3169:
912:
1178:
59:
1067:
different atomic species. In a body-centered cubic structure, there would be translational symmetry along the direction. In the caesium chloride structure, translation along the direction results in a change of species. The structure can also be thought of as two separate simple cubic structures, one of each species, that are superimposed within each other. The corner of the chloride cube is the center of the caesium cube, and vice versa.
3592:
51:
3781:
265:
258:
6027:
5985:
1071:
1055:
5980:
5960:
3661:
31:
5970:
5950:
5940:
251:
6047:
6037:
6017:
5996:
1066:
One structure is the "interpenetrating primitive cubic" structure, also called a "caesium chloride" or B2 structure. This structure is often confused for a body-centered cubic structure because the arrangement of atoms is the same. However, the caesium chloride structure has a basis composed of two
1085:
is eight. The central cation is coordinated to 8 anions on the corners of a cube as shown, and similarly, the central anion is coordinated to 8 cations on the corners of a cube. Alternately, one could view this lattice as a simple cubic structure with a secondary atom in its
3199:(β-ZnS). As in the rock-salt structure, the two atom types form two interpenetrating face-centered cubic lattices. However, it differs from rock-salt structure in how the two lattices are positioned relative to one another. The zincblende structure has
277:
point on each corner of the cube; this means each simple cubic unit cell has in total one lattice point. Each atom at a lattice point is then shared equally between eight adjacent cubes, and the unit cell therefore contains in total one atom
1097:
when prepared at low temperatures or high pressures. Generally, this structure is more likely to be formed from two elements whose ions are of roughly the same size (for example, ionic radius of Cs = 167 pm, and Cl = 181 pm).
4025:
1036:) often have crystal structures based on the cubic crystal system. Some of the more common ones are listed here. These structures can be viewed as two or more interpenetrating sublattices where each sublattice occupies the
304:
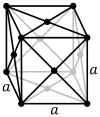
The face-centered cubic lattice (cF) has lattice points on the faces of the cube, that each gives exactly one half contribution, in addition to the corner lattice points, giving a total of 4 lattice points per unit cell
1212:
In the rock-salt structure, each of the two atom types forms a separate face-centered cubic lattice, with the two lattices interpenetrating so as to form a 3D checkerboard pattern. The rock-salt structure has
4290:
J. Aigueperse, P. Mollard, D. Devilliers, M. Chemla, R. Faron, R. Romano, J. P. Cuer, "Fluorine
Compounds, Inorganic" (section 4) in Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005.
1231:
Examples of compounds with this structure include sodium chloride itself, along with almost all other alkali halides, and "many divalent metal oxides, sulfides, selenides, and tellurides". According to the
3881:
De Wolff, P. M.; Belov, N. V.; Bertaut, E. F.; Buerger, M. J.; Donnay, J. D. H.; Fischer, W.; Hahn, Th.; Koptsik, V. A.; MacKay, A. L.; Wondratschek, H.; Wilson, A. J. C.; Abrahams, S. C. (1985).
1078:
It works the same way for the NaCl structure described in the next section. If you take out the Cl atoms, the leftover Na atoms still form an FCC structure, not a simple cubic structure.
1074:
This graphic shows the interlocking simple cubic lattices of cesium and chlorine. You can see them separately and as they are interlocked in what looks like a body-centered cubic arrangement
925:
As a rule, since atoms in a solid attract each other, the more tightly packed arrangements of atoms tend to be more common. (Loosely packed arrangements do occur, though, for example if the
3150:
m structure but has 1:2 ratio of ions. The anti-fluorite structure is nearly identical, except the positions of the anions and cations are switched in the structure. They are designated
291:
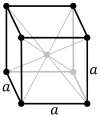
The body-centered cubic lattice (cI) has one lattice point in the center of the unit cell in addition to the eight corner points. It has a net total of two lattice points per unit cell (
3883:"Nomenclature for crystal families, Bravais-lattice types and arithmetic classes. Report of the International Union of Crystallography Ad-Hoc Committee on the Nomenclature of Symmetry"
170:
stands for a face-centered-cubic
Bravais lattice, which is not necessarily close-packed when a motif is set onto the lattice points. E.g. the diamond and the zincblende lattices are
3214:
structure, but with alternating types of atoms at the different lattice sites. The structure can also be described as an FCC lattice of zinc with sulfur atoms occupying half of the
357:(APF) is the fraction of volume that is occupied by atoms. The cP lattice has an APF of about 0.524, the cI lattice an APF of about 0.680, and the cF lattice an APF of about 0.740.
4021:
4502:
Hayashi, J; Shirotani, I; Hirano, K; Ishimatsu, N; Shimomura, O; Kikegawa, T (2003). "Structural phase transition of ScSb and YSb with a NaCl-type structure at high pressures".
4804:
Hayashi, J.; Shirotani, I.; Tanaka, Y.; Adachi, T.; Shimomura, O.; Kikegawa, T. (2000). "Phase transitions of LnSb (Ln=lanthanide) with NaCl-type structure at high pressures".
351:(CN) is the number of nearest neighbors of a central atom in the structure. Each sphere in a cP lattice has coordination number 6, in a cI lattice 8, and in a cF lattice 12.
1224:. In sodium chloride there is a 1:1 ratio of sodium to chlorine atoms. The structure can also be described as an FCC lattice of sodium with chlorine occupying each
5468:"Synthesis of Binary Transition Metal Nitrides, Carbides and Borides from the Elements in the Laser-Heated Diamond Anvil Cell and Their Structure-Property Relations"
3823:
formed by methane, propane, and carbon dioxide at low temperatures have a structure in which water molecules lie at the nodes of the Weaire–Phelan structure and are
1049:
1243:) in some rock-salt-structure crystals are: 2.3 Å (2.3 × 10 m) for NaF, 2.8 Å for NaCl, and 3.2 Å for SnTe. Most of the
5893:
3655:
338:
Attempting to create a base-centered cubic lattice (i.e., putting an extra lattice point in the center of each horizontal face) results in a simple
4073:
Saccone, A.; Delfino, S.; Macció, D.; Ferro, R. (1993). "Magnesium-rare earth phase diagrams: Experimental investigation of the Ho-Mg system".
1236:, this structure is more likely to be formed if the cation is somewhat smaller than the anion (a cation/anion radius ratio of 0.414 to 0.732).
5763:
5419:
4548:
4426:
3985:
3931:
3163:
4375:
Louail, L.; Haddadi, K.; Maouche, D.; Ali
Sahraoui, F.; Hachemi, A. (2008). "Electronic band structure of calcium selenide under pressure".
4231:
Abrahams, S. C.; Bernstein, J. L. (1965). "Accuracy of an automatic diffractometer. Measurement of the sodium chloride structure factors".
1172:
5010:
Kruger, O.L.; Moser, J.B. (1967). "Lattice constants and melting points of actinide-group IVA-VIA compounds with NaCl-type structures".
3133:
920:
4666:
Natali, F.; Ruck, B.J.; Plank, N.O.V.; Trodahl, H.J.; Granville, S.; Meyer, C.; Lambrecht, W.R.L. (2013). "Rare-earth mononitrides".
5818:
5611:
5139:
4459:
3210:. In zinc sulfide the ratio of zinc to sulfur is 1:1. Altogether, the arrangement of atoms in zincblende structure is the same as
5383:
Wachter, P. (1972). "The optical electrical and magnetic properties of the europium chalcogenides and the rare earth pnictides".
3249:
915:
Visualisation of a diamond cubic unit cell: 1. Components of a unit cell, 2. One unit cell, 3. A lattice of 3 x 3 x 3 unit cells
5641:
5303:
Smolensky, G. A.; Adamjan, V. E.; Loginov, G. M. (1968). "Antiferromagnetic
Properties of Light Rare Earth Monochalcogenides".
4180:
4057:
3846:
933:.) Accordingly, the primitive cubic structure, with especially low atomic packing factor, is rare in nature, but is found in
157:
5657:
Wang, L.D.; Kwok, H.S. (2000). "Cubic aluminum nitride and gallium nitride thin films prepared by pulsed laser deposition".
3185:
1202:
1114:
428:
380:
5918:
4309:
Broch, Einar (1927-06-01). "Präzisionsbestimmungen der
Gitterkonstanten der Verbindungen MgO, MgS, MgSe, MnO und MnSe".
3441:
366:
5257:
Didchenko, R.; Gortsema, F.P. (1963). "Some electric and magnetic properties of rare earth monosulfides and nitrides".
3789:
5955:
3677:
3639:
1206:
1118:
4108:
Kanematu, K; T. Alfieri, G.; Banks, E. (1969). "Magnetic
Studies of Rare Earth Zinc Compounds with CsCl Structure".
5886:
5850:
3445:
3206:: Each atom's nearest neighbors consist of four atoms of the opposite type, positioned like the four vertices of a
42:). The crystal structure of pyrite is primitive cubic, and this is reflected in the cubic symmetry of its natural
5965:
5945:
5840:
4866:
Taylor, J. B.; Calvert, L. D.; Wang, Y. (1979). "Powder data for some new europium antimonides and bismuthides".
2504:
1233:
1220:: Each atom's nearest neighbors consist of six atoms of the opposite type, positioned like the six vertices of a
339:
3646:. Together with the closely related half-Heusler and inverse-Huesler compounds, there are hundreds of examples.
2509:
6068:
5975:
5934:
5879:
3168:
2499:
329:
5855:
5845:
3691:
Examples occur among the transition metal silicides and germanides, as well as a few other compounds such as
2902:
2880:
2858:
2662:
2531:
3767:
3681:
2897:
2853:
2740:
2526:
2380:
1763:
1758:
1128:
2892:
2870:
2848:
2836:
2762:
2605:
2575:
1902:
1897:
5195:
Roedhammer, P.; Reichardt, W.; Holtzberg, F. (1978). "Soft-Mode
Behavior in the Phonon Dispersion of YS".
4233:
4196:
Sundquist, J. J.; Lin, C. C. (1981). "Electronic structure of the F centre in a sodium fluoride crystal".
3717:
3103:
2924:
2875:
2831:
2794:
2753:
2716:
2681:
2653:
2643:
2618:
2600:
2570:
2487:
2372:
2356:
2298:
2293:
2268:
2263:
2238:
2233:
2077:
1788:
1748:
1703:
1698:
2919:
2789:
2707:
2445:
2343:
2283:
2110:
2105:
1892:
1843:
1643:
1638:
1542:
1484:
3977:
3762:
3359:
3349:
2914:
2731:
2726:
2691:
2595:
2565:
2521:
2461:
2348:
2288:
2208:
2203:
2193:
2155:
2072:
2052:
2047:
1962:
1957:
1932:
1927:
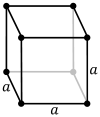
1783:
1753:
1693:
1673:
1547:
926:
642:
475:
354:
3111:
2338:
2328:
2323:
2145:
2100:
2022:
2017:
1733:
1728:
1633:
1512:
5066:
Vogt, O.; Mattenberger, K. (1995). "The magnetism of localized or nearly localized 4f and 5f shells".
3498:
3409:
2313:
2198:
2150:
2042:
1922:
1572:
332:(hcp) system, where two systems differ only in the relative placements of their hexagonal layers. The
5701:
5662:
5479:
5312:
5266:
5204:
5169:
5019:
4943:
4813:
4511:
4384:
4348:
study of phase stability, lattice dynamics and thermodynamic properties of magnesium chalcogenides".
