151:
492:
electrochemical voltage window of the graphite-aqueous acid interface, and thus the elimination of the mixing dilution, detrimental in Cr–Fe RFBs. More importantly for commercial success is the near-perfect match of the voltage window of carbon/aqueous acid interface with that of vanadium redox-couples. This extends the life of the low-cost carbon electrodes and reduces the impact of side reactions, such as H2 and O2 evolutions, resulting in many year durability and many cycle (15,000–20,000) lives, which in turn results in a record low
599:
space. One unresolved issue is zinc buildup on the negative electrode that can permeate the membrane, reducing efficiency. Because of the Zn dendrite formation, Zn-halide batteries cannot operate at high current density (> 20 mA/cm) and thus have limited power density. Adding alcohol to the electrolyte of the ZnI battery can help. The drawbacks of Zn/I RFB lie are the high cost of Iodide salts (> $ 20 / Kg); limited area capacity of Zn deposition, reducing the decoupled energy and power; and Zn dendrite formation.
1068:) with the decoupled energy-power advantage of flow batteries. SEB(ROTS) RFBs have advantages compared to semi-solid RFBs, such as no need to pump viscous slurries, no precipitation/clogging, higher area-specific power, longer durability, and wider chemical design space. However, because of double energy losses (one in the stack and another in the tank between the SEB(ROTS) and a mediator), such batteries suffer from poor energy efficiency. On a system-level, the practical specific energy of traditional
1053:, positive and negative electrode particles are suspended in a carrier liquid. The suspensions are flow through a stack of reaction chambers, separated by a barrier such as a thin, porous membrane. The approach combines the basic structure of aqueous-flow batteries, which use electrode material suspended in a liquid electrolyte, with the chemistry of lithium-ion batteries in both carbon-free suspensions and slurries with a conductive carbon network. The carbon-free semi-solid RFB is also referred to as
496:(LCOE, system cost divided by usable energy, cycle life, and round-trip efficiency). These long lifetimes allow for the amortization of their relatively high capital cost (driven by vanadium, carbon felts, bipolar plates, and membranes). The LCOE is on the order of a few tens cents per kWh, much lower than of solid-state batteries and near the targets of 5 cents stated by US and EC government agencies. Major challenges include: low abundance and high costs of V
1034:
688:. During charging, PFB combines hydrogen ions produced from splitting water with electrons and metal particles in one electrode of a fuel cell. The energy is stored in the form of a metal hydride solid. Discharge produces electricity and water when the process is reversed and the protons are combined with ambient oxygen. Metals less expensive than lithium can be used and provide greater energy density than lithium cells.
33:
1649:
1616:– Because flow batteries can be rapidly "recharged" by replacing the electrolyte, they can be used for applications where the vehicle needs to take on energy as fast as a gas vehicle. A common problem with most RFB chemistries in EV applications is their low energy density which translated into a short driving range. Zinc-chlorine batteries and batteries with highly soluble halates are a notable exception.
4879:
1635:
898:(de)hydrogenation demonstration cell operated continuously for 120 days over 1,111 charging cycles at room temperature without a catalyst, retaining 97% percent capacity. The cell offered more than double the energy density of vanadium-based systems. The major challenge was the lack of a stable catholyte, holding energy densities below 5 Wh/L. Alkaline AORFBs use excess
971:) dissolved in water were the active electrode material. The size-selective nanoporous membrane worked like a strainer and is produced much more easily and at lower cost than conventional ion-selective membranes. It block the big "spaghetti"-like polymer molecules, while allowing small counterions to pass. The concept may solve the high cost of traditional
705:
electrode materials, while the latter use inorganic materials for either anode or cathode. In larger-scale energy storage, lower solvent cost and higher conductivity give AORFBs greater commercial potential, as well as offering the safety advantages of water-based electrolytes. NAORFBs instead provide a much larger voltage window and occupy less space.
4588:
105:(50–80%). This drawback stems from the need to operate flow batteries at high (>= 100 mA/cm2) current densities to reduce the effect of internal crossover (through the membrane/separator) and to reduce the cost of power (size of stacks). Also, most flow batteries (Zn-Cl2, Zn-Br2 and H2-LiBrO3 are exceptions) have lower
1021:
chemical reaction can be reversed to recharge the battery – a first for a membraneless design. One such membraneless flow battery announced in August 2013 produced a maximum power density of 795 kW/cm, three times more than other membraneless systems—and an order of magnitude higher than lithium-ion batteries.
1020:
that can destroy the membrane. Both materials are available at low cost. The design uses a small channel between two electrodes. Liquid bromine flows through the channel over a graphite cathode and hydrobromic acid flows under a porous anode. At the same time, hydrogen gas flows across the anode. The
819:
exceeding 6,000 W/m at 13,000 A/m. Cycling showed > 99% storage capacity retention per cycle. Volumetric energy density was over 20 Wh/L. Anthraquinone-2-sulfonic acid and anthraquinone-2,6-disulfonic acid on the negative side and 1,2-dihydrobenzoquinone- 3,5-disulfonic acid on
598:
store 325 Wh/L. The zinc–polyiodide battery is claimed to be safer than other flow batteries given its absence of acidic electrolytes, nonflammability and operating range of −4 to 122 °F (−20 to 50 °C) that does not require extensive cooling circuitry, which would add weight and occupy
1015:
Membranes are often the most costly and least reliable battery components, as they are subject to corrosion by repeated exposure to certain reactants. The absence of a membrane enables the use of a liquid bromine solution and hydrogen: this combination is problematic when membranes are used, because
96:
Flow batteries have certain technical advantages over conventional rechargeable batteries with solid electroactive materials, such as independent scaling of power (determined by the size of the stack) and of energy (determined by the size of the tanks), long cycle and calendar life, and potentially
577:
aqueous solution. The two electrolytes of different pH are separated by a bipolar membrane. The system demonstrated good reversibility and high efficiencies in coulomb (95%), energy (84%), and voltage (88%). They reported improvements with increased current density, inclusion of larger 100 cm
737:
V, and, possibly, lowest capital cost ($ 180/kWh) reported for AORFBs as of 2015. The aqueous liquid electrolytes were designed as a drop-in replacement without replacing infrastructure. A 600-milliwatt test battery was stable for 100 cycles with nearly 100 percent efficiency at current densities
704:
Organic redox flow batteries can be further classified into aqueous (AORFBs) and non-aqueous (NAORFBs). AORFBs use water as solvent for electrolyte materials while NAORFBs employ organic solvents. AORFBs and NAORFBs can be further divided into total and hybrid systems. The former use only organic
423:
than to conventional batteries. The main reason fuel cells are not considered to be batteries, is because originally (in the 1800s) fuel cells emerged as a means to produce electricity directly from fuels (and air) via a non-combustion electrochemical process. Later, particularly in the 1960s and
520:
The hybrid flow battery (HFB) uses one or more electroactive components deposited as a solid layer. The major disadvantage is that this reduces decoupled energy and power. The cell contains one battery electrode and one fuel cell electrode. This type is limited in energy by the electrode surface
1063:
Flow batteries with redox-targeted solids (ROTS), also known as solid energy boosters (SEBs) either the posolyte or negolyte or both (a.k.a. redox fluids), come in contact with one or more solid electroactive materials (SEM). The fluids comprise one or more redox couples, with redox potentials
491:
Vanadium redox flow batteries are the commercial leaders. They use vanadium at both electrodes, so they do not suffer cross-contamination. The limited solubility of vanadium salts, however, offsets this advantage in practice. This chemistry's advantages include four oxidation states within the
459:
Cr–Fe chemistry has disadvantages, including hydrate isomerism (i.e. the equilibrium between electrochemically active Cr3+ chloro-complexes and inactive hexa-aqua complex and hydrogen evolution on the negode. Hydrate isomerism can be alleviated by adding chelating amino-ligands, while hydrogen
2498:
1551:
Technical merits make redox flow batteries well-suited for large-scale energy storage. Flow batteries are normally considered for relatively large (1 kWh – 10 MWh) stationary applications with multi-hour charge-discharge cycles. Flow batteries are not cost-efficient for shorter
1024:
In 2018, a macroscale membraneless RFB capable of recharging and recirculation of the electrolyte streams was demonstrated. The battery was based on immiscible organic catholyte and aqueous anolyte liquids, which exhibited high capacity retention and
Coulombic efficiency during cycling.
874:
or combi-molecules allow the same material to be used in both tanks. In one tank it is an electron donor, while in the other it is an electron recipient. This has advantages such as diminishing crossover effects. Thus, quinone diaminoanthraquinone and indigo-based molecules as well as
2129:
4680:
Tolmachev, Yuriy V.; Piatkivskyi, Andrii; Ryzhov, Victor V.; Konev, Dmitry V.; Vorotyntsev, Mikhail A. (2015). "Energy cycle based on a high specific energy aqueous flow battery and its potential use for fully electric vehicles and for direct solar-to-chemical energy conversion".
717:
as a supporting electrolyte. At pH neutral conditions, organic and organometallic molecules are more stable than at corrosive acidic and alkaline conditions. For example, K4, a common catholyte used in AORFBs, is not stable in alkaline solutions but is at pH neutral conditions.
