.2 uW/cm of electricity. Although this system was very functional, it was still not at optimum output due to the low accessible
249:
Furthermore, the protein matrix surrounding the active site provides many vital functions; selectivity for the substrate, internal electron coupling, acidic/basic properties and the ability to bind to other proteins (or the electrode). Enzymes are more stable in the absence of
373:) does not react with the fuel. The electrodes are preferably made from carbon which is abundant, renewable and can be modified in many ways or adsorb enzymes with high affinity. The hydrogenase is attached to a surface which also extends the lifetime of the enzyme.
262:
to use three-dimensional electrodes or immobilization on conducting carbon supports which provide high surface area. These electrodes are extended into three-dimensional space which greatly increases the surface area for enzymes to bind thus increasing the current.
364:
With regards to structural advantages, hydrogenase is highly selective for its substrate. The lack of need for a membrane simplifies the biofuel cell design to be small and compact, given that hydrogenase does not react with oxygen (an
847:
Palmore, G.Tayhas R. (1998). "A methanol/dioxygen biofuel cell that uses NAD+-dependent dehydrogenases as catalysts: application of an electro-enzymatic method to regenerate nicotinamide adenine dinucleotide at low overpotentials".
488:
can also be used for a support for hydrogenase on the electrode due to their ability to assemble in large porous and conductive networks. These hybrids have been prepared using and hydrogenases. The hydrogenase isolated from
477:
the film. This was successful because the hydrogenase density was high in the films and the redox mediator helped to connect all enzyme molecules for catalysis which was about the same power output as hydrogenase in solution.
113:
the cell's fuel and most of the liberated electrons will not reach the electrodes, compromising its effectiveness. Even with immobilization, a means must also be provided for electrons to be transferred to and from the
51:
cells, while currently confined to research facilities, are widely prized for the promise they hold in terms of their relatively inexpensive components and fuels, as well as a potential power source for
138:
variety. Research on using enzymes directly for oxidation in biofuel cells began in the early 1960s, with the first enzymatic biofuel cell being produced in 1964. This research began as a product of
242:
With respect to fuel cells, enzymes have several advantages to their incorporation. An important enzymatic property to consider is the driving force or potential necessary for successful reaction
381:
There are several difficulties to consider associated with the incorporation of hydrogenase in biofuel cells. These factors must be taken into account to produce an efficient fuel cell.
93:
as catalysts, the enzymatic biofuel cell uses enzymes derived from living cells (although not within living cells; fuel cells that use whole cells to catalyze fuel are called
497:
with direct electron transfer without a redox mediator with a 10-fold higher catalytic current with stationary CNT-coated electrodes than with bare electrodes.
398:
of the enzyme on graphite electrodes or covalent attachment to the electrode. Another solution includes the entrapment of hydrogenase in a conductive polymer.
209:
has advanced over the past decade non-academic organizations have shown an increasing amount of interest in practical applications for the devices. In 2007,
559:
333:
In addition to the advantages previously mentioned associated with incorporating enzymes in fuel cells, hydrogenase is a very efficient catalyst for H
306:
as fuel with use on demand. This can be demonstrated through the chemical storage of electricity obtained from a renewable source (e.g. solar, wind,
298:
In recent years, research on hydrogenases has grown significantly due to scientific and technological interest in hydrogen. The bidirectional or
932:
Cracknell, J.A.; Vincent, K.A.; Armstrong, F.A. (2008). "Enzymes as working or inspirational electrocatalysts for fuel cells and electrolysis".
258:
organisms, thus offering a wider range of operational temperatures. Operating conditions is generally between 20-50 °C and pH 4.0 to 8.0.
1209:
190:, which is an absolute requirement in fuel cells not of the enzymatic type. This allowed the team to produce a fuel cell that produces 1.1
161:
However, many of these problems were resolved in 1998. In that year, it was announced that researchers had managed to completely oxidize
226:
1247:
174:
to immobilize enzymes directly. Other research has gone into exploiting some of the strengths of the enzymatic design to dramatically
519:
levels, the lower catalytic activity of the oxygen tolerant hydrogenases and the lower density of catalysts on the flat electrodes.