4205:
4152:
4117:
3524:
2998:
2590:
2560:
2429:
2318:
2012:
1947:
1887:
1793:
1743:
1723:
1577:
1436:
709:
533:
462:
423:
376:
4922:"Tunable electronic structure and topological properties of LnPn (Ln=Ce, Pr, Sm, Gd, Yb; Pn=Sb, Bi)"
6022:
3747:
3722:
3514:
3384:
3354:
3203:
3079:
2826:
2672:
2634:
2482:
2253:
2223:
2067:
1952:
1838:
1778:
1688:
1217:
1182:
1094:
1082:
1081:
In the unit cell of CsCl, each ion is at the center of a cube of ions of the opposite kind, so the
796:
629:
348:
5353:
Kershner, C.J.; DeSando, R.J.; Heidelberg, R.F.; Steinmeyer, R.H. (1966). "Rare earth polonides".
5126:. Handbook on the Physics and Chemistry of Rare Earths. Vol. 17. Elsevier. pp. 245–300.
5862:
5834:
5466:
Friedrich, Alexandra; Winkler, Björn; Juarez-Arellano, Erick A.; Bayarjargal, Lkhamsuren (2011).
4933:
4693:
4675:
4326:
4090:
3937:
3856:
3816:
3550:
3519:
3291:
3286:
3245:
3139:
3127:
3075:
3071:
3067:
2477:
2389:
2258:
2228:
2095:
1992:
1987:
1942:
1882:
1858:
1828:
1668:
1628:
1586:
1537:
1479:
1369:
1364:
1125:
930:
5122:
Benedict, U.; Holzapfel, W.B. (1993). "Chapter 113 High-pressure studies — Structural aspects".
1008:. Unlike fcc and bcc, this structure is not a lattice, since it contains multiple atoms in its
6042:
5814:
5759:
5727:
5607:
5507:
5425:
5415:
5135:
4612:
Schmidt, F.A.; McMasters, O.D.; Lichtenberg, R.R. (1969). "The yttrium-bismuth alloy system".
4544:
4538:
4455:
4422:
3981:
3927:
3820:
3808:
3732:
3692:
3688:
properties. There are four atoms of each element for a total of eight atoms in the unit cell.
3665:
3631:
3576:
3540:
3508:
3429:
3404:
3344:
3296:
3230:
3215:
3151:
3115:
2983:
2978:
2585:
2278:
2248:
2218:
2062:
2037:
1977:
1917:
1833:
1773:
1683:
1658:
1532:
1474:
1399:
1394:
1374:
1359:
1225:
1160:
1087:
1037:
911:
244:
87:
5601:
4768:
Yoshihara, K.; Taylor, J.B.; Calvert, L.D.; Despault, J.G. (1975). "Rare-earth bismuthides".
4416:
3191:
The
Zincblende structure (also written "zinc blende") is named after the mineral zincblende (
5785:
5781:
5745:
5717:
5709:
5670:
5534:
5497:
5487:
5448:
5392:
5362:
5320:
5274:
5212:
5177:
5127:
5075:
5027:
4951:
4902:
4875:
4848:
4821:
4777:
4729:
4685:
4621:
4594:
4519:
4484:
4447:
4392:
4357:
4318:
4292:
4268:
4241:
4213:
4160:
4125:
4082:
3969:
3919:
3894:
3752:
3627:
3617:
3596:
3566:
3545:
3424:
3399:
3314:
3301:
3226:
3222:
3091:
3087:
3063:
3054:
3038:
3013:
3008:
2968:
2963:
2935:
2090:
2007:
1982:
1853:
1823:
1718:
1663:
1623:
1517:
1507:
1424:
1404:
1389:
1379:
1309:
1304:
1255:
1148:
1144:
1140:
1106:
1059:
438:
388:
4143:
Buschow, K. H. J. (1974). "Magnetic properties of CsCl‐type rare‐earth cadmium compounds".
3591:
1177:
58:
6012:
5913:
5645:
4184:
4061:
3800:
3571:
3534:
3488:
3419:
3394:
3107:
3099:
3028:
3023:
2993:
2188:
2140:
2032:
1972:
1912:
1653:
1567:
1527:
1502:
1469:
1419:
1409:
1339:
1334:
1314:
1299:
1239:
The interatomic distance (distance between cation and anion, or half the unit cell length
1033:
484:
342:
274:
191:
67:
50:
5705:
5688:
Oseki, Masaaki; Okubo, Kana; Kobayashi, Atsushi; Ohta, Jitsuo; Fujioka, Hiroshi (2014).
5666:
5483:
5316:
5270:
5208:
5173:
5023:
4947:
4920:
Duan, Xu; Wu, Fan; Chen, Jia; Zhang, Peiran; Liu, Yang; Yuan, Huiqiu; Cao, Chao (2018).
4817:
4515:
4388:
4209:
4156:
4121:
174:
but not close-packed. Each is subdivided into other variants listed below. Although the
6073:
6032:
5902:
5780:
Moyer, Harvey V. (1956). "Chemical
Properties of Polonium". In Moyer, Harvey V. (ed.).
5755:
5722:
5689:
5576:
5502:
5467:
3780:
3737:
3560:
3493:
3379:
3083:
2308:
2002:
1713:
1562:
1344:
1329:
1319:
1152:
1009:
822:
558:
222:
179:
83:
5674:
5131:
4825:
4523:
4451:
4418:
The
Aqueous Chemistry of Polonium and the Practical Application of its Thermochemistry
4361:
4217:
896:
6062:
5366:
5278:
5181:
5079:
5031:
4839:
Gschneidner, K. A.; Calderwood, F. W. (1986). "The As−Eu (Arsenic-Europium) system".
4781:
4733:
4697:
4625:
4598:
4585:
Ono, S.; Despault, J.G.; Calvert, L.D.; Taylor, J.B. (1970). "Rare-earth arsenides".
4475:
Gschneidner, K. A.; Calderwood, F. W. (1986). "The As−Sc (Arsenic-Scandium) system".
4330:
4272:
3941:
3824:
3482:
3374:
3211:
3095:
1497:
1349:
1156:
989:
5784:(Report). Oak Ridge, Tenn.: United States Atomic Energy Commission. pp. 33–96.
5637:
5552:
4720:
Ono, S.; Nomura, K.; Hayakawa, H. (1974). "Syntheses of new rare-earth phosphides".
4177:
4094:
4054:
1093:
In addition to caesium chloride itself, the structure also appears in certain other
391:), type, examples, international tables for crystallography space group number, and
5160:
Leger, J.M.; Yacoubi, N.; Loriers, J. (1981). "Synthesis of rare earth monoxides".
3804:
3685:
3455:
3369:
3317:
3237:
3196:
2396:
1557:
1439:
1262:
1244:
1132:
333:
264:
257:
62:
The primitive and cubic close-packed (also known as face-centered cubic) unit cells
4689:
17:
5749:
5626:
3923:
3444:
family of compounds, most of which can be made in both the zincblende (cubic) or
6026:
5984:
4396:
3851:
3452:
3234:
3207:
3200:
3177:
1190:
1102:
413:
392:
5216:
4906:
5396:
4956:
4921:
4879:
4540:
Scandium Its Occurrence, Chemistry Physics, Metallurgy, Biology and Technology
4245:
3899:
3882:
3192:
1221:
1214:
1070:
1013:
730:
505:
480:
5525:
Venkatraman, M.; Neumann, J. P. (1990). "The C-Cr (Carbon-Chromium) System".
5429:
4296:
4322:
3827:
together, and the larger gas molecules are trapped in the polyhedral cages.
3812:
3310:
1181:
The rock-salt crystal structure. Each atom has six nearest neighbours, with
1136:
1021:
1001:
970:
945:, with their higher densities, are both quite common in nature. Examples of
5731:
5511:
4344:
Mir, Showkat H.; Jha, Prakash C.; Dabhi, Shweta; Jha, Prafulla K. (2016). "
5979:
5959:
3660:
1054:
30:
5452:
2393:
1593:
1590:
1017:
958:
954:
934:
433:
384:
5443:
D'Eye, R. W. M.; Sellman, P. G. (1954). "The thorium–tellurium system".
4129:
395:
are listed in the table below. There are a total 36 cubic space groups.
5969:
5949:
5538:
4852:
4488:
4259:
Kao, W.; Peretti, E. (1970). "The ternary subsystem Sn4As3-SnAs-SnTe".
4086:
3954:
3836:
3241:
2942:
2938:
1265:
1247:
997:
962:
652:
647:
99:
95:
5939:
5713:
5492:
5324:
4164:
1062:
unit cell. The two colors of spheres represent the two types of atoms.
3259:
3256:
3221:
Examples of compounds with this structure include zincblende itself,
1269:
1251:
1194:
993:
982:
974:
900:
831:
827:
603:
563:
35:
27:
Crystallographic system where the unit cell is in the shape of a cube
5790:
4938:
3154:
4a and 8c whereas the rock-salt structure positions are 4a and 4b.
1205:), or "225" (in the International Tables for Crystallography). The
1117:), or "221" (in the International Tables for Crystallography). The
5871:
4680:
3779:
3699:
Transition metal silicides and germanides with the FeSi structure
3659:
3590:
3167:
1176:
1069:
1053:
910:
57:
49:
43:
29:
6046:
6036:
6016:
5995:
5866:
5830:
978:
950:
250:
91:
5875:
1151:. Other compounds showing caesium chloride like structure are
1005:
94:. This is one of the most common and simplest shapes found in
3098:). The early actinoid monocarbides also have this structure (
335:
plane of a face-centered cubic lattice is a hexagonal grid.
178:
in these crystals are conventionally taken to be cubes, the
196:
The three Bravais latices in the cubic crystal system are:
328:
The face-centered cubic lattice is closely related to the
5690:"Field-effect transistors based on cubic indium nitride"
4442:
Hulliger, F. (1979). "Chapter 33 Rare earth pnictides".
3672:
The space group of the iron monosilicide structure is P2
1131:
that crystallize in the CsCl structure, including many
1032:
Compounds that consist of more than one element (e.g.
3956:
Crystallography and Minerals Arranged by Crystal Form
4444:
Handbook on the Physics and Chemistry of Rare Earths
3188:), or 216. The Strukturbericht designation is "B3".
1254:
have the rock salt structure, though a few have the
6005:
5927:
3622:
The Heusler structure, based on the structure of Cu
3233:), and a wide array of other binary compounds. The
105:There are three main varieties of these crystals:
5252:
5250:
5248:
5246:
4893:Okamoto, H. (1999). "Bi-Yb (bismuth-ytterbium)".
3248:, and their zincblende forms are less well known
988:Another important cubic crystal structure is the
895:Other terms for hexoctahedral are: normal class,
273:The primitive cubic lattice (cP) consists of one
5414:. Dordrecht: Springer Netherlands. p. 237.
5298:
5296:
5294:
5292:
5290:
5288:
5244:
5242:
5240:
5238:
5236:
5234:
5232:
5230:
5228:
5226:
5117:
5115:
5113:
5111:
5109:
3240:usually have a zincblende structure, though the
5348:
5346:
5344:
5342:
5340:
5338:
5336:
5334:
5107:
5105:
5103:
5101:
5099:
5097:
5095:
5093:
5091:
5089:
1012:. Other cubic elemental structures include the
568:
5809:Hurlbut, Cornelius S.; Klein, Cornelis, 1985,
5412:Synthesis of Lanthanide and Actinide Compounds
5385:C R C Critical Reviews in Solid State Sciences
5378:
5376:
5005:
5003:
5001:
4999:
4997:
4995:
4993:
4991:
4989:
4987:
758:
699:
596:
523:
5887:
4985:
4983:
4981:
4979:
4977:
4975:
4973:
4971:
4969:
4967:
4763:
4000:The original discovery was in J. Chem. Phys.