2008:
E. R. Sum, M.; Skyllas-Kazacos, M., J Power
Sources, 16 (2), 85-95 (1985); E. S.-K. Sum, M., J Power Sources, 15 (2-3), 179-190 (1985); M. Rychcik and M. Skyllas-Kazacos, J Power Sources, 19 (1), 45-54 (1987); M. Rychcik and M. Skyllas-Kazacos, J Power Sources, 22 (1), 59-67
742:
mA, at which about 70% of the battery's original voltage was retained. Neutral AORFBs can be more environmentally friendly than acid or alkaline alternatives, while showing electrochemical performance comparable to corrosive RFBs. The MV/TEMPO AORFB has an energy density of
2517:
223:
and groups in Japan and elsewhere selected Cr–Fe chemistry for further development. Mixed solutions (i.e. comprising both chromium and iron species in the negolyte and in the posolyte) were used in order to reduce the effect of time-varying concentration during cycling.
2475:
700:
batteries. Organic redox flow batteries advantage is the tunable redox properties of its active components. As of 2021, organic RFB experienced low durability (i.e. calendar or cycle life, or both) and have not been demonstrated on a commercial scale.
636:). The battery produces power by pumping liquid across the stack where the liquids mix. Inside the stack, zinc ions pass through a selective membrane and change into metallic zinc on the stack's negative side. To increase energy density, bromide ions (
4586:, Spaziante, Placido Maria; Kampanatsanyakorn, Krisada & Zocchi, Andrea, "System for storing and/or transforming energy from sources at variable voltage and frequency", published 2003-05-22, assigned to Squirrel Holdings Ltd.
218:
Walther Kangro, an
Estonian chemist working in Germany in the 1950s, was the first to demonstrate flow batteries based on dissolved transition metal ions: Ti–Fe and Cr–Fe. After initial experimentations with Ti–Fe redox flow battery (RFB) chemistry,
3755:
Janoschka, Tobias; Martin, Norbert; Martin, Udo; Friebe, Christian; Morgenstern, Sabine; Hiller, Hannes; Hager, Martin D.; Schubert, Ulrich S. (2015). "An aqueous, polymer-based redox-flow battery using non-corrosive, safe, and low-cost materials".
3703:
Janoschka, Tobias; Martin, Norbert; Martin, Udo; Friebe, Christian; Morgenstern, Sabine; Hiller, Hannes; Hager, Martin D.; Schubert, Ulrich S. (2015). "An aqueous, polymer-based redox-flow battery using non-corrosive, safe, and low-cost materials".
1011:
in which two liquids are pumped through a channel, where they undergo electrochemical reactions to store or release energy. The solutions pass in parallel, with little mixing. The flow naturally separates the liquids, without requiring a membrane.
1045:
eliminates the requirement that charge moves in and out of particles that are in direct contact with a conducting plate. Instead, the nanoparticle network allows electricity to flow throughout the liquid. This allows more energy to be extracted.
1622:– An example of this is in cellphone base stations where no grid power is available. The battery can be used alongside solar or wind power sources to compensate for their fluctuating power levels and alongside a generator to save fuel.
288:
Electrolyte is stored externally, generally in tanks, and is typically pumped through the cell (or cells) of the reactor. Flow batteries can be rapidly "recharged" by replacing discharged electrolyte liquid (analogous to refueling
1569:
to store power during off-peak hours and release it during peak demand periods. The common problem limiting this use of most flow battery chemistries is their low areal power (operating current density) which translates into high
2303:
Cho, Kyu Taek; Tucker, Michael C.; Ding, Markus; Ridgway, Paul; Battaglia, Vincent S.; Srinivasan, Venkat; Weber, Adam Z. (2015). "Cyclic
Performance Analysis of Hydrogen/Bromine Flow Batteries for Grid-Scale Energy Storage".
770:
and their derivatives are the basis of many organic redox systems. In one study, 1,2-dihydrobenzoquinone-3,5-disulfonic acid (BQDS) and 1,4-dihydrobenzoquinone-2-sulfonic acid (BQS) were employed as cathodes, and conventional
3395:
Winsberg, Jan; Stolze, Christian; Muench, Simon; Liedl, Ferenc; Hager, Martin D.; Schubert, Ulrich S. (11 November 2016). "TEMPO/Phenazine Combi-Molecule: A Redox-Active
Material for Symmetric Aqueous Redox-Flow Batteries".
3063:
Liu, Tianbiao; Wei, Xiaoliang; Nie, Zimin; Sprenkle, Vincent; Wang, Wei (1 November 2015). "A Total
Organic Aqueous Redox Flow Battery Employing a Low Cost and Sustainable Methyl Viologen Anolyte and 4-HO-TEMPO Catholyte".
578:
electrodes, and series operation. Preliminary data using a fluctuating simulated power input tested the viability toward kWh scale storage. In 2016, a high energy density Mn(VI)/Mn(VII)-Zn hybrid flow battery was proposed.
4186:
Qi, Zhaoxiang; Liu, Aaron L.; Koenig Jr, Gary M. (20 February 2017). "Carbon-free Solid
Dispersion LiCoO2 Redox Couple Characterization and Electrochemical Evaluation for All Solid Dispersion Redox Flow Batteries".
2107:
952:
redox-species were proposed to reduce crossover, while allowing low-cost membranes. Such redox-active oligomers are known as redoxymers. One system uses organic polymers and a saline solution with a
2374:
Tolmachev, Yuriy V. (2015). "Energy cycle based on a high specific energy aqueous flow battery and its potential use for fully electric vehicles and for direct solar-to-chemical energy conversion".
391:). However a high power of 1.4 W/cm was demonstrated for hydrogen–bromine flow batteries, and a high specific energy (530 Wh/kg at the tank level) was shown for hydrogen–bromate flow batteries
540:
hybrid flow battery with an experimental OCV of 1.93 V and operating voltage of 1.70 V, relatively high values. It consists of a graphite felt positive electrode operating in a mixed solution of
387:
Traditional flow battery chemistries have both low specific energy (which makes them too heavy for fully electric vehicles) and low specific power (which makes them too expensive for stationary
2971:; Almheiri, Saif (2017). "The potential of non-aqueous redox flow batteries as fast-charging capable energy storage solutions: demonstration with an iron–chromium acetylacetonate chemistry".
239:
chemistry UNSW filed several patents related to VRFBs, that were later licensed to
Japanese, Thai and Canadian companies, which tried to commercialize this technology with varying success.
1057:. Dissolving a material changes its chemical behavior significantly. However, suspending bits of solid material preserves the solid's characteristics. The result is a viscous suspension.
4445:
Li, Zheng; Sam Pan, Menghsuan; Su, Liang; Tsai, Ping-Chun; Badel, Andres F.; Valle, Joseph M.; Eiler, Stephanie L.; Xiang, Kai; Brushett, Fikile R.; Chiang, Yet-Ming (11 October 2017).
3543:
Feng, Ruozhu; Zhang, Xin; Murugesan, Vijayakumar; Hollas, Aaron; Chen, Ying; Shao, Yuyan; Walter, Eric; Wellala, Nadeesha P. N.; Yan, Litao; Rosso, Kevin M.; Wang, Wei (21 May 2021).
778:
was the anolyte in a hybrid acid AORFB. Quinones accept two units of electrical charge, compared with one in conventional catholyte, implying twice as much energy in a given volume.
2127:, Fujii, Toshinobu; Hirose, Takashi & Kondou, Naoki, "Metallohalogen secondary battery", published 1981-06-13, assigned to Meidensha Electric Mfg. Co. Ltd.
859:
V. Cell efficiency exceeded 99%, while round-trip efficiency measured 84%. The battery offered an expected lifetime of at least 1,000 cycles. Its theoretic energy density was 19
154:
The log number of publications related to electrochemical power sources by year. Also shown as the magenta line is the inflation-adjusted oil price in US$ /liter in linear scale
71:
Various flow batteries have been demonstrated, including inorganic and organic forms. Flow battery design can be further classified into full flow, semi-flow, and membraneless.
3992:; Hashaikeh, Raed; Almheiri, Saif (2018). "Cyclable membraneless redox flow batteries based on immiscible liquid electrolytes: Demonstration with all-iron redox chemistry".
2268:
Xu, Q.; Ji, Y.N.; Qin, L.Y.; Leung, P.K.; Qiao, F.; Li, Y.S.; Su, H.N. (2018). ""Evaluation of redox flow batteries goes beyond round-trip efficiency: A technical review"".
2889:
Andrews, J.; Seif
Mohammadi, S. (2014). "Towards a 'proton flow battery': Investigation of a reversible PEM fuel cell with integrated metal-hydride hydrogen storage".
4358:
Tolmachev, Yuriy, and
Svetlana V. Starodubceva. "Flow batteries with solid energy boosters." Journal of Electrochemical Science and Engineering 12.4 (2022): 731-766.
1598:– Because all cells share the same electrolyte(s), the electrolytes may be charged using a given number of cells and discharged with a different number. As battery
956:
membrane. A prototype underwent 10,000 charging cycles while retaining substantial capacity. The energy density was 10 Wh/L. Current density reached ,1 amperes/cm.
781:
Another quinone 9,10-Anthraquinone-2,7-disulfonic acid (AQDS), was evaluated. AQDS undergoes rapid, reversible two-electron/two-proton reduction on a glassy carbon
4077:
3357:
Carretero-González, Javier; Castillo-Martínez, Elizabeth; Armand, Michel (2016). "Highly water-soluble three-redox state organic dyes as bifunctional analytes".