97:). This offers a couple of advantages for enzymatic biofuel cells: Enzymes are relatively easy to mass-produce and so benefit from
540:
device utilized a lactate oxidase-based biofuel cell, allowing real-time monitoring of lactate in sweat for on-body applications.
898:
1390:
1345:
85:
conventional fuel cells are the catalysts they use and the fuels that they accept. Whereas most fuel cells use metals like
1385:
302:
catalyzed by hydrogenase is a solution to the challenge in the development of technologies for the capture and storage of
282:
for H2 oxidation in which molecular hydrogen is split into electrons and protons. In the case of H2/O2 biofuel cells, the
1464:
1268:
1438:
1355:
1298:
341:
is typically the catalyst for this reaction however, the activity of hydrogenases are comparable without the issue of
182:. One research team took advantage of the extreme selectivity of the enzymes to completely remove the barrier between
234:
price of portable energy sources goes up, or if they can be successfully integrated into electronic human implants.
1375:
1237:
727:
Yahiro, A. T.; Lee, S. M.; Kimble, D. O. (1964). "Bioelectrochemistry: I. Enzyme utilizing bio-fuel cell studies".
549:
246:. Many enzymes operate at potentials close to their substrates which is most suitable for fuel cell applications.
1365:
1283:
1242:
1370:
1278:
1202:
358:
106:
763:
213:
announced that it had developed an enzymatic biofuel cell that can be linked in sequence and used to power an
1360:
1293:
1273:
1380:
1288:
1252:
564:
1303:
1072:
Katz, Eugenii; Bückmann, Andreas F.; Willner, Itamar (2001). "Self-Powered Enzyme-Based
Biosensors".
94:
64:
1459:
1324:
1195:
569:
459:, a proton exchange membrane can be used to separate the anode and cathode compartments such that O
299:
135:
419:
matrix. The graphite particles then can collect and transport electrons to the electrode surface.
1232:
1171:
709:
342:
179:
98:
1146:
1097:
1089:
1054:
949:
786:
744:
663:
522:
This system was then later improved by adding a MWCNT network to increase the electrode area.
394:
clusters are used as an electron relay to exchange electrons with its natural redox partner.
1425:
1420:
1415:
1410:
1136:
1128:
1081:
1046:
996:
941:
857:
827:
817:
778:
736:
699:
655:
607:
485:
366:
303:
171:
151:
102:
832:
321:
The use of hydrogen in energy converting devices has gained interest due to being a clean
271:
205:
While enzymatic biofuel cells are not currently in use outside of the laboratory, as the
646:
Moehlenbrock, Michael J.; Shelley D. Minteer (2008). "Extended
Lifetime Biofuel Cells".
134:
Early work with biofuel cells, which began in the early 20th century, was purely of the
1141:
1116:
537:
322:
167:
17:
861:
1453:
1340:
740:
713:
1350:
307:
255:
230:
178:
the fuel cells, a process that must occur if these cells are ever to be used with
463:
is unable to diffuse to and destructively modify the active site of hydrogenase.
440:
275:
175:
155:
143:
118:
77:
1000:
782:
1165:
554:
214:
206:
147:
1093:
1218:
876:
432:
251:
243:
114:
32:
1150:
1101:
1058:
953:
790:
748:
667:
1117:"Stretchable biofuel cells as wearable textile-based self-powered sensors"
1402:
1037:
Lubitz, W.; Ogata, H.; Rudiger, O.; Reijerse, E. (2014). "Hydrogenases".
412:
338:
218:
162:
122:
86:
40:
1132:
612:
595:
416:
370:
287:
283:
222:
187:
69:
48:
44:
36:
1085:
1050:
945:
822:
805:
704:
687:
659:
427:
In a biofuel cell, hydrogenase is exposed to two oxidizing threats. O
90:
73:
53:
806:"Recent Advances in Enzymatic Fuel Cells: Experiments and Modeling"
688:"Pyruvate/Air Enzymatic Biofuel Cell Capable of Complete Oxidation"
536:
cells, acting as wearable self-powered sensors, was described. The
390:
279:
199:
183:
63:
452:
causing even which was previously O2-tolerant, to be affected.