3066:monoxides also have the rock salt structure (
1020:, and the extremely complicated structure of
8:
5155:
5153:
5151:
5061:
4761:
4759:
4757:
4755:
4753:
4751:
4749:
4747:
4745:
4743:
4410:
4408:
4406:
3684:structure, and is sometimes associated with
1197:(sodium chloride) structure is denoted as Fm
845:
5059:
5057:
5055:
5053:
5051:
5049:
5047:
5045:
5043:
5041:
4580:
4446:. Vol. 4. Elsevier. pp. 153–236.
1050:Category:Caesium chloride crystal structure
54:A network model of a primitive cubic system
5894:
5880:
5872:
5355:Journal of Inorganic and Nuclear Chemistry
5259:Journal of Physics and Chemistry of Solids
5012:Journal of Physics and Chemistry of Solids
4799:
4797:
4795:
4793:
4791:
4661:
4659:
4657:
4655:
4578:
4576:
4574:
4572:
4570:
4568:
4566:
4564:
4562:
4560:
3876:
3874:
3872:
2933:
2387:
1584:
1434:
1260:
5789:
5721:
5501:
5491:
4955:
4937:
4679:
4653:
4651:
4649:
4647:
4645:
4643:
4641:
4639:
4637:
4635:
4198:Journal of Physics C: Solid State Physics
3898:
3656:Category:Iron monosilicide structure type
1163:, AlCo, AgZn, BeCu, MgCe, RuAl and SrTl.
742:
4110:Journal of the Physical Society of Japan
3916:International Tables for Crystallography
3697:
3450:
3308:
3254:
3225:, many compound semiconductors (such as
859:
397:
198:
166:structure occurring in metals. However,
4286:
4284:
4282:
4037:
4035:
3868:
3843:unit cell, with vertical body diagonal.
3815:where it is usually known as a "type I
3180:of the Zincblende structure is called F
3138:Much like the rock salt structure, the
5627:Birkbeck College, University of London
4715:
4713:
4711:
4709:
4707:
2934:
2388:
1585:
1435:
1261:
3799:It has three orientations of stacked
3164:Category:Zincblende crystal structure
315: × 8 from the corners plus
7:
4311:Zeitschrift für Physikalische Chemie
4016:
4014:
4012:
4010:
3807:cells in the gaps. It is found as a
1173:Category:Rock salt crystal structure
34:A rock containing three crystals of
3134:Category:Fluorite crystal structure
5640:. Naval Research Laboratory, U.S.
4868:Journal of Applied Crystallography
4022:"Cubic Lattices and Close Packing"
921:Periodic table (crystal structure)
25:
5603:Quantum Theory of the Solid State
4770:Journal of the Less Common Metals
4722:Journal of the Less Common Metals
4614:Journal of the Less Common Metals
4587:Journal of the Less Common Metals
4543:. Elsevier Science. p. 273.
4415:Brown, S.A.; Brown, P.L. (2019).
4362:10.1016/j.matchemphys.2016.02.066
4261:Journal of the Less Common Metals
3839:: building which is a model of a
156:is often used in synonym for the
6045:
6035:
6025:
6015:
5994:
5983:
5978:
5968:
5958:
5948:
5938:
5527:Bulletin of Alloy Phase Diagrams
5162:Journal of Solid State Chemistry
5124:Lanthanides/Actinides: Physics I
4841:Bulletin of Alloy Phase Diagrams
4477:Bulletin of Alloy Phase Diagrams
4421:. Elsevier Science. p. 25.
4028:from the original on 2020-11-01.
3887:Acta Crystallographica Section A
3626:MnAl, is a common structure for
3584:This group is also known as the
3440:This group is also known as the
325: × 6 from the faces).
263:
256:
249:
5068:Journal of Alloys and Compounds
4350:Materials Chemistry and Physics
4145:The Journal of Chemical Physics
992:structure, which can appear in
3458:with the zincblende structure
3320:with the zincblende structure
3262:with the zincblende structure
1139:, and with elements in groups
375:class names, point groups (in
1:
5675:10.1016/s0169-4332(99)00372-4
5638:The Zincblende (B3) Structure
5132:10.1016/s0168-1273(05)80030-3
4826:10.1016/s0038-1098(00)00113-7
4690:10.1016/j.pmatsci.2013.06.002
4668:Progress in Materials Science
4524:10.1016/s0038-1098(02)00889-x
4452:10.1016/s0168-1273(79)04006-x
2945:with the rock salt structure
2399:with the rock salt structure
1596:with the rock salt structure
1442:with the rock salt structure
1272:with the rock salt structure
1109:(CsCl) structure is called Pm
5919:Crystallographic point group
5367:10.1016/0022-1902(66)80054-4
5279:10.1016/0022-3697(63)90062-3
5182:10.1016/0022-4596(81)90436-9
5080:10.1016/0925-8388(94)09005-x
5032:10.1016/0022-3697(67)90257-0
4782:10.1016/0022-5088(75)90038-7
4734:10.1016/0022-5088(74)90055-1
4626:10.1016/0022-5088(69)90159-3
4599:10.1016/0022-5088(70)90175-x
4273:10.1016/0022-5088(70)90174-8
3924:10.1107/97809553602060000001
899:, ditesseral central class,
786:
672:
619:
498:
452:
418:
367:Crystallographic point group
220:
4895:Journal of Phase Equilibria
4397:10.1016/j.physb.2008.03.009
4377:Physica B: Condensed Matter
4218:10.1088/0022-3719/14/32/016
4075:Journal of Phase Equilibria
3678:Strukturbericht designation
3650:Iron monosilicide structure
3640:Strukturbericht designation
3634:. It has the space group Fm
1207:Strukturbericht designation
1124:There are nearly a hundred
1119:Strukturbericht designation
6090:
5661:. 154–155 (1–4): 439–443.
5305:Journal of Applied Physics
5217:10.1103/physrevlett.40.465
4907:10.1361/105497199770335640
4806:Solid State Communications
4504:Solid State Communications
3653:
3615:
3161:
3131:
3125:
2510:Praseodymium monotelluride
1170:
1047:
1044:Caesium chloride structure
918:
835:
364:
301: × 8 + 1).
189:
5909:
5751:Chemistry of the Elements
5748:; Earnshaw, Alan (1984).
5644:October 19, 2008, at the
5397:10.1080/10408437208244865
4957:10.1038/s42005-018-0074-8
4880:10.1107/s0021889879012309
4246:10.1107/S0365110X65002244
3974:Chemistry of the Elements
3972:; Earnshaw, Alan (1997).
3900:10.1107/S0108767385000587
3758:
3743:
3728:
3713:
3708:
3705:
3703:
3556:
3530:
3504:
3478:
3473:
3470:
3467:
3464:
3462:
3335:
3332:
3329:
3326:
3324:
3277:
3274:
3271:
3268:
3266:
2954:
2951:
2949:
2749:
2614:
2505:Praseodymium monoselenide
2425:
2417:
2414:
2411:
2408:
2405:
2403:
1873:
1614:
1611:
1608:
1605:
1602:
1600:
1460:
1457:
1454:
1451:
1448:
1446:
1428:
1415:
1385:
1355:
1325:
1295:
1290:
1287:
1284:
1281:
1278:
1276:
907:Single element structures
729:
727:
725:
722:
716:
708:
705:
646:
641:
639:
636:
633:
628:
625:
562:
557:
555:
552:
540:
532:
529:
479:
474:
472:
469:
466:
461:
458:
412:
409:
406:
403:
400:
116:and alternatively called
5976:trigonal & hexagonal
5863:Making crystal structure
5606:. Springer. p. 32.
5600:Kantorovich, L. (2004).
4297:10.1002/14356007.a11_307
3914:Prince, E., ed. (2006).
3186:Hermann–Mauguin notation
2500:Praseodymium monosulfide
1203:Hermann–Mauguin notation
1115:Hermann–Mauguin notation
1028:Multi-element structures
381:Hermann–Mauguin notation
373:isometric crystal system
5659:Applied Surface Science
5197:Physical Review Letters
4537:Horovitz, C.T. (2012).
4323:10.1515/zpch-1927-12724
4178:The NaCl (B1) Structure
4055:The CsCl (B2) Structure
4043:Modern Theory of Solids
3790:Weaire–Phelan structure
3784:Weaire–Phelan structure
3776:Weaire–Phelan structure
3244:are more common in the
2903:Americium monotelluride
2881:Plutonium monotelluride
2859:Neptunium monotelluride
2663:Dysprosium monopolonide
2532:Neodymium monotelluride
1764:Praseodymium bismuthide
1759:Praseodymium antimonide
1129:intermetallic compounds
4926:Communications Physics
3785:
3768:Chromium(IV) germanide
3718:Manganese monosilicide
3669:
3608:
3173:
3172:A zincblende unit cell
2898:Americium monoselenide
2876:Plutonium monoselenide
2854:Neptunium monoselenide
2741:Ytterbium monopolonide
2654:Dysprosium monosulfide
2619:Gadolinium monosulfide
2601:Europium monotelluride
2571:Samarium monotelluride
2527:Neodymium monoselenide
2381:Californium bismuthide
1749:Praseodymium phosphide
1186:
1075:
1063:
916:
330:hexagonal close packed
63:
55:
47:
3978:Butterworth-Heinemann
3970:Greenwood, Norman N.
3783:
3763:Chromium(IV) silicide
3676:3 (No. 198), and the
3663:
3638:m (No. 225), and the
3595:The structure of the
3594:
3588:family of compounds.
3171:
2893:Americium monosulfide
2871:Plutonium monosulfide
2849:Neptunium monosulfide
2837:Uranium monotelluride
2763:Lutetium monopolonide
2732:Ytterbium monosulfide
2606:Europium monopolonide
2596:Europium monoselenide
2576:Samarium monopolonide
2566:Samarium monoselenide
2522:Neodymium monosulfide
2462:Lanthanum monosulfide
2194:Uranium monophosphide
1963:Dysprosium bismuthide
1958:Dysprosium antimonide
1903:Gadolinium bismuthide
1898:Gadolinium antimonide
1754:Praseodymium arsenide
1180:
1073:
1057:
927:orbital hybridization
914:
365:Further information:
355:Atomic packing factor
190:Further information:
90:is in the shape of a
61:
53:
33:
5811:Manual of Mineralogy
5746:Greenwood, Norman N.