2104:
4635:
2566:
Spagnuolo, G.; Petrone, G.; Mattavelli, P.; Guarnieri, M. (2016). "Vanadium Redox Flow Batteries: Potentials and Challenges of an Emerging Storage Technology".
730:
3006:
Luo, J.; Sam, A.; Hu, B.; DeBruler C.; Liu, T. L. (2017). "Unraveling pH Dependent Cycling Stability of Ferricyanide / Ferrocyanide in Redox Flow Batteries".
4310:
Single-Molecule Redox-Targeting Reactions for a pH-Neutral Aqueous Organic Redox Flow Battery. Angewandte Chemie-International Edition 2020, 59, 14286-14291.
372:
Lower energy efficiency, because they operate at higher current densities to minimize the effects of cross-over (internal self-discharge) and to reduce cost.
68:
inside the cell (accompanied by current flow through an external circuit) occurs across the membrane while the liquids circulate in their respective spaces.
882:
Another approach adopted a Blatter radical as the donor/recipient. It endured 275 charge and discharge cycles in tests, although it was not water-soluble.
979:
redox-species have not demonstrated competitive area-specific power. Low operating current density may be an intrinsic feature of large redox-molecules.
352:
Some types offer easy state-of-charge determination (through voltage dependence on charge), low maintenance and tolerance to overcharge/overdischarge.
150:
3192:
Xu, Yan; Wen, Yue-Hua; Cheng, Jie; Cao, Gao-Ping; Yang, Yu-Sheng (2010). "A study of tiron in aqueous solutions for redox flow battery application".
4808:
303:
due to their low inherent activity toward many redox couples. The amount of electricity that can be generated depends on the volume of electrolyte.
210:
733:
as a catholyte at pH neutral conditions, plus NaCL and a low-cost anion exchange membrane. This MV/TEMPO system has the highest cell voltage, 1.25
3807:
Bamgbopa, Musbaudeen O.; Almheiri, Saif; Sun, Hong (2017). "Prospects of recently developed membraneless cell designs for redox flow batteries".
4431:
2840:
Weng, Guo-Ming (2017). "Unlocking the capacity of iodide for high-energy-density zinc/polyiodide and lithium/polyiodide redox flow batteries".
3301:
1860:
Redox Active Inorganic Materials for Redox Flow Batteries in Encyclopedia of Inorganic and Bioinorganic Chemistry: Inorganic Battery Materials
3228:
113:. The heavier weight results mostly from the need to use a solvent (usually-water) to maintain the redox active species in the liquid phase.
5301:
4602:
3254:"Harvard team demonstrates new metal-free organic–inorganic aqueous flow battery; potential breakthrough for low-cost grid-scale storage"
1054:
5079:
4966:
2515:, Keefer, Richard Mackay, "Redox fuel cell regenerated with sugar", published 1972-08-08, assigned to Electrocell Ltd.
1509:
529:
5339:
355:
They are safe because they typically do not contain flammable electrolytes, and electrolytes can be stored away from the power stack.
4538:
3851:
1064:
flanking the redox potential of the SEM. Such SEB/RFBs combine the high specific energy advantage of conventional batteries (such as
4033:
Journal of Vacuum Science & Technology B, Nanotechnology and Microelectronics: Materials, Processing, Measurement, and Phenomena
2646:
Leung, P. K.; Ponce-De-León, C.; Low, C. T. J.; Shah, A. A.; Walsh, F. C. (2011). "Characterization of a zinc–cerium flow battery".
1711:
Journal of Vacuum Science & Technology B, Nanotechnology and Microelectronics: Materials, Processing, Measurement, and Phenomena
1562:
759:
AORFB were reported to be stable for 1000 cycles at an energy density of 10 Wh/L, the most stable, energy-dense AORFB to that date.
117:
5119:
215:
on September 29, 1879. Zn-Br2 batteries have relatively high specific energy, and were demonstrated in electric cars in the 1970s.
4765:
4371:
Badwal, Sukhvinder P. S.; Giddey, Sarbjit S.; Munnings, Christopher; Bhatt, Anand I.; Hollenkamp, Anthony F. (24 September 2014).
1754:
Badwal, Sukhvinder P. S.; Giddey, Sarbjit S.; Munnings, Christopher; Bhatt, Anand I.; Hollenkamp, Anthony F. (24 September 2014).
2255:
299:. Many flow batteries use carbon felt electrodes due to its low cost and adequate electrical conductivity, despite their limited
5129:
1426:
602:
When the battery is fully discharged, both tanks hold the same electrolyte solution: a mixture of positively charged zinc ions (
472:
5482:
2473:, Borchers, William, "Process of transforming chemical energy of fuel into electrical energy", published 1896-09-22
918:
2814:
Borghino, Dario (27 February 2015). "High-performance flow battery could rival lithium-ions for EVs and grid storage". Gizmag.
933:
coordinated to 1,3-propanediaminetetraacetate (PDTA), gave cell potentials of 1.62 V vs. ferrocyanide and a record 2.13 V vs.
322:
Independent scaling of energy (tanks) and power (stack), which allows for a cost/weight/etc. optimization for each application
89:(where new charged negolyte (a.k.a. reducer or fuel) and charged posolyte (a.k.a. oxidant) are added to the system) or like a
5437:
5057:
925:
would otherwise precipitate. By blocking the coordination of water to the metal, organic ligands can inhibit metal-catalyzed
595:
2254:
Tolmachev, Yuriy. "Flow batteries from 1879 to 2022 and beyond." Journal of Electrochemical Science and Engineering (2022) (
525:
201:
1060:
In 2022, Influit Energy announced a flow battery electrolyte consisting of a metal oxide suspended in an aqueous solution.
594:
flow battery demonstrated an energy density of 167 Wh/L. Older zinc–bromide cells reach 70 Wh/L. For comparison,
5477:
5139:
4620:
3518:
2826:
232:
996:
5360:
4801:
1589:
5104:
5530:
5447:
5390:
4971:
2732:"Investigations of High Voltage Vanadium-Metal Hydride Flow Battery toward kWh Scale Storage with 100 cm 2 Electrodes"
1662:
307:
5144:
5114:
5099:
5067:
2924:
Brushett, Fikile; Vaughey, John; Jansen, Andrew (2012). "An All-Organic Non-aqueous Lithium-Ion Redox Flow Battery".
138:. The energy capacity is a function of the electrolyte volume and the power is a function of the surface area of the
36:
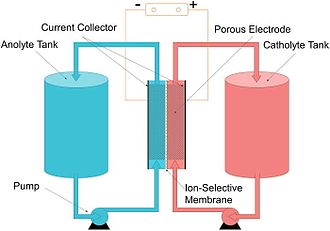
A typical flow battery consists of two tanks of liquids which are pumped past a membrane held between two electrodes.
3109:"Unprecedented Capacity and Stability of Ammonium Ferrocyanide Catholyte in pH Neutral Aqueous Redox Flow Batteries"
2215:"Engineering aspects of the design, construction and performance of modular redox flow batteries for energy storage"
5467:
5329:
4776:
4721:
1578:
290:
5159:
4906:
4328:
Redox Targeting of Energy Materials for Energy Storage and Conversion. Advanced Materials 2021, 2104562 (2104519).
1813:
Alotto, P.; Guarnieri, M.; Moro, F. (2014). "Redox Flow Batteries for the storage of renewable energy: a review".
5556:
5457:
5375:
5334:
1607:
1595:
789:. An aqueous flow battery with inexpensive carbon electrodes, combining the quinone/hydroquinone couple with the
5174:
4564:
1471:
992:
5462:
5370:
5294:
5072:
3677:
3652:
2427:
Shiokawa, Y.; Yamana, H.; Moriyama, H. (2000). "An Application of Actinide Elements for a Redox Flow Battery".
2339:
Yu; Tolmachev, V. (2013). "Hydrogen-halogen electrochemical cells: A review of applications and technologies".
1978:
Yuriy V. Tolmachev "Review—Flow Batteries from 1879 to 2022 and Beyond." 2023 J. Electrochem. Soc. 170 030505.
1966:
Yuriy V. Tolmachev "Review—Flow Batteries from 1879 to 2022 and Beyond." 2023 J. Electrochem. Soc. 170 030505.
1619:
681:
493:
5179:
5124:
5109:
5042:
5001:
4976:
4956:
4936:
2214:
1443:
988:
281:. Electroactive elements are "elements in solution that can take part in an electrode reaction or that can be
49:
3282:
5551:
5452:
5385:
5365:
5062:
4794:
2867:
1654:
1050:
476:
335:
No need for "equalisation" charging (the overcharging of a battery to ensure all cells have an equal charge)
98:
3492:
5566:
5472:
5411:
5268:
5169:
1405:
1383:
1359:
1334:
899:
468:
236:
121:
5030:
1999:
W. Kangro Dr, 1949.; W. Kangro Dr, 1954.;W. Kangro and H. Pieper, Electrochim Acta, 7 (4), 435-448 (1962)
1494:
533:
5380:
5344:
5134:
4951:
4781:
2170:
McCreery, Richard L. (July 2008). "Advanced Carbon Electrode Materials for Molecular Electrochemistry".