318:
can be oxidized to produce electricity which is very efficient.
210:
195:
191:
139:
1191:
877:"Sony Develops 'Bio Battery' Generating Electricity from Sugar"
439:
to the active site followed by destructive modification of the
314:
during periods of low energy demands. When energy is desired, H
121:
are close to the enzyme's surface, but doing so presents fewer
1187:
431:
inactivates most hydrogenases with the exception of through
270:
As per the definition of biofuel cells, enzymes are used as
899:"The Bio-Battery: Converting Sugar into Electrical Energy"
101:, whereas precious metals must be mined and so have an
278:-based biofuel cells, hydrogenases are present at the
68:
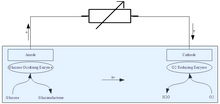
A general diagram for an enzymatic biofuel cell using
254:, while heat resistant enzymes can be extracted from
1401:
1333:
1312:
1261:
1225:
290:enzymes which then convert the protons into water.
389:Since the hydrogenase-based biofuel cell hosts a
47:its fuel, rather than precious metals. Enzymatic
411:electrode. An alternative is to form films with
686:Sokic-Lazic, Daria; Shelley D. Minteer (2009).
1203:
493:(thermophilic bacteria) was able to oxidize H
415:particles adsorbed with hydrogenase inside a
150:, as well as a component of the quest for an
8:
981:
979:
977:
975:
973:
971:
969:
967:
965:
963:
927:
925:
923:
921:
919:
481:Immobilizing hydrogenase on carbon nanotubes
560:Electrochemical reduction of carbon dioxide
505:Hydrogenase-based biofuel cell applications
337:consumption forming electrons and protons.
1210:
1196:
1188:
1032:
1030:
1140:
1028:
1026:
1024:
1022:
1020:
1018:
1016:
1014:
1012:
1010:
831:
821:
703:
611:
1115:Jeerapan, Itthipon; et al. (2016).
1074:Journal of the American Chemical Society
692:Electrochemical and Solid-State Letters
681:
679:
677:
594:Atanassov, Plamen; et al. (2007).
581:
329:Feasibility of hydrogenase as catalysts
170:to their designs, including the use of
1164:Itthipon Jeerapan (29 December 2018),
850:Journal of Electroanalytical Chemistry
589:
587:
585:
1167:Sweat-powered Sensor for Sweaty Socks
764:"Biofuel cells and their development"
641:
639:
637:
635:
633:
631:
629:
627:
625:
623:
600:The Electrochemical Society Interface
472:Entrapment of hydrogenase in polymers
369:) and the cathode enzymes (typically
7:
142:interest in finding ways to recycle
762:Bullen, R. A.; et al. (2006).
325:and potential transportation fuel.
238:Feasibility of enzymes as catalysts
126:required levels can pose problems.
1248:Proton-exchange membrane fuel cell
804:Ivanov, Ivan; et al. (2010).
274:at both the cathode and anode. In
25:
1121:Journal of Materials Chemistry A
1391:Unitized regenerative fuel cell
1174:from the original on 2021-12-21
294:Hydrogenase as an energy source
266:Hydrogenase-based biofuel cells
833:11858/00-001M-0000-0013-9052-C
510:chamber with a mixture of 3% H
359:production of greenhouse gases
198:in a space of just 0.01 cubic
76:. The blue area indicates the
1:
1386:Solid oxide electrolyzer cell
862:10.1016/S0022-0728(97)00393-8
771:Biosensors and Bioelectronics
729:Biochimica et Biophysica Acta
1269:Direct borohydride fuel cell
741:10.1016/0926-6577(64)90192-5
361:where the product is water.