5453:10.1039/jr9540003760
3525:Aluminium antimonide
3158:Zincblende structure
2925:Curium monotelluride
2832:Uranium monoselenide
2795:Thorium monoselenide
2754:Lutetium monosulfide
2717:Thulium monopolonide
2682:Holmium monopolonide
2644:Terbium monopolonide
2591:Europium monosulfide
2561:Samarium monosulfide
2488:Cerium monotelluride
2430:Scandium monosulfide
2373:Californium arsenide
2357:Berkelium bismuthide
2299:Americium bismuthide
2294:Americium antimonide
2269:Plutonium bismuthide
2264:Plutonium antimonide
2239:Neptunium bismuthide
2234:Neptunium antimonide
2078:Ytterbium antimonide
1948:Dysprosium phosphide
1888:Gadolinium phosphide
1794:Neodymium bismuthide
1789:Neodymium antimonide
1744:Praseodymium nitride
1704:Lanthanum bismuthide
1699:Lanthanum antimonide
1437:Alkaline earth metal
1193:of the rock-salt or
1135:of rare earths with
180:primitive unit cells
5813:, 20th ed., Wiley,
5706:2014NatSR...4E3951O
5667:2000ApSS..154..439W
5484:2011Mate....4.1648F
5317:1968JAP....39..786S
5271:1963JPCS...24..863D
5209:1978PhRvL..40..465R
5174:1981JSSCh..36..261L
5024:1967JPCS...28.2321K
4948:2018CmPhy...1...71D
4818:2000SSCom.114..561H
4516:2003SSCom.125..543H
4389:2008PhyB..403.3022L
4210:1981JPhC...14.4797S
4157:1974JChPh..61.4666B
4130:10.1143/jpsj.26.244
4122:1969JPSJ...26..244K
3748:Cobalt monosilicide
3723:Manganese germanide
3700:
3515:Aluminium phosphide
3459:
3355:Beryllium telluride
3321:
3263:
3208:regular tetrahedron
2946:
2920:Curium monoselenide
2827:Uranium monosulfide
2790:Thorium monosulfide
2708:Thulium monosulfide
2673:Holmium monosulfide
2635:Terbium monosulfide
2483:Cerium monoselenide
2446:Yttrium monosulfide
2400:
2344:Berkelium phosphide
2284:Americium phosphide
2254:Plutonium phosphide
2224:Neptunium phosphide
2111:Lutetium bismuthide
2106:Lutetium antimonide
2068:Ytterbium phosphide
1953:Dysprosium arsenide
1893:Gadolinium arsenide
1844:Samarium bismuthide
1839:Samarium antimonide
1779:Neodymium phosphide
1689:Lanthanum phosphide
1644:Scandium bismuthide
1639:Scandium antimonide
1597:
1543:Strontium telluride
1485:Magnesium telluride
1443:
1273:
1258:structure instead.
1183:octahedral geometry
1167:Rock-salt structure
1159:, high-temperature
1083:coordination number
377:Schönflies notation
349:Coordination number
138:Face-centered cubic
124:Body-centered cubic
5694:Scientific Reports
5539:10.1007/bf02841701
4853:10.1007/bf02869009
4489:10.1007/bf02873011
4183:2008-10-19 at the
4087:10.1007/bf02668225
4060:2008-09-15 at the
4024:. 3 October 2013.
3857:Reciprocal lattice
3796:n (223) symmetry.
3786:
3698:
3680:is B20. This is a
3670:
3609:
3551:Gallium antimonide
3520:Aluminium arsenide
3451:
3448:(hexagonal) form.
3360:Beryllium polonide
3350:Beryllium selenide
3309:
3292:Copper(I) chloride
3287:Copper(I) fluoride
3255:
3246:wurtzite structure
3174:
3140:fluorite structure
3128:Fluorite structure
3122:Fluorite structure
2915:Curium monosulfide
2727:Ytterbium monoxide
2692:Erbium monosulfide
2478:Cerium monosulfide
2349:Berkelium arsenide
2289:Americium arsenide
2259:Plutonium arsenide
2229:Neptunium arsenide
2209:Uranium bismuthide
2204:Uranium antimonide
2156:Thorium antimonide
2096:Lutetium phosphide
2073:Ytterbium arsenide
2053:Thulium bismuthide
2048:Thulium antimonide
1993:Holmium bismuthide
1988:Holmium antimonide
1943:Dysprosium nitride
1933:Terbium bismuthide
1928:Terbium antimonide
1883:Gadolinium nitride
1859:Europium phosphide
1829:Samarium phosphide
1784:Neodymium arsenide
1694:Lanthanum arsenide
1674:Yttrium bismuthide
1669:Yttrium antimonide
1629:Scandium phosphide
1548:Strontium polonide
1538:Strontium selenide
1480:Magnesium selenide
1370:Potassium chloride
1365:Potassium fluoride
1222:regular octahedron
1187:
1076:
1064:
1038:interstitial sites
917:
159:cubic close-packed
64:
56:
48:
18:Face-centred cubic
6056:
6055:
5765:978-0-08-022057-4
5714:10.1038/srep03951
5493:10.3390/ma4101648
5478:(10): 1648–1692.
5421:978-94-011-3758-4
5410:Meyer, G (1991).
5325:10.1063/1.2163619
5018:(11): 2321–2325.
4550:978-0-323-14451-3
4428:978-0-12-819309-9
4383:(18): 3022–3026.
4234:Acta Crystallogr.
4204:(32): 4797–4805.
4165:10.1063/1.1681788
4151:(11): 4666–4670.
3987:978-0-08-037941-8
3933:978-1-4020-4969-9
3809:crystal structure
3773:
3772:
3733:Iron monosilicide
3693:gallium palladide
3666:iron monosilicide
3632:transition metals
3628:ternary compounds
3612:Heusler structure
3597:Heusler compounds
3582:
3581:
3577:Indium antimonide
3541:Gallium phosphide
3509:Aluminium nitride
3438:
3437:
3430:Mercury telluride
3405:Cadmium telluride
3345:Beryllium sulfide
3307:
3306:
3297:Copper(I) bromide
3231:cadmium telluride
3216:tetrahedral voids
3152:Wyckoff positions
3060:
3059:
3043:(CoSn structure)
2984:Zirconium nitride
2979:Zirconium carbide
2932:
2931:
2799:(CsCl structure)
2586:Europium monoxide
2386:
2385:
2339:Berkelium nitride
2329:Curium bismuthide
2324:Curium antimonide
2279:Americium nitride
2249:Plutonium nitride
2219:Neptunium nitride
2160:(CsCl structure)
2146:Thorium phosphide
2101:Lutetium arsenide
2063:Ytterbium nitride
2038:Thulium phosphide
2023:Erbium bismuthide
2018:Erbium antimonide
1978:Holmium phosphide
1918:Terbium phosphide
1834:Samarium arsenide
1774:Neodymium nitride
1734:Cerium bismuthide
1729:Cerium antimonide
1684:Lanthanum nitride
1659:Yttrium phosphide
1634:Scandium arsenide
1583:
1582:
1533:Strontium sulfide
1513:Calcium telluride
1489:(NiAs structure)
1475:Magnesium sulfide
1433:
1432:
1429:(CsCl structure)
1400:Rubidium chloride
1395:Rubidium fluoride
1375:Potassium bromide
1360:Potassium hydride
1234:radius ratio rule
893:
892:
790:
762:
703:
676:
623:
600:
527:
502:
456:
288: × 8).
271:
270:
16:(Redirected from
6081:
6049:
6039:
6029:
6019:
5998:
5987:
5982:
5972:
5962:
5952:
5942:
5928:Seven 3D systems
5896:
5889:
5882:
5873:
5797:
5795:
5793:
5777:
5771:
5769:
5742:
5736:
5735:
5725:
5685:
5679:
5678:
5654:
5648:
5635:
5629:
5624:
5618:
5617:
5597:
5591:
5590:
5588:
5587:
5573:
5567:
5566:
5564:
5563:
5549:
5543:
5542:
5522:
5516:
5515:
5505:
5495:
5463:
5457:
5456:
5440:
5434:
5433:
5407:
5401:
5400:
5380:
5371:
5370:
5361:(8): 1581–1588.
5350:
5329:
5328:
5300:
5283:
5282:
5254:
5221:
5220:
5192:
5186:
5185:
5157:
5146:
5145:
5119:
5084:
5083:
5063:
5036:
5035:
5007:
4962:
4961:
4959:
4941:
4917:
4911:
4910:
4890:
4884:
4883:
4863:
4857:
4856:
4836:
4830:
4829:
4801:
4786:
4785:
4765:
4738:
4737:
4728:(2–3): 119–130.
4717:
4702:
4701:
4683:
4674:(8): 1316–1360.
4663:
4630:
4629:
4609:
4603:
4602:
4582:
4555:
4554:
4534:
4528:
4527:
4499:
4493:
4492:
4472:
4466:
4465:
4439:
4433:
4432:
4412:
4401:
4400:
4372:
4366:
4365:
4341:
4335:
4334:
4306:
4300:
4288:
4277:
4276:
4256:
4250:
4249:
4228:
4222:
4221:
4193:
4187:
4175:
4169:
4168:
4140:
4134:
4133:
4105:
4099:
4098:
4070:
4064:
4052:
4046:
4039:
4030:
4029:
4018:
4005:
3998:
3992:
3991:
3976:(2nd ed.).
3966:
3960:
3952:
3946:
3945:
3911:
3905:
3904:
3902:
3878:
3801:tetradecahedrons
3795:
3753:Cobalt germanide
3701:
3637:
3618:Heusler compound
3567:Indium phosphide
3546:Gallium arsenide
3499:Boron antimonide
3460:
3425:Mercury selenide
3410:Cadmium polonide
3400:Cadmium selenide
3322:
3302:Copper(I) iodide
3264:
3227:gallium arsenide
3223:lead(II) nitrate
3183:
3149:
3064:transition metal
3055:Chromium nitride
3039:Tantalum carbide
3014:Vanadium nitride
3009:Vanadium carbide
2969:Titanium nitride
2964:Titanium carbide
2947:
2936:Transition metal
2401:
2314:Curium phosphide
2199:Uranium arsenide
2151:Thorium arsenide
2091:Lutetium nitride
2043:Thulium arsenide
2008:Erbium phosphide
1983:Holmium arsenide
1923:Terbium arsenide
1854:Europium nitride
1824:Samarium nitride
1719:Cerium phosphide
1664:Yttrium arsenide
1624:Scandium nitride
1598:
1573:Barium telluride
1518:Calcium polonide
1508:Calcium selenide
1444:
1425:Caesium fluoride
1405:Rubidium bromide
1390:Rubidium hydride
1380:Potassium iodide
1310:Lithium chloride
1305:Lithium fluoride
1274:
1256:caesium chloride
1200:
1133:binary compounds
1112:
1107:caesium chloride
1060:caesium chloride
1034:binary compounds
929:demands certain
888:
884:
877:
873:
869:
863:
855:
849:
843:
839:
813:
807:
788:
782:
775:
768:
760:
754:
746:
738:
719:
701:
674:
621:
616:
607:
598:
593:
587:
583:
577:
572:
549:
544:
525:
500:
454:
398:
389:Coxeter notation
324:
323:
319:
314:
313:
309:
300:
299:
295:
287:
286:
282:
267:
260:
253:
202:Bravais lattice
199:
186:Bravais lattices
21:
6089:
6088:
6084:
6083:
6082:
6080:
6079:
6078:
6069:Crystal systems
6059:
6058:
6057:
6052:
6006:Four 2D systems
6001:
5923:
5914:Bravais lattice
5905:
5903:Crystal systems
5900:
5835:Graz University
5833:simulations by
5827:
5806:
5804:Further reading
5801:
5800:
5791:10.2172/4367751
5779:
5778:
5774:
5766:
5758:. p. 899.