2124:
1682:
1672:
1667:
1073:
1069:
381:
228:
110:
102:
5395:
4996:
4583:
824:. The battery was claimed to last 1,000 cycles without degradation. It has a low cell voltage (ca. 0.55
369:
Low charge and discharge rates. This implies large electrodes and membrane separators, increasing cost.
124:(H01M8/18), even though it is more appropriate to consider fuel cells as a subclass of flow batteries.
2512:
2078:
1990:
C. J. Amato, in "1973 International Automotive Engineering Congress and Exposition", p. 11, 1973-02-01
403:
cell uses redox-active species in fluid (liquid or gas) media. Redox flow batteries are rechargeable (
5012:
4981:
4817:
4495:
4458:
4384:
4283:
Redox-Targeting-Based Flow Batteries for Large-Scale Energy Storage. Advanced Materials 2018, 30, 13.
4158:
4147:"A carbon-free lithium-ion solid dispersion redox couple with low viscosity for redox flow batteries"
4111:
4040:
3946:
3873:
3816:
3765:
3713:
3624:
3556:
3120:
3038:
3011:
2933:
2898:
2694:
2655:
2620:
2489:
2470:
2436:
2277:
2229:
1822:
1767:
1718:
1111:
960:
504:(> $ 30 / Kg); parasitic reactions including hydrogen and oxygen evolution; and precipitation of V
270:
206:
90:
61:
53:
4654:
1603:
5561:
5416:
5287:
5164:
5149:
5084:
5052:
5047:
4863:
4603:"Electric Vehicle Refuelling System (EVRS) used in conjunction with Vanadium Redox Flow Technology"
4215:
1979:
1967:
1556:
855:
tanks. The increased electrical resistance in the membrane was compensated increased voltage to 1.2
844:
574:
127:
74:
The fundamental difference between conventional and flow batteries is that energy is stored in the
5324:
5154:
5025:
4878:
4836:
4698:
4519:
4127:
3970:
3936:
3897:
3863:
3789:
3737:
3588:
3473:
3089:
2949:
2593:
2548:
2452:
2391:
2356:
2321:
1890:
922:
3927:
Braff, W. A.; Bazant, M. Z.; Buie, C. R. (2013). "Membrane-less hydrogen bromine flow battery".
2773:
1606:. In addition, if the number of cells is continuously changed (on the input and/or output side)
5228:
4946:
4916:
4614:
4511:
4447:"Air-Breathing Aqueous Sulfur Flow Battery for Ultralow-Cost Long-Duration Electrical Storage"
4412:
4220:
4058:
4009:
3962:
3889:
3832:
3781:
3729:
3580:
3572:
3545:"Reversible ketone hydrogenation and dehydrogenation for aqueous organic redox flow batteries"
3465:
3457:
3413:
3374:
3209:
3081:
2988:
2796:
2751:
2712:
2195:
2187:
2060:
2052:
1795:
1736:
937:. Metal-organic flow batteries may be known as coordination chemistry flow batteries, such as
648:) are used as the complexing agent to stabilize the free iodine, forming iodine–bromide ions (
412:
408:
404:
3253:
3148:"Nonaqueous redox-flow batteries: organic solvents, supporting electrolytes, and redox pairs"
2683:"Developments in soluble lead flow batteries and remaining challenges: An illustrated review"
1874:
5517:
5512:
5507:
5502:
5094:
5089:
4901:
4841:
4733:
4690:
4662:
4503:
4466:
4402:
4392:
4259:
4196:
4166:
4119:
4048:
4001:
3954:
3881:
3824:
3773:
3721:
3632:
3564:
3447:
3405:
3366:
3201:
3159:
3128:
3108:
3073:
3019:
2980:
2941:
2906:
2849:
2788:
2743:
2702:
2663:
2628:
2583:
2575:
2540:
2444:
2383:
2348:
2313:
2285:
2237:
2179:
2152:
2044:
1947:
1882:
1838:
1830:
1785:
1775:
1726:
1613:
1610:
can also be AC/DC, AC/AC, or DC–AC with the frequency limited by that of the switching gear.
1137:
1017:
836:
772:
453:
326:
318:
Redox flow batteries, and to a lesser extent hybrid flow batteries, have the advantages of:
4717:
Talk by John Davis of Deeya energy about their flow battery's use in the telecomms industry
4961:
4888:
4542:
4486:
Service, R.F. (2 November 2018). "Advances in flow batteries promise cheap backup power".
2731:
2730:
Weng, Guo-Ming; Li, Chi-Ying Vanessa; Chan, Kwong-Yu; Lee, Cheuk-Wing; Zhong, Jin (2016).
2111:
1677:
1566:
938:
926:
714:
274:
131:
106:
57:
3268:
2611:
Bartolozzi, M. (1989). "Development of redox flow batteries. A historical bibliography".
959:
Another oligomer RFB employed viologen and TEMPO redoxymers in combination with low-cost
4499:
4462:
4388:
4292:
Redox targeting-based flow batteries. Journal of Physics D-Applied Physics 2019, 52, 17.
4162:
4115:
4044:
3950:
3877:
3820:
3769:
3717:
3628:
3560:
3124:
3015:
2937:
2902:
2698:
2659:
2624:
2440:
2281:
2233:
2143:
Aaron, Douglas (2013). "In Situ Kinetics Studies in All-Vanadium Redox Flow Batteries".
1826:
1771:
1722:
1033:
5197:
4851:
4407:
4372:
3989:
3678:"Chemists present an innovative redox-flow battery based on organic polymers and water"
2968:
2493:
2032:
1875:"Status and Prospects of Organic Redox Flow Batteries towards Renewable Energy Storage"
1790:
1755:
1592:, where the battery is used if the main power fails to provide an uninterrupted supply.
964:
388:
363:
342:
253:
began operating a 400 MWh, 100 MW vanadium flow battery, then the largest of its type.
65:
3544:
5545:
5432:
4831:
4338:
4319:
Redox targeting of energy materials. Current Opinion in Electrochemistry 2021, 29, 7.
3592:
3477:
3329:
2632:
1894:
1640:
921:, and can enable the electrolyte to be in neutral or alkaline conditions under which
816:
786:
677:
537:
300:
4702:
4523:
4200:
4131:
4005:
3974:
3901:
3205:
3107:
Luo, J.; Hu, B.; DeBruler C.; Zhao, Y.; Yuan B.; Hu, M.; Wu, W.; Liu, T. L. (2019).
3093:
2953:
2597:
2552:
2531:
Kummer, J. T.; Oei, D. -G. (1985). "A chemically regenerative redox fuel cell. II".
2456:
2395:
2360:
2325:
2018:
G. Kear, A. A. Shah, and F. C. Walsh, Int. J. Energy Res., 36 (11), 1105-1120 (2012)
1909:
5035:
4991:
4926:
4868:
4171:
4146:
3793:
3741:
2910:
2667:
1042:
1008:
903:
864:
848:
756:
116:
Patent Classifications for flow batteries had not been fully developed as of 2021.
3409:
2496:, "Brennstoffelement mit unangreifbaren Elektroden ", published 1912-06-15
2448:
1886:
483:. Redox fuel cells are less common commercially although many have been proposed.
32:
4507:
3023:
2774:"High energy density MnO4−/MnO42− redox couple for alkaline redox flow batteries"
2033:"Electrolyte Lifetime in Aqueous Organic Redox Flow Batteries: A Critical Review"
894:
molecules can be reengineered to increase water solubility. In 2021 a reversible
293:) while recovering the spent material for recharging. They can also be recharged
93:(where an electric power source drives regeneration of the reducer and oxidant).
5263:
5248:
4986:
4911:
4471:
4446:
3637:
3612:
3132:
2048:
1648:
1065:
870:
Integrating both anolyte and catholyte in the same molecule, i.e., bifunctional
416:
278:
266:
79:
78:
material in conventional batteries, while in flow batteries it is stored in the
3828:
1834:
1602:
is proportional to the number of cells used, the battery can act as a powerful
64:
in liquids that are pumped through the system on separate sides of a membrane.
5207:
4941:
4921:
4770:
4715:
4694:
4235:
4078:"Nanoparticle Networks Promise Cheaper Batteries for Storing Renewable Energy"
2707:
2682:
2387:
2352:
2289:
2241:
1630:
891:
591:
377:
282:
17:
4734:"Performance Testing of Zinc–Bromine Flow Batteries for Remote Telecom Sites"
4397:
4062:
4013:
3836:
3576:
3461:
3434:
Potash, Rebecca A.; McKone, James R.; Conte, Sean; Abruña, Héctor D. (2016).
3417:
3378:
3213:
3085:
2992:
2755:
2716:
2681:
Krishna, M.; Fraser, E. J.; Wills, R. G. A.; Walsh, F. C. (1 February 2018).
2579:
2191:
2056:
1780:
1740:
5310:
5253:
5243:
5233:
5202:
4858:
3613:"Chelated Chromium Electrolyte Enabling High-Voltage Aqueous Flow Batteries"
3568:
1634:
1574:
968:
953:
914:
876:
782:
752:
685:
420:
262:
139:
86:
75:
4515:
4416:
4123:
3966:
3893:
3852:"New rechargeable flow battery enables cheaper, large-scale energy storage"
3785:
3733:
3584:
3180:
World Non-Grid-Connected Wind Power and Energy Conference, 2009. WNWEC 2009
3077:
2945:
2800:
2317:
2199:
1799:
879:
are potential electrolytes for such symmetric redox-flow batteries (SRFB).