146:into usable energy on board
1356:Membrane electrode assembly
1299:Reformed methanol fuel cell
1481:
1376:Protonic ceramic fuel cell
1346:Electro-galvanic fuel cell
1238:Molten carbonate fuel cell
1001:10.1016/j.crci.2012.11.006
783:10.1016/j.bios.2006.01.030
550:Bioelectrochemical reactor
455:To avoid inactivation by O
349:S and CO. In the case of H
1434:
1366:Photoelectrochemical cell
1284:Direct methanol fuel cell
1243:Phosphoric acid fuel cell
596:"Enzymatic Biofuel Cells"
194:operating at over half a
1371:Proton-exchange membrane
1279:Direct-ethanol fuel cell
648:Chemical Society Reviews
357:fuel cells, there is no
229:was planning to conduct
107:renewable energy sources
1361:Membraneless Fuel Cells
1294:Metal hydride fuel cell
1274:Direct carbon fuel cell
531:Self-powered biosensors
18:Enzymatic Biofuel Cells
1381:Regenerative fuel cell
1320:Enzymatic biofuel cell
81:
31:is a specific type of
29:enzymatic biofuel cell
1289:Formic acid fuel cell
1253:Solid oxide fuel cell
989:Comptes Rendus Chimie
897:Hurley, Christopher.
565:Electromethanogenesis
385:Enzyme immobilization
67:
95:microbial fuel cells
1465:Bioelectrochemistry
1325:Microbial fuel cell
1127:(47): 18342–18353.
1080:(43): 10752–10753.
570:Microbial fuel cell
300:reversible reaction
225:announced that the
180:implantable devices
1233:Alkaline fuel cell
1133:10.1039/C6TA08358G
903:Armed with Science
613:10.1149/2.F04072IF
343:catalyst poisoning
227:Defense Department
99:economies of scale
82:
1447:
1446:
1086:10.1021/ja0167102
1051:10.1021/cr4005814
946:10.1021/cr0680639
823:10.3390/en3040803
705:10.1149/1.3170904
217:, and in 2010 an
16:(Redirected from
1472:
1304:Zinc–air battery
1212:
1205:
1198:
1189:
1182:
1181:
1180:
1179:
1161:
1155:
1154:
1144:
1112:
1106:
1105:
1069:
1063:
1062:
1045:(8): 2081–4148.
1034:
1005:
1004:
983:
958:
957:
940:(7): 2439–2461.
929:
914:
913:
911:
909:
894:
888:
887:
885:
883:
872:
866:
865:
844:
838:
837:
835:
825:
801:
795:
794:
768:
759:
753:
752:
724:
718:
717:
707:
683:
672:
671:
660:10.1039/b708013c
643:
618:
617:
615:
591:
486:Carbon nanotubes
423:Oxidative damage
304:renewable energy
272:electrocatalysts
221:employed by the
172:carbon nanotubes
152:artificial heart
119:activation sites
103:inelastic supply
21:
1480:
1479:
1475:
1474:
1473:
1471:
1470:
1469:
1450:
1449:
1448:
1443:
1430:
1397:
1329:
1308:
1257:
1221:
1216:
1186:
1185:
1177:
1175:
1163:
1162:
1158:
1114:
1113:
1109:
1071:
1070:
1066:
1036:
1035:
1008:
985:
984:
961:
931:
930:
917:
907:
905:
896:
895:
891:
881:
879:
874:
873:
869:
846:
845:
841:
803:
802:
798:
777:(11): 2015–45.
766:
761:
760:
756:
726:
725:
721:
685:
684:
675:
645:
644:
621:
593:
592:
583:
578:
546:
533:
528:
518:
513:
507:
496:
483:
474:
469:
462:
458:
451:
446:
438:
430:
425:
404:
387:
379:
356:
352:
348:
336:
331:
317:
313:
296:
286:is coated with
268:
240:
132:
62:
23:
22:
15:
12:
11:
5:
1478:
1476:
1468:
1467:
1462:
1452:
1451:
1445:
1444:
1442:
1441:
1435:
1432:
1431:
1429:
1428:
1423:
1418:
1413:
1407:
1405:
1399:
1398:
1396:
1395:
1394:
1393:
1388:
1378:
1373:
1368:
1363:
1358:
1353:
1348:
1343:
1337:
1335:
1331:
1330:
1328:
1327:
1322:
1316:
1314:
1310:
1309:
1307:
1306:
1301:
1296:
1291:
1286:
1281:
1276:
1271:
1265:
1263:
1259:
1258:
1256:
1255:
1250:
1245:
1240:
1235:
1229:
1227:
1226:By electrolyte
1223:
1222:
1217:
1215:
1214:
1207:
1200:
1192:
1184:
1183:
1156:
1107:
1064:
1006:
995:(5): 491–505.