5744:
5743:
5739:
5687:
5686:
5682:
5656:
5655:
5651:
5646:Wayback Machine
5636:
5632:
5625:
5621:
5614:
5599:
5598:
5594:
5585:
5583:
5575:
5574:
5570:
5561:
5559:
5551:
5550:
5546:
5524:
5523:
5519:
5465:
5464:
5460:
5442:
5441:
5437:
5422:
5409:
5408:
5404:
5382:
5381:
5374:
5352:
5351:
5332:
5302:
5301:
5286:
5256:
5255:
5224:
5194:
5193:
5189:
5159:
5158:
5149:
5142:
5121:
5120:
5087:
5065:
5064:
5039:
5009:
5008:
4965:
4919:
4918:
4914:
4892:
4891:
4887:
4865:
4864:
4860:
4838:
4837:
4833:
4812:(11): 561–565.
4803:
4802:
4789:
4767:
4766:
4741:
4719:
4718:
4705:
4665:
4664:
4633:
4611:
4610:
4606:
4584:
4583:
4558:
4551:
4536:
4535:
4531:
4510:(10): 543–546.
4501:
4500:
4496:
4474:
4473:
4469:
4462:
4441:
4440:
4436:
4429:
4414:
4413:
4404:
4374:
4373:
4369:
4343:
4342:
4338:
4308:
4307:
4303:
4289:
4280:
4258:
4257:
4253:
4230:
4229:
4225:
4195:
4194:
4190:
4185:Wayback Machine
4176:
4172:
4142:
4141:
4137:
4107:
4106:
4102:
4072:
4071:
4067:
4062:Wayback Machine
4053:
4049:
4040:
4033:
4020:
4019:
4008:
3999:
3995:
3988:
3968:
3967:
3963:
3953:
3949:
3934:
3913:
3912:
3908:
3880:
3879:
3870:
3865:
3833:
3825:hydrogen bonded
3793:
3778:
3675:
3664:Diagram of the
3658:
3652:
3645:
3635:
3625:
3620:
3614:
3606:
3602:
3572:Indium arsenide
3535:Gallium nitride
3489:Boron phosphide
3420:Mercury sulfide
3395:Cadmium sulfide
3218:or vice versa.
3195:), one form of
3181:
3166:
3160:
3147:
3146:) is also an Fm
3145:
3136:
3130:
3124:
3029:Niobium nitride
3024:Niobium carbide
2999:Hafnium nitride
2994:Hafnium carbide
2319:Curium arsenide
2189:Uranium nitride
2141:Thorium nitride
2033:Thulium nitride
2013:Erbium arsenide
1973:Holmium nitride
1913:Terbium nitride
1870:
1866:
1724:Cerium arsenide
1654:Yttrium nitride
1578:Barium polonide
1568:Barium selenide
1528:Strontium oxide
1503:Calcium sulfide
1470:Magnesium oxide
1420:Caesium hydride
1410:Rubidium iodide
1340:Sodium chloride
1335:Sodium fluoride
1315:Lithium bromide
1300:Lithium hydride
1228:or vice versa.
1226:octahedral void
1198:
1175:
1169:
1110:
1052:
1046:
1040:of the others.
1030:
923:
909:
886:
882:
875:
871:
867:
861:
853:
847:
841:
837:
823:centrosymmetric
811:
809:
805:
800:
780:
773:
766:
752:
744:
736:
717:
713:
706:Hextetrahedral
695:
686:
682:
665:
658:
614:
605:
591:
585:
581:
575:
570:
559:centrosymmetric
547:
545:
542:
537:
519:
509:
485:Sodium chlorate
369:
363:
361:Crystal classes
343:Bravais lattice
321:
317:
316:
311:
307:
306:
297:
293:
292:
284:
280:
279:
216:
211:
206:
194:
192:Bravais lattice
188:
182:often are not.
152:Note: the term
110:Primitive cubic
68:crystallography
41:
28:
23:
22:
15:
12:
11:
5:
6087:
6085:
6077:
6076:
6071:
6061:
6060:
6054:
6053:
6051:
6050:
6040:
6030:
6020:
6009:
6007:
6003:
6002:
6000:
5999:
5988:
5973:
5963:
5953:
5943:
5931:
5929:
5925:
5924:
5922:
5921:
5916:
5910:
5907:
5906:
5901:
5899:
5898:
5891:
5884:
5876:
5870:
5869:
5860:
5859:
5858:
5853:
5848:
5843:
5826:
5825:External links
5823:
5822:
5821:
5805:
5802:
5799:
5798:
5772:
5764:
5756:Pergamon Press
5737:
5680:
5649:
5630:
5619:
5612:
5592:
5568:
5544:
5533:(2): 152–159.
5517:
5458:
5435:
5420:
5402:
5391:(2): 189–241.
5372:
5330:
5311:(2): 786–790.
5284:
5265:(7): 863–870.
5222:
5203:(7): 465–468.
5187:
5168:(3): 261–270.
5147:
5140:
5085:
5074:(2): 226–236.
5037:
4963:
4912:
4885:
4874:(2): 249–251.
4858:
4847:(3): 279–283.
4831:
4787:
4776:(2): 329–337.
4739:
4703:
4631:
4620:(3): 215–220.
4604:
4556:
4549:
4529:
4494:
4483:(4): 348–349.
4467:
4460:
4434:
4427:
4402:
4367:
4336:
4317:(1): 446–454.
4301:
4278:
4251:
4240:(5): 926–932.
4223:
4188:
4170:
4135:
4116:(2): 244–248.
4100:
4081:(3): 280–287.
4065:
4047:
4031:
4006:
3993:
3986:
3961:
3947:
3932:
3906:
3867:
3866:
3864:
3861:
3860:
3859:
3854:
3849:
3844:
3832:
3829:
3777:
3774:
3771:
3770:
3765:
3760:
3756:
3755:
3750:
3745:
3741:
3740:
3738:Iron germanide
3735:
3730:
3726:
3725:
3720:
3715:
3711:
3710:
3707:
3704:
3673:
3651:
3648:
3643:
3623:
3616:Main article:
3613:
3610:
3604:
3600:
3599:with formula X
3580:
3579:
3574:
3569:
3564:
3561:Indium nitride
3558:
3554:
3553:
3548:
3543:
3538:
3532:
3528:
3527:
3522:
3517:
3512:
3506:
3502:
3501:
3496:
3494:Boron arsenide
3491:
3486:
3480:
3476:
3475:
3472:
3469:
3466:
3463:
3436:
3435:
3432:
3427:
3422:
3417:
3413:
3412:
3407:
3402:
3397:
3392:
3388:
3387:
3382:
3380:Zinc telluride
3377:
3372:
3367:
3363:
3362:
3357:
3352:
3347:
3342:
3338:
3337:
3334:
3331:
3328:
3325:
3305:
3304:
3299:
3294:
3289:
3284:
3280:
3279:
3276:
3273:
3270:
3267:
3159:
3156:
3143:
3126:Main article:
3123:
3120:
3058:
3057:
3052:
3049:
3045:
3044:
3041:
3036:
3032:
3031:
3026:
3021:
3017:
3016:
3011:
3006:
3002:
3001:
2996:
2991:
2987:
2986:
2981:
2976:
2972:
2971:
2966:
2961:
2957:
2956:
2953:
2950:
2930:
2929:
2927:
2922:
2917:
2912:
2908:
2907:
2905:
2900:
2895:
2890:
2886:
2885:
2883:
2878:
2873:
2868:
2864:
2863:
2861:
2856:
2851:
2846:
2842:
2841:
2839:
2834:
2829:
2824:
2820:
2819:
2816:
2813:
2810:
2807:
2803:
2802:
2800:
2797:
2792:
2787:
2783:
2782:
2779:
2776:
2773:
2770:
2766:
2765:
2760:
2758:
2756:
2751:
2748:
2744:
2743:
2738:
2736:
2734:
2729:
2724:
2720:
2719:
2714:
2712:
2710:
2705:
2701:
2700:
2698:
2696:
2694:
2689:
2685:
2684:
2679:
2677:
2675:
2670:
2666:
2665:
2660:
2658:
2656:
2651:
2647:
2646:
2641:
2639:
2637:
2632:
2628:
2627:
2625:
2623:
2621:
2616:
2613:
2609:
2608:
2603:
2598:
2593:
2588:
2583:
2579:
2578:
2573:
2568:
2563:
2558:
2554:
2553:
2550:
2547:
2544:
2541:
2537:
2536:
2534:
2529:
2524:
2519:
2515:
2514:
2512:
2507:
2502:
2497:
2493:
2492:
2490:
2485:
2480:
2475:
2471:
2470:
2468:
2466:
2464:
2459:
2455:
2454:
2452:
2450:
2448:
2443:
2439:
2438:
2436:
2434:
2432:
2427:
2424:
2420:
2419:
2416:
2413:
2410:
2407:
2404:
2384:
2383:
2378:
2375:
2370:
2367:
2364:
2360:
2359:
2354:
2351:
2346:
2341:
2336:
2332:
2331:
2326:
2321:
2316:
2311:
2309:Curium nitride
2306:
2302:
2301:
2296:
2291:
2286:
2281:
2276:
2272:
2271:
2266:
2261:
2256:
2251:
2246:
2242:
2241:
2236:
2231:
2226:
2221:
2216:
2212:
2211:
2206:
2201:
2196:
2191:
2186:
2182:
2181:
2178:
2175:
2172:
2169:
2166:
2162:
2161:
2158:
2153:
2148:
2143:
2138:
2134:
2133:
2130:
2127:
2124:
2121:
2118:
2114:
2113:
2108:
2103:
2098:
2093:
2088:
2084:
2083:
2080:
2075:
2070:
2065:
2060:
2056:
2055:
2050:
2045:
2040:
2035:
2030:
2026:
2025:
2020:
2015:
2010:
2005:
2003:Erbium nitride
2000:
1996:
1995:
1990:
1985:
1980:
1975:
1970:
1966:
1965:
1960:
1955:
1950:
1945:
1940:
1936:
1935:
1930:
1925:
1920:
1915:
1910:
1906:
1905:
1900:
1895:
1890:
1885:
1880:
1876:
1875:
1872:
1868:
1864:
1861:
1856:
1851:
1847:
1846:
1841:
1836:
1831:
1826:
1821:
1817:
1816:
1813:
1810:
1807:
1804:
1801:
1797:
1796:
1791:
1786:
1781:
1776:
1771:
1767:
1766:
1761:
1756:
1751:
1746:
1741:
1737:
1736:
1731:
1726:
1721:
1716:
1714:Cerium nitride
1711:
1707:
1706:
1701:
1696:
1691:
1686:
1681:
1677:
1676:
1671:
1666:
1661:
1656:
1651:
1647:
1646:
1641:
1636:
1631:
1626:
1621:
1617:
1616:
1613:
1610:
1607:
1604:
1601:
1581:
1580:
1575:
1570:
1565:
1563:Barium sulfide
1560:
1555:
1551:
1550:
1545:
1540:
1535:
1530:
1525:
1521:
1520:
1515:
1510:
1505:
1500:
1495:
1491:
1490:
1487:
1482:
1477:
1472:
1467:
1463:
1462:
1459:
1456:
1453:
1450:
1447:
1431:
1430:
1427:
1422:
1417:
1413:
1412:
1407:
1402:
1397:
1392:
1387:
1383:
1382:
1377:
1372:
1367:
1362:
1357:
1353:
1352:
1347:
1345:Sodium bromide
1342:
1337:
1332:
1330:Sodium hydride
1327:
1323:
1322:
1320:Lithium iodide
1317:
1312:
1307:
1302:
1297:
1293:
1292:
1289:
1286:
1283:
1280:
1277:
1168:
1165:
1095:alkali halides
1045:
1042:
1029:
1026:
1010:primitive cell
965:. Examples of
908:
905:
891:
890:
879:
857:
834:
825:
820:
818:
815:
802:
798:
794:
793:Hexoctahedral
791:
785:
784:
777:
770:
763:
757:
756:
749:
740:
733:
728:
726:
724:
721:
715:
711:
707:
704:
698:
697:
693:
690:
688:
684:
680:
677:
671:
670:
667:
663:
660:
656:
650:
645:
643:enantiomorphic
640:
638:
635:
632:
627:
624:
618:
617:
611:
609:
601:
595:
594:
588:
578:
566:
561:
556:
554:
551:
539:
535:
531:
528:
522:
521:
517:
514:
512:
507:
503:
497:
496:
493:
490:
487:
478:
476:enantiomorphic
473:
471:
468:
465:
460:
457:
451:
450:
449:Body-centered
447:
446:Face-centered
444:
441:
436:
431:
426:
421:
417:
416:
411:
408:
405:
402:
362:
359:
269:
268:
261:
254:
247:
241:
240:
235:
230:
225:
223:Pearson symbol
219:
218:
213:
208:
203:
187:
184:
150:
149:
135:
121:
84:crystal system
80:crystal system
44:crystal facets
39:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
6086:
6075:
6072:
6070:
6067:
6066:
6064:
6048:
6044:
6041:
6038:
6034:
6031:
6028:
6024:
6021:
6018:
6014:
6011:
6010:
6008:
6004:
5997:
5992:
5989:
5986:
5981:
5977:
5974:
5971:
5967:
5964:
5961:
5957:
5954:
5951:
5947:
5944:
5941:
5936:
5933:
5932:
5930:
5926:
5920:
5917:
5915:
5912:
5911:
5908:
5904:
5897:
5892:
5890:
5885:
5883:
5878:
5877:
5874:
5868:
5864:
5861:
5857:
5854:
5852:
5849:
5847:
5844:
5842:
5839:
5838:
5836:
5832:
5829:
5828:
5824:
5820:
5819:0-471-80580-7
5816:
5812:
5808:
5807:
5803:
5792:
5787:
5783:
5776:
5773:
5767:
5761:
5757:
5753:
5752:
5747:
5741:
5738:
5733:
5729:
5724:
5719:
5715:
5711:
5707:
5703:
5699:
5695:
5691:
5684:
5681:
5676:
5672:
5668:
5664:
5660:
5653:
5650:
5647:
5643:
5639:
5634:
5631:
5628:
5623:
5620:
5615:
5613:1-4020-2153-4
5609:
5605:
5604:
5596:
5593:
5582:
5578:
5572:
5569:
5558:
5554:
5548:
5545:
5540:
5536:
5532:
5528:
5521:
5518:
5513:
5509:
5504:
5499:
5494:
5489:
5485:
5481:
5477:
5473:
5469:
5462:
5459:
5454:
5450:
5447:: 3760–3766.