4102:
Duduta, Mihai (May 2011). "Semi-Solid Lithium Rechargeable Flow Battery".
3493:"Symmetrical flow battery may strike right balance for grid-scale storage"
2408:
Linden, D.; Reddy, T.B. (2002). Handbook of Batteries (Eds.). McGraw-Hill.
5494:
4931:
4029:"Review Article: Flow battery systems with solid electroactive materials"
3452:
3435:
3318:
Alkaline quinone flow battery Lin et al. Science 2015 349 (6255), p. 1529
3269:"New water-based organic battery is cheap, rechargeable and eco-friendly"
2747:
2588:
1843:
1707:"Review Article: Flow battery systems with solid electroactive materials"
1217:
1199:
1163:
1133:
1107:
976:
949:
930:
871:
748:
722:
464:
overvoltage and Au salts for catalyzing the chromium electrode reaction.
269:
containing one or more dissolved electroactive elements flows through an
4786:
4655:"A Zinc-Chloride Battery - The Missing Link to a Practical Electric Car"
4260:"Influit moves to commercialize its ultra-high density liquid batteries"
3777:
3725:
2156:
5238:
3958:
3885:
3370:
3330:"Greener, safer flow battery could store renewable energy on the cheap"
3164:
3147:
2984:
2853:
2792:
2544:
1599:
1159:
934:
929:, resulting in higher voltage aqueous systems. For example, the use of
852:
767:
726:
480:
295:
4053:
4028:
3519:"Candle compound brings high density to grid-scale battery technology"
3469:
2183:
2064:
1952:
1935:
1731:
1706:
696:
Compared to inorganic redox flow batteries, such as vanadium and Zn-Br
4373:"Emerging electrochemical energy conversion and storage technologies"
1756:"Emerging electrochemical energy conversion and storage technologies"
972:
910:
895:
738:
ranging from 20 to 100 mA/cm, with optimal performance rated at 40–50
246:
4359:
3352:
3350:
3039:"New flow battery projected to cost 60% less than existing standard"
4760:
4666:
4636:"nanoFLOWCELL-powered Quant e-Limo approved for german road trials"
713:
pH neutral AORFBs are operated at pH 7 conditions, typically using
624:). When charged, one tank holds another negative ion, polyiodide, (
325:
Long cycle and calendar lives (because there are no solid-to-solid
5223:
4432:"Room-temperature flow battery uses liquid sodium-potassium alloy"
4301:
Redox Targeting Improves Flow Batteries. Joule 2019, 3, 2066-2067.
3941:
3868:
3302:"Flow Battery Could Smooth Irregular Wind and Solar Energy Supply"
3283:"A rechargeable battery to power a home from rooftop solar panels"
1032:
812:
400:
250:
31:
1980:
https://iopscience.iop.org/article/10.1149/1945-7111/acb8de/meta
1968:
https://iopscience.iop.org/article/10.1149/1945-7111/acb8de/meta
1858:
Hu, B.; Luo, J.; DeBruler C.; Hu, M; Wu, W.; Liu, T. L. (2019).
867:'s chemical stability in high pH KOH solution was not verified.
587:
536:, and iron-salt flow batteries. Weng et al. reported a vanadium–
449:
220:
135:
5283:
4790:
3611:
Robb, Brian H.; Farrell, Jason M.; Marshak, Michael P. (2019).
2031:
Kwabi, David G.; Ji, Yunlong; Aziz, Michael J. (22 July 2020).
460:
evolution can be mitigated by adding Pb salts to increase the H
204:(Zn-Br2) was the original flow battery. John Doyle file patent
5258:
4339:"130+ million publications organized by topic on ResearchGate"
3850:
Braff, William A.; Bazant, Martin Z.; Buie, Cullen R. (2013).
2772:
Colli, Alejandro N.; Peljo, Pekka; Girault, Hubert H. (2016).
1181:
376:
Flow batteries typically have a higher energy efficiency than
5279:
2079:"World's largest flow battery connected to the grid in China"
1559:- short and/or long-term energy storage for use by the grid
366:(large tanks are required to store useful amounts of energy)
3178:
Xu, Y.; Wen, Y.; Cheng, J.; Yanga, Y.; Xie, Z.; Cao, G. In
2213:
Arenas, L.F.; Ponce de León, C.; Walsh, F.C. (June 2017).
2827:"New flow battery to keep big cities lit, green and safe"
1873:
Luo, J.; Hu, B.; Hu, M.; Liu, T. L. (13 September 2019).
1584:
Peak shaving, where demand spikes are met by the battery.
3429:
3427:
668:) as a means to free up iodide ions for charge storage.
306:
Flow batteries are governed by the design principles of
134:
and ranges, in practical applications, from 1.0 to 2.43
4568:
2417:
C. Y. Sun and H. Zhang, ChemSusChem, 15 (1), 15 (2022)
4145:
Qi, Zhaoxiang; Koenig Jr., Gary M. (15 August 2016).
1934:
Yuriy V. Tolmachev; Svetlana V. Starodubceva (2022).
3146:
Gong, K; Fang, Q; Gu, S; Li, F.S.Y.; Yan, Y (2015).
851:
was less corrosive, allowing the use of inexpensive
5493:
5425:
5404:
5353:
5317:
5216:
5188:
5010:
4886:
4824:
3436:"On the Benefits of a Symmetric Redox Flow Battery"
3390:
3388:
747:
Wh/L with the limitation on the TEMPO side. In 2019
467:Traditional redox flow battery chemistries include
1573:Shifting energy from intermittent sources such as
1072:is larger than that of SEB(ROTS)-flow versions of
329:, which degrade lithium-ion and related batteries)
4609:. Archived from the original on 10 December 2011.
1041:A lithium–sulfur system arranged in a network of
101:,. However, flow batteries suffer from low cycle
913:to improve redox properties. The ligands can be
820:the positive side avoids the use of hazardous Br
2868:"Proton flow battery simplifies hydrogen power"
1552:charge/discharge times. Market niches include:
1705:Qi, Zhaoxiang; Koenig, Gary M. (12 May 2017).
448:, such as unitized regenerative fuel cells in
242:Organic redox flow batteries emerged in 2009.
5295:
4802:
731:4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl
8:
2767:
2765:
902:catholyte because of the stability issue of
573:, and a metal hydride negative electrode in
1936:"Flow batteries with solid energy boosters"
1700:
1698:
5302:
5288:
5280:
4809:
4795:
4787:
4766:Research on the uranium redox flow battery
1815:Renewable & Sustainable Energy Reviews
1083:
751:-based flow batteries using an ultralight
4470:
4406:
4396:
4170:
4052:
3940:
3867:
3636:
3451:
3229:"From Harvard, a Cheaper Storage Battery"
3163:
2706:
2587:
2429:Journal of Nuclear Science and Technology
1951:
1842:
1789:
1779:
1730:
909:Metal-organic flow batteries use organic
3922:
3920:
3918:
3809:Renewable and Sustainable Energy Reviews
2891:International Journal of Hydrogen Energy
1085:Comparison of flow battery compositions
676:Proton flow batteries (PFB) integrate a
149:
4683:Journal of Solid State Electrochemistry
4027:Qi, Zhaoxiang; Koenig, Gary M. (2017).
2376:Journal of Solid State Electrochemistry
1940:Electrochemical Science and Engineering
1694:
60:is provided by two chemical components
4612:
4565:"Redflow – Sustainable Energy Storage"
3440:Journal of the Electrochemical Society
2736:Journal of the Electrochemical Society
1581:for use during periods of peak demand.
1096:Average electrode power density (W/m)
987:Other flow-type batteries include the
839:with a less toxic alkaline solution (1
613:) and negatively charged iodide ion, (
4782:South Australian Flow Battery Project
4214:Chandler, David L. (23 August 2011).
3606:
3604:
3602:
3328:Borghino, Dario (30 September 2015).
2026:
2024:
1055:solid dispersion redox flow batteries
424:1990s, rechargeable fuel cells (i.e.
7:
4216:"Go with the Flow – Cambridge Crude"
2568:IEEE Industrial Electronics Magazine
680:storage electrode into a reversible
348:Recycling of electroactive materials
227:In the late 1980s, Sum, Rychcik and
3227:WALD, MATTHEW L. (8 January 2014).
2825:White, Frances (25 February 2015).
2533:Journal of Applied Electrochemistry
2341:Russian Journal of Electrochemistry
828:V) and a low energy density (< 4
5340:Proton-exchange membrane fuel cell
4237:Darpa Nanoelectrofuel Flow Battery
3359:Energy & Environmental Science
3037:Moss, Richard (22 December 2015).
2842:Energy & Environmental Science
1908:Clark, Elliot (17 November 2023).
85:A flow battery may be used like a
25:
4634:Antony Ingram (11 October 2016).
4360:https://hrcak.srce.hr/file/410594
3300:Matthew Gunther, ChemistryWorld.
1565:– the battery is attached to the
1007:A membraneless battery relies on
261:A flow battery is a rechargeable
235:(UNSW) in Australia demonstrated
118:Cooperative Patent Classification
4877:
4653:Amato, C. J. (1 February 1973).
3182:IEEE: Nanjing, China, 2009, p 1.