959:
915:
889:
867:
856:(1): 155–161.
839:
816:(4): 803–846.
796:
754:
735:(2): 375–383.
719:
673:
654:(6): 1188–96.
619:
580:
579:
577:
574:
573:
572:
567:
562:
557:
552:
545:
542:
532:
529:
527:
524:
516:
511:
506:
503:
494:
482:
479:
473:
470:
468:
465:
460:
456:
449:
444:
436:
428:
424:
421:
403:
400:
386:
383:
378:
375:
354:
350:
346:
334:
330:
327:
323:energy carrier
315:
311:
295:
292:
267:
264:
239:
236:
168:nanotechnology
131:
128:
61:
58:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1477:
1466:
1463:
1461:
1458:
1457:
1455:
1440:
1437:
1436:
1433:
1427:
1424:
1422:
1419:
1417:
1414:
1412:
1409:
1408:
1406:
1404:
1400:
1392:
1389:
1387:
1384:
1383:
1382:
1379:
1377:
1374:
1372:
1369:
1367:
1364:
1362:
1359:
1357:
1354:
1352:
1349:
1347:
1344:
1342:
1339:
1338:
1336:
1332:
1326:
1323:
1321:
1318:
1317:
1315:
1313:Biofuel cells
1311:
1305:
1302:
1300:
1297:
1295:
1292:
1290:
1287:
1285:
1282:
1280:
1277:
1275:
1272:
1270:
1267:
1266:
1264:
1260:
1254:
1251:
1249:
1246:
1244:
1241:
1239:
1236:
1234:
1231:
1230:
1228:
1224:
1220:
1213:
1208:
1206:
1201:
1199:
1194:
1193:
1190:
1173:
1169:
1168:
1160:
1157:
1152:
1148:
1143:
1138:
1134:
1130:
1126:
1122:
1118:
1111:
1108:
1103:
1099:
1095:
1091:
1087:
1083:
1079:
1075:
1068:
1065:
1060:
1056:
1052:
1048:
1044:
1040:
1033:
1031:
1029:
1027:
1025:
1023:
1021:
1019:
1017:
1015:
1013:
1011:
1007:
1002:
998:
994:
990:
982:
980:
978:
976:
974:
972:
970:
968:
966:
964:
960:
955:
951:
947:
943:
939:
935:
928:
926:
924:
922:
920:
916:
904:
900:
893:
890:
878:
871:
868:
863:
859:
855:
851:
843:
840:
834:
829:
824:
819:
815:
811:
807:
800:
797:
792:
788:
784:
780:
776:
772:
765:
758:
755:
750:
746:
742:
738:
734:
730:
723:
720:
715:
711:
706:
701:
697:
693:
689:
682:
680:
678:
674:
669:
665:
661:
657:
653:
649:
642:
640:
638:
636:
634:
632:
630:
628:
626:
624:
620:
614:
609:
605:
601:
597:
590:
588:
586:
582:
575:
571:
568:
566:
563:
561:
558:
556:
553:
551:
548:
547:
543:
541:
539:
538:smart textile
530:
525:
523:
520:
504:
502:
498:
492:
487:
480:
478:
471:
466:
464:
453:
442:
434:
422:
420:
418:
414:
408:
401:
399:
395:
392:
384:
382:
376:
374:
372:
368:
362:
360:
344:
340:
328:
326:
324:
319:
309:
305:
301:
293:
291:
289:
285:
281:
277:
273:
265:
263:
259:
257:
253:
247:
245:
237:
235:
232:
228:
224:
220:
216:
212:
208:
203:
201:
197:
193:
189:
185:
181:
177:
173:
169:
164:
159:
157:
153:
149:
145:
141:
137:
129:
127:
124:
120:
116:
110:
108:
104:
100:
96:
92:
88:
79:
75:
71:
66:
59:
57:
55:
50:
46:
42:
38:
34:
30:
19:
1351:Flow battery
1319:
1176:, retrieved
1166:
1159:
1124:
1120:
1110:
1077:
1073:
1067:
1042:
1038:
992:
988:
937:
933:
906:. Retrieved
902:
892:
880:. Retrieved
870:
853:
849:
842:
813:
809:
799:
774:
770:
757:
732:
728:
722:
695:
691:
651:
647:
606:(2): 28–31.