5446:
5445:J. Chem. Soc.
5439:
5436:
5431:
5427:
5423:
5417:
5413:
5406:
5403:
5398:
5394:
5390:
5386:
5379:
5377:
5373:
5368:
5364:
5360:
5356:
5349:
5347:
5345:
5343:
5341:
5339:
5337:
5335:
5331:
5326:
5322:
5318:
5314:
5310:
5306:
5299:
5297:
5295:
5293:
5291:
5289:
5285:
5280:
5276:
5272:
5268:
5264:
5260:
5253:
5251:
5249:
5247:
5245:
5243:
5241:
5239:
5237:
5235:
5233:
5231:
5229:
5227:
5223:
5218:
5214:
5210:
5206:
5202:
5198:
5191:
5188:
5183:
5179:
5175:
5171:
5167:
5163:
5156:
5154:
5152:
5148:
5143:
5141:9780444815026
5137:
5133:
5129:
5125:
5118:
5116:
5114:
5112:
5110:
5108:
5106:
5104:
5102:
5100:
5098:
5096:
5094:
5092:
5090:
5086:
5081:
5077:
5073:
5069:
5062:
5060:
5058:
5056:
5054:
5052:
5050:
5048:
5046:
5044:
5042:
5038:
5033:
5029:
5025:
5021:
5017:
5013:
5006:
5004:
5002:
5000:
4998:
4996:
4994:
4992:
4990:
4988:
4986:
4984:
4982:
4980:
4978:
4976:
4974:
4972:
4970:
4968:
4964:
4958:
4953:
4949:
4945:
4940:
4935:
4931:
4927:
4923:
4916:
4913:
4908:
4904:
4900:
4896:
4889:
4886:
4881:
4877:
4873:
4869:
4862:
4859:
4854:
4850:
4846:
4842:
4835:
4832:
4827:
4823:
4819:
4815:
4811:
4807:
4800:
4798:
4796:
4794:
4792:
4788:
4783:
4779:
4775:
4771:
4764:
4762:
4760:
4758:
4756:
4754:
4752:
4750:
4748:
4746:
4744:
4740:
4735:
4731:
4727:
4723:
4716:
4714:
4712:
4710:
4708:
4704:
4699:
4695:
4691:
4687:
4682:
4677:
4673:
4669:
4662:
4660:
4658:
4656:
4654:
4652:
4650:
4648:
4646:
4644:
4642:
4640:
4638:
4636:
4632:
4627:
4623:
4619:
4615:
4608:
4605:
4600:
4596:
4592:
4588:
4581:
4579:
4577:
4575:
4573:
4571:
4569:
4567:
4565:
4563:
4561:
4557:
4552:
4546:
4542:
4541:
4533:
4530:
4525:
4521:
4517:
4513:
4509:
4505:
4498:
4495:
4490:
4486:
4482:
4478:
4471:
4468:
4463:
4461:9780444852168
4457:
4453:
4449:
4445:
4438:
4435:
4430:
4424:
4420:
4419:
4411:
4409:
4407:
4403:
4398:
4394:
4390:
4386:
4382:
4378:
4371:
4368:
4363:
4359:
4355:
4351:
4347:
4340:
4337:
4332:
4328:
4324:
4320:
4316:
4313:(in German).
4312:
4305:
4302:
4298:
4294:
4287:
4285:
4283:
4279:
4274:
4270:
4266:
4262:
4255:
4252:
4247:
4243:
4239:
4236:
4235:
4227:
4224:
4219:
4215:
4211:
4207:
4203:
4199:
4192:
4189:
4186:
4182:
4179:
4174:
4171:
4166:
4162:
4158:
4154:
4150:
4146:
4139:
4136:
4131:
4127:
4123:
4119:
4115:
4111:
4104:
4101:
4096:
4092:
4088:
4084:
4080:
4076:
4069:
4066:
4063:
4059:
4056:
4051:
4048:
4044:
4038:
4036:
4032:
4027:
4023:
4017:
4015:
4013:
4011:
4007:
4004:, 569 (1946).
4003:
3997:
3994:
3989:
3983:
3979:
3975:
3971:
3965:
3962:
3958:
3957:
3951:
3948:
3943:
3939:
3935:
3929:
3925:
3921:
3917:
3910:
3907:
3901:
3896:
3892:
3888:
3884:
3877:
3875:
3873:
3869:
3862:
3858:
3855:
3853:
3850:
3848:
3847:Close-packing
3845:
3842:
3838:
3835:
3834:
3830:
3828:
3826:
3822:
3818:
3814:
3810:
3806:
3802:
3797:
3791:
3782:
3775:
3769:
3766:
3764:
3761:
3757:
3754:
3751:
3749:
3746:
3742:
3739:
3736:
3734:
3731:
3727:
3724:
3721:
3719:
3716:
3712:
3702:
3696:
3694:
3689:
3687:
3683:
3679:
3667:
3662:
3657:
3649:
3647:
3641:
3633:
3629:
3619:
3611:
3603:YZ (e. g., Co
3598:
3593:
3589:
3587:
3578:
3575:
3573:
3570:
3568:
3565:
3562:
3559:
3555:
3552:
3549:
3547:
3544:
3542:
3539:
3536:
3533:
3529:
3526:
3523:
3521:
3518:
3516:
3513:
3510:
3507:
3503:
3500:
3497:
3495:
3492:
3490:
3487:
3484:
3483:Boron nitride
3481:
3477:
3461:
3457:
3456:pnictogenides
3454:
3449:
3447:
3443:
3433:
3431:
3428:
3426:
3423:
3421:
3418:
3415:
3414:
3411:
3408:
3406:
3403:
3401:
3398:
3396:
3393:
3390:
3389:
3386:
3385:Zinc polonide
3383:
3381:
3378:
3376:
3375:Zinc selenide
3373:
3371:
3368:
3365:
3364:
3361:
3358:
3356:
3353:
3351:
3348:
3346:
3343:
3340:
3339:
3323:
3319:
3318:chalcogenides
3316:
3312:
3303:
3300:
3298:
3295:
3293:
3290:
3288:
3285:
3282:
3281:
3265:
3261:
3258:
3253:
3251:
3247:
3243:
3239:
3238:pnictogenides
3236:
3232:
3228:
3224:
3219:
3217:
3213:
3212:diamond cubic
3209:
3205:
3202:
3198:
3194:
3189:
3187:
3179:
3170:
3165:
3157:
3155:
3153:
3141:
3135:
3129:
3121:
3119:
3117:
3113:
3109:
3105:
3101:
3097:
3093:
3089:
3085:
3081:
3077:
3073:
3069:
3065:
3056:
3053:
3050:
3047:
3046:
3042:
3040:
3037:
3034:
3033:
3030:
3027:
3025:
3022:
3019:
3018:
3015:
3012:
3010:
3007:
3004:
3003:
3000:
2997:
2995:
2992:
2989:
2988:
2985:
2982:
2980:
2977:
2974:
2973:
2970:
2967:
2965:
2962:
2959:
2958:
2948:
2944:
2940:
2937:
2928:
2926:
2923:
2921:
2918:
2916:
2913:
2910:
2909:
2906:
2904:
2901:
2899:
2896:
2894:
2891:
2888:
2887:
2884:
2882:
2879:
2877:
2874:
2872:
2869:
2866:
2865:
2862:
2860:
2857:
2855:
2852:
2850:
2847:
2844:
2843:
2840:
2838:
2835:
2833:
2830:
2828:
2825:
2822:
2821:
2817:
2814:
2811:
2808:
2806:Protactinium
2805:
2804:
2801:
2798:
2796:
2793:
2791:
2788:
2785:
2784:
2780:
2777:
2774:
2771:
2768:
2767:
2764:
2761:
2759:
2757:
2755:
2752:
2746:
2745:
2742:
2739:
2737:
2735:
2733:
2730:
2728:
2725:
2722:
2721:
2718:
2715:
2713:
2711:
2709:
2706:
2703:
2702:
2699:
2697:
2695:
2693:
2690:
2687:
2686:
2683:
2680:
2678:
2676:
2674:
2671:
2668:
2667:
2664:
2661:
2659:
2657:
2655:
2652:
2649:
2648:
2645:
2642:
2640:
2638:
2636:
2633:
2630:
2629:
2626:
2624:
2622:
2620:
2617:
2611:
2610:
2607:
2604:
2602:
2599:
2597:
2594:
2592:
2589:
2587:
2584:
2581:
2580:
2577:
2574:
2572:
2569:
2567:
2564:
2562:
2559:
2556:
2555:
2551:
2548:
2545:
2542:
2539:
2538:
2535:
2533:
2530:
2528:
2525:
2523:
2520:
2517:
2516:
2513:
2511:
2508:
2506:
2503:
2501:
2498:
2496:Praseodymium
2495:
2494:
2491:
2489:
2486:
2484:
2481:
2479:
2476:
2473:
2472:
2469:
2467:
2465:
2463:
2460:
2457:
2456:
2453:
2451:
2449:
2447:
2444:
2441:
2440:
2437:
2435:
2433:
2431:
2428:
2422:
2421:
2402:
2398:
2397:chalcogenides
2395:
2391:
2382:
2379:
2376:
2374:
2371:
2368:
2365:
2362:
2361:
2358:
2355:
2352:
2350:
2347:
2345:
2342:
2340:
2337:
2334:
2333:
2330:
2327:
2325:
2322:
2320:
2317:
2315:
2312:
2310:
2307:
2304:
2303:
2300:
2297:
2295:
2292:
2290:
2287:
2285:
2282:
2280:
2277:
2274:
2273:
2270:
2267:
2265:
2262:
2260:
2257:
2255:
2252:
2250:
2247:
2244:
2243:
2240:
2237:
2235:
2232:
2230:
2227:
2225:
2222:
2220:
2217:
2214:
2213:
2210:
2207:
2205:
2202:
2200:
2197:
2195:
2192:
2190:
2187:
2184:
2183:
2179:
2176:
2173:
2170:
2167:
2165:Protactinium
2164:
2163:
2159:
2157:
2154:
2152:
2149:
2147:
2144:
2142:
2139:
2136:
2135:
2131:
2128:
2125:
2122:
2119:
2116:
2115:
2112:
2109:
2107:
2104:
2102:
2099:
2097:
2094:
2092:
2089:
2086:
2085:
2081:
2079:
2076:
2074:
2071:
2069:
2066:
2064:
2061:
2058:
2057:
2054:
2051:
2049:
2046:
2044:
2041:
2039:
2036:
2034:
2031:
2028:
2027:
2024:
2021:
2019:
2016:
2014:
2011:
2009:
2006:
2004:
2001:
1998:
1997:
1994:
1991:
1989:
1986:
1984:
1981:
1979:
1976:
1974:
1971:
1968:
1967:
1964:
1961:
1959:
1956:
1954:
1951:
1949:
1946:
1944:
1941:
1938:
1937:
1934:
1931:
1929:
1926:
1924:
1921:
1919:
1916:
1914:
1911:
1908:
1907:
1904:
1901:
1899:
1896:
1894:
1891:
1889:
1886:
1884:
1881:
1878:
1877:
1862:
1860:
1857:
1855:
1852:
1849:
1848:
1845:
1842:
1840:
1837:
1835:
1832:
1830:
1827:
1825:
1822:
1819:
1818:
1814:
1811:
1808:
1805:
1802:
1799:
1798:
1795:
1792:
1790:
1787:
1785:
1782:
1780:
1777:
1775:
1772:
1769:
1768:
1765:
1762:
1760:
1757:
1755:
1752:
1750:
1747:
1745:
1742:
1740:Praseodymium
1739:
1738:
1735:
1732:
1730:
1727:
1725:
1722:
1720:
1717:
1715:
1712:
1709:
1708:
1705:
1702:
1700:
1697:
1695:
1692:
1690:
1687:
1685:
1682:
1679:
1678:
1675:
1672:
1670:
1667:
1665:
1662:
1660:
1657:
1655:
1652:
1649:
1648:
1645:
1642:
1640:
1637:
1635:
1632:
1630:
1627:
1625:
1622:
1619:
1618:
1599:
1595:
1592:
1588:
1579:
1576:
1574:
1571:
1569:
1566:
1564:
1561:
1559:
1556:
1553:
1552:
1549:
1546:
1544:
1541:
1539:
1536:
1534:
1531:
1529:
1526:
1523:
1522:
1519:
1516:
1514:
1511:
1509:
1506:
1504:
1501:
1499:
1498:Calcium oxide
1496:
1493:
1492:
1488:
1486:
1483:
1481:
1478:
1476:
1473:
1471:
1468:
1465:
1464:
1445:
1441:
1440:chalcogenides
1438:
1426:
1423:
1421:
1418:
1414:
1411:
1408:
1406:
1403:
1401:
1398:
1396:
1393:
1391:
1388:
1384:
1381:
1378:
1376:
1373:
1371:
1368:
1366:
1363:
1361:
1358:
1354:
1351:
1350:Sodium iodide
1348:
1346:
1343:
1341:
1338:
1336:
1333:
1331:
1328:
1324:
1321:
1318:
1316:
1313:
1311:
1308:
1306:
1303:
1301:
1298:
1294:
1275:
1271:
1267:
1264:
1259:
1257:
1253:
1249:
1246:
1242:
1237:
1235:
1229:
1227:
1223:
1219:
1216:
1210:
1208:
1204:
1196:
1192:
1184:
1179:
1174:
1166:
1164:
1162:
1158:
1154:
1150:
1146:
1142:
1138:
1134:
1130:
1127:
1122:
1120:
1116:
1108:
1104:
1099:
1096:
1091:
1089:
1084:
1079:
1072:
1068:
1061:
1056:
1051:
1043:
1041:
1039:
1035:
1027:
1025:
1023:
1019:
1015:
1014:A15 structure
1011:
1007:
1003:
999:
995:
991:
990:diamond cubic
986:
984:
980:
976:
972:
968:
964:
960:
956:
952:
948:
944:
940:
936:
932:
928:
922:
913:
906:
904:
902:
898:
880:
865:
858:
851:
833:
829:
826:
824:
821:
819:
816:
803:
801:
795:
792:
787:
778:
771:
764:
759:
750:
748:
741:
734:
732:
714:
700:
691:
689:
678:
673:
668:
661:
654:
651:
649:
644:
631:
620:
612:
610:
608:
602:
597:
589:
579:
573:
567:
565:
560:
538:
524:
515:
513:
511:
504:
499:
494:
491:
488:
486:
482:
477:
464:
453:
448:
445:
442:
440:
437:
435:
432:
430:
427:
425:
422:
419:
415:
399:
396:
394:
390:
386:
382:
378:
374:
368:
360:
358:
356:
352:
350:
346:
344:
341:
336:
334:
331:
326:
302:
289:
276:
266:
262:
259:
255:
252:
248:
246:
243:
242:
239:
236:
234:
231:
229:
226:
224:
221:
215:Face-centered
214:
210:Body-centered
209:
204:
201:
200:
197:
193:
185:
183:
181:
177:
173:
169:
165:
161:
160:
155:
147:
143:
140:(abbreviated
139:
136:
133:
129:
126:(abbreviated
125:
122:
119:
115:
112:(abbreviated
111:
108:
107:
106:
103:
101:
97:
93:
89:
85:
81:
77:
73:
69:
60:
52:
45:
37:
32:
19:
5993:(isometric)
5990:
5956:orthorhombic
5841:Simple cubic
5810:
5775:
5750:
5740:
5697:
5693:
5683:
5658:
5652:
5633:
5622:
5602:
5595:
5584:. Retrieved
5580:
5571:
5560:. Retrieved
5556:
5547:
5530:
5526:
5520:
5475:
5471:
5461:
5444:
5438:
5411:
5405:
5388:
5384:
5358:
5354:
5308:
5304:
5262:
5258:
5200:
5196:
5190:
5165:
5161:
5123:
5071:
5067:
5015:
5011:
4929:
4925:
4915:
4898:
4894:
4888:
4871:
4867:
4861:
4844:
4840:
4834:
4809:
4805:
4773:
4769:
4725:
4721:
4671:
4667:
4617:
4613:
4607:
4593:(1): 51–59.
4590:
4586:
4539:
4532:
4507:
4503:
4497:
4480:
4476:
4470:
4443:
4437:
4417:
4380:
4376:
4370:
4353:
4349:
4345:
4339:
4314:
4310:
4304:
4264:
4260:
4254:
4237:
4232:
4226:
4201:
4197:
4191:
4173:
4148:
4144:
4138:
4113:
4109:
4103:
4078:
4074:
4068:
4050:
4045:(1940), p.49
4042:
4001:
3996:
3973:
3964:
3959:, Webmineral
3955:
3950:
3915:
3909:
3890:
3886:
3852:Dislocations
3840:
3821:Gas hydrates
3819:structure".
3805:pyritohedral
3798:
3787:
3690:
3686:helimagnetic
3671:
3621:
3585:
3583:
3474:Antimonides
3439:
3370:Zinc sulfide
3220:
3204:coordination
3197:zinc sulfide
3190:
3175:
3137:
3061:
2363:Californium
1615:Bismuthides
1612:Antimonides
1558:Barium oxide
1263:Alkali metal
1245:alkali metal
1240:
1238:
1230:
1218:coordination
1211:
1188:
1123:
1100:
1092:
1080:
1077:
1065:
1031:
987:
966:
946:
942:
938:
924:
894:
459:Tetartoidal
414:Space groups
404:Point group
393:space groups
372:
370:
353:
347:
337:
327:
303:
290:
272:
237:
232:
227:
195:
175:
171:
167:
163:
158:
153:
151:
145:
141:
137:
131:
127:
123:
118:simple cubic
117:
113:
109:
104:
79:
75:
71:
65:
6023:rectangular
5937:(anorthic)
5794:. TID-5221.
5700:(1): 3951.
5577:"Rock Salt"
3709:Germanides
3468:Phosphides
3333:Tellurides
3235:boron group
3201:tetrahedral
3178:space group
3051:(unstable)
2750:(unstable)
2650:Dysprosium
2615:(unstable)
2612:Gadolinium
2540:Promethium
2426:(unstable)
2415:Tellurides
2082:(unstable)
1939:Dysprosium
1879:Gadolinium
1874:(unstable)
1871:structure)
1800:Promethium
1606:Phosphides
1458:Tellurides
1191:space group
1103:space group
931:bond angles
6063:Categories
5966:tetragonal
5946:monoclinic
5754:. Oxford:
5586:2020-05-22
5562:2020-05-22
5553:"Fluorite"
4939:1802.04554
4901:(4): 453.
3893:(3): 278.
3863:References
3714:Manganese
3706:Silicides
3668:structure.
3654:See also:
3630:involving
3505:Aluminium
3471:Arsenides
3341:Beryllium
3336:Polonides
3330:Selenides
3272:Chlorides
3269:Fluorides
3250:polymorphs
3193:sphalerite
3162:See also:
3132:See also:
2975:Zirconium
2889:Americium
2867:Plutonium
2845:Neptunium
2723:Ytterbium
2518:Neodymium
2458:Lanthanum
2418:Polonides
2412:Selenides
2390:Rare-earth
2335:Berkelium
2275:Americium
2245:Plutonium
2215:Neptunium
2059:Ytterbium
1770:Neodymium
1680:Lanthanum
1609:Arsenides
1587:Rare-earth
1524:Strontium
1466:Magnesium
1461:Polonides
1455:Selenides
1356:Potassium
1285:Chlorides
1282:Fluorides
1215:octahedral
1171:See also:
1126:rare earth
1088:cubic void
1048:See also:
919:See also:
897:holohedral
731:Sphalerite
530:Diploidal
481:Ullmannite
443:Primitive
340:tetragonal
176:unit cells
86:where the
6043:hexagonal
5935:triclinic
5581:aflow.org
5557:aflow.org
5472:Materials
5430:840310000
4932:(1): 71.