3152:Energy and Environmental Science
2973:Journal of Materials Chemistry A
1647:
1633:
596:lithium iron phosphate batteries
130:is chemically determined by the
120:considers RFBs as a subclass of
5483:Unitized regenerative fuel cell
4761:Electropaedia on Flow Batteries
4201:10.1016/j.electacta.2017.01.061
4006:10.1016/j.electacta.2018.02.063
3653:"Energy Storage: GridStar Flow"
3206:10.1016/j.electacta.2009.09.031
815:couple, yields a peak galvanic
409:heterogeneous electron transfer
4172:10.1016/j.jpowsour.2016.05.033
4076:Kevin Bullis (24 April 2014).
3491:Lavars, Nick (17 March 2022).
3267:Szondy, David (29 June 2014).
2911:10.1016/j.ijhydene.2013.11.010
2870:. Gizmag.com. 13 February 2014
2668:10.1016/j.jpowsour.2011.01.095
1510:Zinc–cerium (methanesulfonate)
1:
5478:Solid oxide electrolyzer cell
3410:10.1021/acsenergylett.6b00413
2926:Advanced Functional Materials
2449:10.1080/18811248.2000.9714891
1887:10.1021/acsenergylett.9b01332
1099:Average fluid energy density
967:(similar to acrylic glass or
941:'s Gridstar Flow technology.
407:) cells. Because they employ
233:University of New South Wales
5361:Direct borohydride fuel cell
4508:10.1126/science.362.6414.508
4430:Bush, Steve (20 July 2018).
4258:Blain, Loz (9 August 2022).
3517:Lavars, Nick (21 May 2021).
3024:10.1016/j.nanoen.2017.10.057
2633:10.1016/0378-7753(89)80037-0
2145:ECS Electrochemistry Letters
1495:Lead–acid (methanesulfonate)
1384:Vanadium–vanadium (sulphate)
358:The main disadvantages are:
27:Type of electrochemical cell
5448:Membrane electrode assembly
5391:Reformed methanol fuel cell
4472:10.1016/j.joule.2017.08.007
3638:10.1016/j.joule.2019.07.002
3133:10.1016/j.joule.2018.10.010
2049:10.1021/acs.chemrev.9b00599
1663:Glossary of fuel cell terms
1406:Vanadium–vanadium (bromide)
308:electrochemical engineering
291:internal combustion engines
5583:
5468:Protonic ceramic fuel cell
5438:Electro-galvanic fuel cell
5330:Molten carbonate fuel cell
4659:SAE Technical Paper Series
4539:"Storing Renewable Energy"
3829:10.1016/j.rser.2016.11.234
1910:"What is a Calendar-life?"
1881:. 2019, 4 (9): 2220–2240.
1835:10.1016/j.rser.2013.08.001
1427:Sodium–bromine polysulfide
1316:Sulfonate viologen (NH4)4
963:membranes. Functionalized
395:Traditional flow batteries
5526:
5458:Photoelectrochemical cell
5376:Direct methanol fuel cell
5335:Phosphoric acid fuel cell
4967:Metal–air electrochemical
4875:
4695:10.1007/s10008-015-2805-z
4619:: CS1 maint: unfit URL (
4104:Advanced Energy Materials
3988:Bamgbopa, Musbaudeen O.;
3066:Advanced Energy Materials
2967:Bamgbopa, Musbaudeen O.;
2708:10.1016/j.est.2017.10.020
2687:Journal of Energy Storage
2388:10.1007/s10008-015-2805-z
2353:10.1134/S1023193513120069
2290:10.1016/j.est.2018.01.005
2270:Journal of Energy Storage
2242:10.1016/j.est.2017.02.007
2222:Journal of Energy Storage
2103:Science-Dictionary.org. "
927:water-splitting reactions
419:they are more similar to
273:that reversibly converts
202:zinc–bromine flow battery
5463:Proton-exchange membrane
5371:Direct-ethanol fuel cell
4669:– via www.sae.org.
4398:10.3389/fchem.2014.00079
4151:Journal of Power Sources
2648:Journal of Power Sources
2613:Journal of Power Sources
2580:10.1109/MIE.2016.2611760
1781:10.3389/fchem.2014.00079
1620:Stand-alone power system
997:hydrogen–bromine battery
682:proton exchange membrane
494:levelized cost of energy
5453:Membraneless Fuel Cells
5386:Metal hydride fuel cell
5366:Direct carbon fuel cell
4772:How flow batteries work
3569:10.1126/science.abd9795
2781:Chemical Communications
2125:JP patent S5671271A
2105:Electroactive Substance
1655:Renewable energy portal
1051:semi-solid flow battery
1037:Semi-solid flow battery
906:in alkaline solutions.
99:total cost of ownership
5473:Regenerative fuel cell
5412:Enzymatic biofuel cell
5269:Semipermeable membrane
5058:Lithium–iron–phosphate
4584:WO patent 03043170
4377:Frontiers in Chemistry
4124:10.1002/aenm.201100152
3078:10.1002/aenm.201501449
2946:10.1002/aenm.201200322
2318:10.1002/cplu.201402043
2110:27 August 2013 at the
1760:Frontiers in Chemistry
1093:Max. cell voltage (V)
1038:
900:potassium ferrocyanide
155:
122:regenerative fuel cell
109:(heavier weight) than
37:
5381:Formic acid fuel cell
5345:Solid oxide fuel cell
5140:Rechargeable alkaline
4818:Electrochemical cells
4082:MIT Technology Review
3929:Nature Communications
3856:Nature Communications
3010:. 2017, 42: 215–221.
2513:US patent 3682704
1683:Microtubular membrane
1673:List of battery types
1668:Hydrogen technologies
1517:< 1,200–2,500
1074:lithium-ion batteries
1070:lithium-ion batteries
1036:
413:solid-state diffusion
382:lithium-ion batteries
153:
111:lithium-ion batteries
35:
5120:Nickel–metal hydride
3453:10.1149/2.0971602jes
2748:10.1149/2.0271601jes
2490:DE patent 264026
2471:US patent 567959
993:zinc–bromine battery
975:membrane. RFBs with
923:metal aquo complexes
338:No harmful emissions
332:Quick response times
285:on the electrode."
271:electrochemical cell
91:rechargeable battery
54:electrochemical cell
5417:Microbial fuel cell
5130:Polysulfide–bromide
4972:Nickel oxyhydroxide
4864:Thermogalvanic cell
4607:REDT Energy Storage
4571:on 9 February 2010.
4500:2018Sci...362..508S
4463:2017Joule...1..306L
4389:2014FrCh....2...79B
4189:Electrochimica Acta
4163:2016JPS...323...97Q
4116:2011AdEnM...1..511D
4045:2017JVSTB..35d0801Q
3994:Electrochimica Acta
3951:2013NatCo...4.2346B
3878:2013NatCo...4.2346B
3821:2017RSERv..70..506B
3778:10.1038/nature15746
3770:2015Natur.527...78J
3726:10.1038/nature15746
3718:2015Natur.527...78J
3629:2019Joule...3.2503R
3561:2021Sci...372..836F
3306:Scientific American
3194:Electrochimica Acta
3125:2019Joule...3..149L
3016:2017NEne...42..215L
2979:(26): 13457–13468.
2938:2012AdEnM...2.1390B
2903:2014IJHE...39.1740A
2787:(97): 14039–14042.
2699:2018JEnSt..15...69K
2660:2011JPS...196.5174L
2625:1989JPS....27..219B
2441:2000JNST...37..253S
2282:2018JEnSt..16..108X
2234:2017JEnSt..11..119A
2157:10.1149/2.001303eel
1827:2014RSERv..29..325A
1772:2014FrCh....2...79B
1723:2017JVSTB..35d0801Q
1458:Sulfur–oxygen-salt
1086:
989:zinc–cerium battery
473:polysulfide–bromide
345:during idle periods
50:reduction–oxidation
5325:Alkaline fuel cell
4893:(non-rechargeable)
4837:Concentration cell
4545:on 1 February 2014
3959:10.1038/ncomms3346
3886:10.1038/ncomms3346
3398:ACS Energy Letters
3371:10.1039/C6EE01883A
3256:. 11 January 2014.
3165:10.1039/C5EE02341F
2985:10.1039/c7ta02022h
2854:10.1039/C6EE03554J
2793:10.1039/C6CC08070G
2742:(1): A5180–A5187.
2545:10.1007/BF01059304
1084:
1039:
456:) were developed.
156:
46:redox flow battery
38:
5539:
5538:
5277:
5276:
4494:(6414): 508–509.
4221:Technology Review
4054:10.1116/1.4983210
3684:. 21 October 2015
3623:(10): 2503–2512.
3555:(6544): 836–840.
3365:(11): 3521–3530.
3158:(12): 3515–3530.
2932:(11): 1390–1396.
2654:(11): 5174–5185.
2184:10.1021/cr068076m
2043:(14): 6467–6489.
1953:10.5599/jese.1363
1732:10.1116/1.4983210
1614:Electric vehicles
1544:
1543:
1526:Zn-Mn(VI)/Mn(VII)
709:pH neutral AORFBs
512:during cycling.