603:
599:
534:
526:Applications
521:
508:
499:
490:
484:
475:
467:Applications
454:
426:
409:
405:
396:
388:
380:
363:
332:
320:
308:hydrothermal
297:
269:
260:
256:thermophilic
248:
241:
231:field trials
204:
160:
133:
111:
83:
28:
26:
1341:Blue energy
491:A. aeolicus
441:active site
402:Enzyme size
276:hydrogenase
200:millimeters
176:miniaturize
156:electricity
144:human waste
78:electrolyte
1460:Fuel cells
1454:Categories
1219:Fuel cells
1178:2019-01-13
908:22 October
882:22 October
698:(9): F26.
576:References
555:Biobattery
377:Challenges
215:mp3 player
207:technology
192:microwatts
148:spacecraft
115:electrodes
56:implants.
35:that uses
1094:0002-7863
1039:Chem. Rev
934:Chem. Rev
433:diffusion
367:inhibitor
252:proteases
244:catalysis
136:microbial
60:Operation
33:fuel cell
1439:Glossary
1403:Hydrogen
1172:archived
1151:28439415
1102:11674014
1059:24655035
954:18620369
810:Energies
791:16569499
749:14249845
714:85345248
668:18497931
544:See also
413:graphite
339:Platinum
219:engineer
163:methanol
123:toxicity
87:platinum
41:catalyst
1426:Vehicle
1421:Storage
1416:Station
1411:Economy
1262:By fuel
1142:5400293
417:polymer
371:laccase
288:oxidase
284:cathode
223:US Army
188:cathode
130:History
70:Glucose
49:biofuel
45:oxidize
37:enzymes
1334:Others
1149:
1139:
1100:
1092:
1057:
952:
875:Sony.
789:
747:
712:
666:
310:) as H
140:NASA's
91:nickel
74:Oxygen
54:bionic
767:(PDF)
710:S2CID
391:redox
280:anode
184:anode
39:as a
1147:PMID
1098:PMID
1090:ISSN
1055:PMID
950:PMID
910:2011
884:2011
787:PMID
745:PMID
664:PMID
435:of O
345:by H
211:Sony
196:volt
186:and
89:and
72:and
1137:PMC
1129:doi
1082:doi
1078:123
1047:doi
1043:114
997:doi
942:doi
938:108
858:doi
854:443
828:hdl
818:doi
779:doi
737:doi
700:doi
656:doi
608:doi
443:. O
43:to
27:An
1456::
1170:,
1145:.
1135:.
1123:.
1119:.
1096:.
1088:.
1076:.
1053:.
1041:.
1009:^
993:16
991:.
962:^
948:.
936:.
918:^
901:.
852:.
826:.
812:.
808:.
785:.
775:21
773:.
769:.
743:.
733:88
731:.
708:.
696:12
694:.
690:.
676:^
662:.
652:37
650:.
622:^
604:16
602:.
598:.
584:^
353:/O
202:.
109:.
1211:e
1204:t
1197:v
1153:.
1131::
1125:4
1104:.
1084::
1061:.
1049::
1003:.
999::
956:.
944::
912:.
886:.
864:.
860::
836:.
830::
820::
814:3
793:.
781::
751:.
739::
716:.
702::
670:.
658::
616:.
610::
517:2
515:H
512:2
495:2
461:2
457:2
450:2
448:O
445:2
437:2
429:2
355:2
351:2
347:2
335:2
316:2
312:2
80:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.