4698:118566136
4681:1208.2410
4356:: 54–61.
4346:Ab initio
4331:100227546
4267:: 39–50.
3942:146060934
3817:clathrate
3813:chemistry
3759:Chromium
3465:Nitrides
3327:Sulfides
3311:Beryllium
3275:Bromides
3048:Chromium
3035:Tantalum
3005:Vanadium
2960:Titanium
2955:Nitrides
2952:Carbides
2769:Actinium
2747:Lutetium
2582:Europium
2557:Samarium
2423:Scandium
2409:Sulfides
2117:Actinium
2087:Lutetium
1850:Europium
1820:Samarium
1620:Scandium
1603:Nitrides
1594:pnictides
1452:Sulfides
1386:Rubidium
1288:Bromides
1279:Hydrides
1209:is "B1".
1137:magnesium
1121:is "B2".
1022:manganese
1016:found in
1002:germanium
971:aluminium
626:Gyroidal
245:Unit cell
205:Primitive
88:unit cell
76:isometric
5782:Polonium
5732:24492240
5642:Archived
5512:28824101
4181:Archived
4095:95011597
4058:Archived
4026:Archived
3831:See also
3531:Gallium
3453:Group 13
3446:wurtzite
3416:Mercury
3391:Cadmium
3315:Group 12
3278:Iodides
3242:nitrides
3020:Niobium
2990:Hafnium
2943:nitrides
2939:carbides
2823:Uranium
2786:Thorium
2704:Thulium
2669:Holmium
2631:Terbium
2442:Yttrium
2394:actinoid
2185:Uranium
2137:Thorium
2029:Thulium
1969:Holmium
1909:Terbium
1650:Yttrium
1591:actinoid
1494:Calcium
1416:Caesium
1296:Lithium
1291:Iodides
1266:hydrides
1248:hydrides
1018:tungsten
969:include
959:tungsten
955:chromium
949:include
935:polonium
662:F432, F4
410:Example
385:orbifold
100:minerals
96:crystals
6013:oblique
5867:Molview
5723:3912472
5702:Bibcode
5663:Bibcode
5503:5448873
5480:Bibcode
5313:Bibcode
5267:Bibcode
5205:Bibcode
5170:Bibcode
5020:Bibcode
4944:Bibcode
4814:Bibcode
4512:Bibcode
4385:Bibcode
4206:Bibcode
4153:Bibcode
4118:Bibcode
4041:Seitz,
3837:Atomium
3744:Cobalt
3557:Indium
3283:Copper
3260:halides
3184:3m (in
2911:Curium
2688:Erbium
2474:Cerium
2406:Oxides
2305:Curium
1999:Erbium
1710:Cerium
1554:Barium
1449:Oxides
1326:Sodium
1270:halides
1252:halides
1105:of the
998:silicon
963:niobium
789:221–230
761:218–220
702:215–217
675:212–214
648:Petzite
622:207–211
599:205–206
526:200–204
501:198–199
455:195–197
320:⁄
310:⁄
296:⁄
283:⁄
275:lattice
6033:square
5817:
5762:
5730:
5720:
5610:
5510:
5500:
5428:
5418:
5138:
4696:
4547:
4458:
4425:
4329:
4093:
3984:
3940:
3930:
3792:has Pm
3682:chiral
3607:MnSi).
3479:Boron
3257:Copper
1201:m (in
1195:halite
1147:, and
1113:m (in
1004:, and
994:carbon
983:silver
975:copper
961:, and
937:. The
903:type.
901:galena
832:Halite
828:Galena
683:32, P4
564:Pyrite
424:Schön.
387:, and
217:cubic
212:cubic
207:cubic
70:, the
36:pyrite
6074:Cubes
5991:cubic
5865:with
4934:arXiv
4694:S2CID
4676:arXiv
4327:S2CID
4091:S2CID
3938:S2CID
3803:with
3729:Iron
3642:is L2
3586:III-V
3442:II-VI
3366:Zinc
3062:Many
885:m, Ia
874:m, Fd
870:c, Fd
840:m, Pn
817:*432
723:*332
669:I432
420:Name
407:Type
82:is a
72:cubic
5831:JMol
5815:ISBN
5760:ISBN
5728:PMID
5608:ISBN
5508:PMID
5426:OCLC
5416:ISBN
5136:ISBN
4545:ISBN
4456:ISBN
4423:ISBN
4315:127U
3982:ISBN
3928:ISBN
3313:and
3229:and
3176:The
2941:and
2392:and
1589:and
1268:and
1250:and
1189:The
1161:RbCl
1153:CsBr
1101:The
981:and
979:gold
951:iron
941:and
866:, Fm
852:, Pn
655:, P4
653:P432
637:432
634:432
584:, Fd
574:, Pn
553:3*2
495:I23
492:F23
489:P23
470:332
439:Cox.
434:Orb.
429:Intl
401:No.
371:The
98:and
92:cube
74:(or
38:(FeS
5856:HCP
5851:FCC
5846:BCC
5786:doi
5718:PMC
5710:doi
5671:doi
5535:doi
5498:PMC
5488:doi
5449:doi
5393:doi
5363:doi
5321:doi
5275:doi
5213:doi
5178:doi
5128:doi
5076:doi
5072:223
5028:doi
4952:doi
4903:doi
4876:doi
4849:doi
4822:doi
4810:114
4778:doi
4730:doi
4686:doi
4622:doi
4595:doi
4520:doi
4508:125
4485:doi
4448:doi
4393:doi
4381:403
4358:doi
4354:175
4319:doi
4293:doi
4269:doi
4242:doi
4214:doi
4161:doi
4126:doi
4083:doi
3920:doi
3895:doi
3841:bcc
3811:in
3142:(AB
3118:).
3116:PuC
3112:NpC
3104:PaC
3100:ThC
3096:CdO
3092:NiO
3088:CoO
3084:FeO
3080:MnO
3076:CrO
3068:TiO
1863:(Na
1157:CsI
1006:tin
967:fcc
947:bcc
943:fcc
939:bcc
844:n,
814:m)
808:2/m
804:4/m
783:3d
776:3c
769:3n
755:3m
739:3m
720:3m
696:32
687:32
666:32
659:32
541:2/m
467:23
172:fcc
168:fcc
164:ccp
162:or
154:fcc
146:fcc
144:or
132:bcc
130:or
66:In
6065::
5837::
5726:.
5716:.
5708:.
5696:.
5692:.
5669:.
5579:.
5555:.
5531:11
5529:.
5506:.
5496:.
5486:.
5474:.
5470:.
5424:.
5387:.
5375:^
5359:28
5357:.
5333:^
5319:.
5309:39
5307:.
5287:^
5273:.
5263:24
5261:.
5225:^
5211:.
5201:40
5199:.
5176:.
5166:36
5164:.
5150:^
5134:.
5088:^
5070:.
5040:^
5026:.
5016:28
5014:.
4966:^
4950:.
4942:.
4928:.
4924:.
4899:20
4897:.
4872:12
4870:.
4843:.
4820:.
4808:.
4790:^
4774:41
4772:.
4742:^
4726:38
4724:.
4706:^
4692:.
4684:.
4672:58
4670:.
4634:^
4618:18
4616:.
4591:22
4589:.
4559:^
4518:.
4506:.
4479:.
4454:.
4405:^
4391:.
4379:.
4352:.
4325:.
4281:^
4265:22
4263:.
4238:18
4212:.
4202:14
4200:.
4159:.
4149:61
4147:.
4124:.
4114:26
4112:.
4089:.
4079:14
4077:.
4034:^
4009:^
4002:14
3980:.
3936:.
3926:.
3918:.
3891:41
3889:.
3885:.
3871:^
3788:A
3695:.
3563:*
3537:*
3511:*
3485:*
3434:–
3252:.
3114:,
3110:,
3108:UC
3106:,
3102:,
3094:,
3090:,
3086:,
3082:,
3078:,
3074:,
3072:VO
3070:,
2818:?
2815:?
2812:?
2809:?
2781:?
2778:?
2775:?
2772:?
2552:?
2549:?
2546:?
2543:?
2377:?
2369:?
2366:?
2353:?
2180:?
2177:?
2174:?
2171:?
2168:?
2132:?
2129:?
2126:?
2123:?
2120:?
1815:?
1812:?
1809:?
1806:?
1803:?
1155:,
1149:13
1145:12
1143:,
1141:11
1090:.
1058:A
1024:.
1000:,
996:,
985:.
977:,
973:,
957:,
953:,
889:d
881:Im
878:c
860:Fm
856:m
846:Pm
836:Pm
830:,
810:(m
747:3m
692:I4
679:P4
613:Ia
604:Pa
580:Fm
569:Pm
550:)
546:(m
520:3
516:I2
506:P2
483:,
383:,
379:,
345:.
238:cF
233:cI
228:cP
142:cF
128:cI
114:cP
102:.
78:)
5895:e
5888:t
5881:v
5796:.
5788::
5770:.
5768:.
5734:.
5712::
5704::
5698:4
5677:.
5673::
5665::
5616:.
5589:.
5565:.
5541:.
5537::
5514:.
5490::
5482::
5476:4
5455:.
5451::
5432:.
5399:.
5395::
5389:3
5369:.
5365::
5327:.
5323::
5315::
5281:.
5277::
5269::
5219:.
5215::
5207::
5184:.
5180::
5172::
5144:.
5130::
5082:.
5078::
5034:.
5030::
5022::
4960:.
4954::
4946::
4936::
4930:1
4909:.
4905::
4882:.
4878::
4855:.
4851::
4845:7
4828:.
4824::
4816::
4784:.
4780::
4736:.
4732::
4700:.
4688::
4678::
4628:.
4624::
4601:.
4597::
4553:.
4526:.
4522::
4514::
4491:.
4487::
4481:7
4464:.
4450::
4431:.
4399:.
4395::
4387::
4364:.
4360::
4333:.
4321::
4299:.
4295::
4275:.
4271::
4248:.
4244::
4220:.
4216::
4208::
4167:.
4163::
4155::
4132:.
4128::
4120::
4097:.
4085::
3990:.
3944:.
3922::
3903:.
3897::
3794:3
3674:1
3644:1
3636:3
3624:2
3605:2
3601:2
3182:4
3148:3
3144:2
1869:2
1867:O
1865:2
1241:a
1199:3
1185:.
1111:3
887:3
883:3
876:3
872:3
868:3
864:m
862:3
854:3
850:n
848:3
842:3
838:3
812:3
806:3
799:h
797:O
781:4
779:I
774:4
772:F
767:4
765:P
753:4
751:I
745:4
743:F
737:4
735:P
718:4
712:d
710:T
694:1
685:1
681:3
664:1
657:2
630:O
615:3
606:3
592:3
590:I
586:3
582:3
576:3
571:3
548:3
543:3
536:h
534:T
518:1
510:3
508:1
463:T
322:2
318:1
312:8
308:1
305:(
298:8
294:1
285:8
281:1
278:(
148:)
134:)
120:)
46:.
40:2
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.