380:, but lower than
327:phase transitions
279:electrical energy
103:energy efficiency
16:(Redirected from
5574:
5557:Electrochemistry
5396:Zinc–air battery
5304:
5297:
5290:
5281:
5073:Lithium–titanate
5018:
4894:
4881:
4842:Electric battery
4811:
4804:
4797:
4788:
4773:
4748:
4747:
4745:
4743:
4738:
4730:
4724:
4718:
4713:
4707:
4706:
4689:(9): 2711–2722.
4677:
4671:
4670:
4650:
4644:
4643:
4631:
4625:
4624:
4618:
4610:
4599:
4593:
4592:
4591:
4587:
4579:
4573:
4572:
4567:. Archived from
4561:
4555:
4554:
4552:
4550:
4541:. Archived from
4534:
4528:
4527:
4483:
4477:
4476:
4474:
4442:
4436:
4435:
4427:
4421:
4420:
4410:
4400:
4368:
4362:
4356:
4350:
4349:
4347:
4345:
4335:
4329:
4326:
4320:
4317:
4311:
4308:
4302:
4299:
4293:
4290:
4284:
4281:
4275:
4274:
4272:
4270:
4255:
4249:
4248:
4247:
4245:
4232:
4226:
4225:
4211:
4205:
4204:
4183:
4177:
4176:
4174:
4142:
4136:
4135:
4099:
4093:
4092:
4090:
4088:
4073:
4067:
4066:
4056:
4024:
4018:
4017:
3985:
3979:
3978:
3944:
3924:
3913:
3912:
3910:
3908:
3871:
3847:
3841:
3840:
3804:
3798:
3797:
3752:
3746:
3745:
3700:
3694:
3693:
3691:
3689:
3674:
3668:
3667:
3665:
3663:
3649:
3643:
3642:
3640:
3608:
3597:
3596:
3540:
3534:
3533:
3531:
3529:
3514:
3508:
3507:
3505:
3503:
3488:
3482:
3481:
3455:
3446:(3): A338–A344.
3431:
3422:
3421:
3392:
3383:
3382:
3354:
3345:
3344:
3342:
3340:
3325:
3319:
3316:
3310:
3309:
3297:
3291:
3290:
3279:
3273:
3272:
3264:
3258:
3257:
3250:
3244:
3243:
3241:
3239:
3224:
3218:
3217:
3189:
3183:
3176:
3170:
3169:
3167:
3143:
3137:
3136:
3104:
3098:
3097:
3060:
3054:
3053:
3051:
3049:
3034:
3028:
3027:
3003:
2997:
2996:
2964:
2958:
2957:
2921:
2915:
2914:
2897:(4): 1740–1751.
2886:
2880:
2879:
2877:
2875:
2864:
2858:
2857:
2837:
2831:
2830:
2822:
2816:
2815:
2811:
2805:
2804:
2778:
2769:
2760:
2759:
2727:
2721:
2720:
2710:
2678:
2672:
2671:
2643:
2637:
2636:
2608:
2602:
2601:
2591:
2563:
2557:
2556:
2528:
2522:
2521:
2520:
2516:
2509:
2503:
2502:
2501:
2497:
2486:
2480:
2479:
2478:
2474:
2467:
2461:
2460:
2424:
2418:
2415:
2409:
2406:
2400:
2399:
2382:(9): 2711–2722.
2371:
2365:
2364:
2336:
2330:
2329:
2300:
2294:
2293:
2265:
2259:
2252:
2246:
2245:
2219:
2210:
2204:
2203:
2178:(7): 2646–2687.
2172:Chemical Reviews
2167:
2161:
2160:
2140:
2134:
2133:
2132:
2128:
2121:
2115:
2101:
2095:
2094:
2092:
2090:
2085:. 3 October 2022
2075:
2069:
2068:
2037:Chemical Reviews
2028:
2019:
2016:
2010:
2006:
2000:
1997:
1991:
1988:
1982:
1976:
1970:
1964:
1958:
1957:
1955:
1931:
1925:
1924:
1922:
1920:
1905:
1899:
1898:
1870:
1864:
1863:
1862:. pp. 1–25.
1855:
1849:
1848:
1846:
1810:
1804:
1803:
1793:
1783:
1751:
1745:
1744:
1734:
1702:
1657:
1652:
1651:
1643:
1638:
1637:
1608:power conversion
1596:Power conversion
1537:
1485:
1444:Sodium–potassium
1417:
1397:
1374:
1349:
1307:
1287:
1267:
1246:
1151:
1138:lithium chlorate
1125:
1087:
1029:Suspension-based
1018:hydrobromic acid
862:
858:
842:
837:hydrobromic acid
831:
827:
811:
810:
809:
800:
799:
798:
746:
741:
736:
667:
666:
665:
658:
657:
647:
646:
645:
635:
634:
633:
623:
622:
621:
612:
611:
610:
572:
571:
570:
562:
561:
551:
550:
549:
454:Helios Prototype
447:
446:
445:
435:
434:
433:
214:
213:
209:
52:), is a type of
21:
5582:
5581:
5577:
5576:
5575:
5573:
5572:
5571:
5542:
5541:
5540:
5535:
5522:
5489:
5421:
5400:
5349:
5313:
5308:
5278:
5273:
5212:
5191:
5184:
5105:Nickel–hydrogen
5063:Lithium–polymer
5019:
5016:
5015:
5006:
4895:
4892:
4891:
4882:
4873:
4820:
4815:
4771:
4757:
4752:
4751:
4741:
4739:
4736:
4732:
4731:
4727:
4716:
4714:
4710:
4679:
4678:
4674:
4661:. Vol. 1.
4652:
4651:
4647:
4633:
4632:
4628:
4611:
4601:
4600:
4596:
4589:
4582:
4580:
4576:
4563:
4562:
4558:
4548:
4546:
4536:
4535:
4531:
4485:
4484:
4480:
4444:
4443:
4439:
4429:
4428:
4424:
4370:
4369:
4365:
4357:
4353:
4343:
4341:
4337:
4336:
4332:
4327:
4323:
4318:
4314:
4309:
4305:
4300:
4296:
4291:
4287:
4282:
4278:
4268:
4266:
4257:
4256:
4252:
4243:
4241:
4240:, 18 March 2022
4234:
4233:
4229:
4213:
4212:
4208:
4185:
4184:
4180:
4144:
4143:
4139:
4101:
4100:
4096:
4086:
4084:
4075:
4074:
4070:
4026:
4025:
4021:
3990:Shao-Horn, Yang
3987:
3986:
3982:
3926:
3925:
3916:
3906:
3904:
3849:
3848:
3844:
3806:
3805:
3801:
3764:(7576): 78–81.
3754:
3753:
3749:
3712:(7576): 78–81.
3702:
3701:
3697:
3687:
3685:
3676:
3675:
3671:
3661:
3659:
3657:Lockheed Martin
3651:
3650:
3646:
3610:
3609:
3600:
3542:
3541:
3537:
3527:
3525:
3516:
3515:
3511:
3501:
3499:
3490:
3489:
3485:
3433:
3432:
3425:
3394:
3393:
3386:
3356:
3355:
3348:
3338:
3336:
3327:
3326:
3322:
3317:
3313:
3299:
3298:
3294:
3281:
3280:
3276:
3266:
3265:
3261:
3252:
3251:
3247:
3237:
3235:
3226:
3225:
3221:
3191:
3190:
3186:
3177:
3173:
3145:
3144:
3140:
3106:
3105:
3101:
3062:
3061:
3057:
3047:
3045:
3036:
3035:
3031:
3005:
3004:
3000:
2969:Shao-Horn, Yang
2966:
2965:
2961:
2923:
2922:
2918:
2888:
2887:
2883:
2873:
2871:
2866:
2865:
2861:
2839:
2838:
2834:
2824:
2823:
2819:
2813:
2812:
2808:
2776:
2771:
2770:
2763:
2729:
2728:
2724:
2680:
2679:
2675:
2645:
2644:
2640:
2610:
2609:
2605:
2565:
2564:
2560:
2530:
2529:
2525:
2518:
2511:
2510:
2506:
2499:
2494:Nernst, Walther
2488:
2487:
2483:
2476:
2469:
2468:
2464:
2426:
2425:
2421:
2416:
2412:
2407:
2403:
2373:
2372:
2368:
2338:
2337:
2333:
2302:
2301:
2297:
2267:
2266:
2262:
2253:
2249:
2217:
2212:
2211:
2207:
2169:
2168:
2164:
2142:
2141:
2137:
2130:
2123:
2122:
2118:
2112:Wayback Machine
2102:
2098:
2088:
2086:
2077:
2076:
2072:
2030:
2029:
2022:
2017:
2013:
2007:
2003:
1998:
1994:
1989:
1985:
1977:
1973:
1965:
1961:
1933:
1932:
1928:
1918:
1916:
1907:
1906:
1902:
1879:ACS Energy Lett
1872:
1871:
1867:
1857:
1856:
1852:
1812:
1811:
1807:
1753:
1752:
1748:
1704:
1703:
1696:
1691:
1678:Redox electrode
1653:
1646:
1639:
1632:
1629:
1604:DC–DC converter
1549:
1535:
1483:
1415:
1395:
1372:
1347:
1305:
1285:
1265:
1244:
1149:
1123:
1112:lithium bromate
1082:
1031:
1005:
985:
947:
939:Lockheed Martin
888:
860:
856:
840:
829:
825:
823:
808:
806:
805:
804:
802:
797:
794:
793:
792:
790:
776:
765:
744:
739:
734:
723:methyl viologen
711:
699:
694:
674:
664:
662:
661:
660:
656:
653:
652:
651:
649:
644:
641:
640:
639:
637:
632:
629:
628:
627:
625:
620:
618:
617:
616:
614:
609:
607:
606:
605:
603:
584:
582:Zinc-polyiodide
569:
566:
565:
564:
560:
557:
556:
555:
553:
548:
545:
544:
543:
541:
518:
511:
507:
503:
499:
489:
463:
444:
441:
440:
439:
437:
432:
429:
428:
427:
425:
397:
316:
275:chemical energy
259:
229:Skyllas-Kazacos
211:
205:
197:
194:
191:
188:
185:
182:
179:
176:
173:
170:
167:
164:
161:
158:
148:
132:Nernst equation
107:specific energy
58:chemical energy
28:
23:
22:
15:
12:
11:
5:
5580:
5578:
5570:
5569:
5564:
5559:
5554:
5552:Flow batteries
5544:
5543:
5537:
5536:
5534:
5533:
5527:
5524:
5523:
5521:
5520:
5515:
5510:
5505:
5499:
5497:
5491:
5490:
5488:
5487:
5486:
5485:
5480:
5470:
5465:
5460:
5455:
5450:
5445:
5440:
5435:
5429:
5427:
5423:
5422:
5420:
5419:
5414:
5408:
5406:
5402:
5401:
5399:
5398:
5393:
5388:
5383:
5378:
5373:
5368:
5363:
5357:
5355:
5351:
5350:
5348:
5347:
5342:
5337:
5332:
5327:
5321:
5319:
5318:By electrolyte
5315:
5314:
5309:
5307:
5306:
5299:
5292:
5284:
5275:
5274:
5272:
5271:
5266:
5261:
5256:
5251:
5246:
5241:
5236:
5231:
5226:
5220:
5218:
5214:
5213:
5211:
5210:
5205:
5200:
5198:Atomic battery
5194:
5192:
5189:
5186:
5185:
5183:
5182:
5177:
5172:
5170:Vanadium redox
5167:
5162:
5157:
5152:
5147:
5145:Silver–cadmium
5142:
5137:
5132:
5127:
5122:
5117:
5115:Nickel–lithium
5112:
5107:
5102:
5100:Nickel–cadmium
5097:
5092:
5087:
5082:
5077:
5076:
5075:
5070:
5068:Lithium–sulfur
5065:
5060:
5055:
5045:
5040:
5039:
5038:
5028:
5022:
5020:
5017:(rechargeable)
5013:Secondary cell
5011:
5008:
5007:
5005:
5004:
4999:
4994:
4989:
4984:
4979:
4974:
4969:
4964:
4959:
4954:
4949:
4944:
4939:
4937:Edison–Lalande
4934:
4929:
4924:
4919:
4914:
4909:
4904:
4898:
4896:
4887:
4884:
4883:
4876:
4874:
4872:
4871:
4866:
4861:
4856:
4855:
4854:
4852:Trough battery
4849:
4839:
4834:
4828:
4826:
4822:
4821:
4816:
4814:
4813:
4806:
4799:
4791:
4785:
4784:
4779:
4768:
4763:
4756:
4755:External links
4753:
4750:
4749:
4725:
4708:
4672:
4667:10.4271/730248
4645:
4626:
4594:
4574:
4556:
4529:
4478:
4457:(2): 306–327.
4437:
4422:
4363:
4351:
4330:
4321:
4312:
4303:
4294:
4285:
4276:
4250:
4227:
4206:
4178:
4137:
4110:(4): 511–516.
4094:
4068:
4019:
3980:
3914:
3842:
3799:
3747:
3695:
3669:
3644:
3598:
3535:
3509:
3483:
3423:
3404:(5): 976–980.
3384:
3346:
3334:www.gizmag.com
3320:
3311:
3292:
3274:
3259:
3245:
3233:New York Times
3219:
3200:(3): 715–720.
3184:
3171:
3138:
3119:(1): 149–163.
3099:
3072:(3): 1501449.
3055:
3043:www.gizmag.com
3029:
2998:
2959:
2916:
2881:
2859:
2848:(3): 735–741.
2832:
2817:
2806:
2761:
2722:
2673:
2638:
2619:(3): 219–234.
2603:
2558:
2539:(4): 619–629.
2523:
2504:
2481:
2462:
2435:(3): 253–256.
2419:
2410:
2401:
2366:
2347:(4): 301–316.
2331:
2312:(2): 402–411.
2295:
2260:
2247:
2205:
2162:
2151:(3): A29–A31.
2135:
2116:
2114:" 14 May 2013.
2096:
2070:
2020:
2011:
2001:
1992:
1983:
1971:
1959:
1946:(4): 731–766.
1926:
1900:
1865:
1850:
1805:
1746:
1693:
1692:
1690:
1687:
1686:
1685:
1680:
1675:
1670:
1665:
1659:
1658:
1644:
1628:
1625:
1624:
1623:
1617:
1611:
1593:
1587:
1586:
1585:
1582:
1571:
1563:Load balancing
1548:
1545:
1542:
1541:
1539:
1532:
1530:
1527:
1523:
1522:
1520:
1518:
1515:
1512:
1506:
1505:
1503:
1500:
1497:
1491:
1490:
1487:
1480:
1477:
1474:
1468:
1467:
1465:
1463:
1461:
1459:
1455:
1454:
1452:
1450:
1448:
1446:
1440:
1439:
1437:
1435:
1432:
1429:
1423:
1422:
1419:
1412:
1410:
1408:
1402:
1401:
1399:
1392:
1389:
1386:
1380:
1379:
1376:
1369:
1366:
1363:
1355:
1354:
1351:
1344:
1341:
1338:
1330:
1329:
1326:
1323:
1320:
1317:
1313:
1312:
1309:
1302:
1300:
1297:
1293:
1292:
1289:
1282:
1280:
1277:
1276:Organic (2015)
1273:
1272:
1269:
1262:
1259:
1256:
1255:Organic (2013)
1252:
1251:
1248:
1241:
1240:< 1000
1238:
1235:
1231:
1230:
1228:
1226:
1223:
1220:
1213:
1212:
1210:
1208:
1205:
1202:
1195:
1194:
1192:
1190:
1187:
1184:
1177:
1176:
1174:
1172:
1169:
1166:
1156:
1155:
1153:
1146:
1143:
1140:
1130:
1129:
1127:
1120:
1117:
1114:
1104:
1103:
1100:
1097:
1094:
1091:
1081:
1078:
1030:
1027:
1004:
1001:
984:
981:
965:macromolecules
946:
943:
887:
884:
821:
807:
795:
774:
764:
761:
710:
707:
697:
693:
690:
673:
670:
663:
654:
642:
630:
619:
608:
583:
580:
567:
558:
546:
517:
514:
509:
505:
501:
497:
488:
485:
461:
442:
430:
396:
393:
389:energy storage
374:
373:
370:
367:
364:energy density
350:
349:
346:
343:self-discharge
339:
336:
333:
330:
323:
315:
312:
258:
255:
147:
144:
26:
24:
18:Flow batteries
14:
13:
10:
9:
6:
4:
3:
2:
5579:
5568:
5567:Battery types
5565:
5563:
5560:
5558:
5555:
5553:
5550:
5549:
5547:
5532:
5529:
5528:
5525:
5519:
5516:
5514:
5511:
5509:
5506:
5504:
5501:
5500:
5498:
5496:
5492:
5484:
5481:
5479:
5476:
5475:
5474:
5471:
5469:
5466:
5464:
5461:
5459:
5456:
5454:
5451:
5449:
5446:
5444:
5441:
5439:
5436:
5434:
5431:
5430:
5428:
5424:
5418:
5415:
5413:
5410:
5409:
5407:
5405:Biofuel cells
5403:
5397:
5394:
5392:
5389:
5387:
5384:
5382:
5379:
5377:
5374:
5372:
5369:
5367:
5364:
5362:
5359:
5358:
5356:
5352:
5346:
5343:
5341:
5338:
5336:
5333:
5331:
5328:
5326:
5323:
5322:
5320:
5316:
5312:
5305:
5300:
5298:
5293:
5291:
5286:
5285:
5282:
5270:
5267:
5265:
5262:
5260:
5257:
5255:
5252:
5250:
5247:
5245:
5242:
5240:
5237:
5235:
5232:
5230:
5227:
5225:
5222:
5221:
5219:
5215:
5209:
5206:
5204:
5201:
5199:
5196:
5195:
5193:
5187:
5181:
5178:
5176:
5173:
5171:
5168:
5166:
5163:
5161:
5160:Sodium–sulfur
5158:
5156:
5153:
5151:
5148:
5146:
5143:
5141:
5138:
5136:
5135:Potassium ion
5133:
5131:
5128:
5126:
5123:
5121:
5118:
5116:
5113:
5111:
5108:
5106:
5103:
5101:
5098:
5096:
5093:
5091:
5088:
5086:
5083:
5081:
5078:
5074:
5071:
5069:
5066:
5064:
5061:
5059:
5056:
5054:
5051:
5050:
5049:
5046:
5044:
5041:
5037:
5034:
5033:
5032:
5029:
5027:
5024:
5023:
5021:
5014:
5009:
5003:
5000:
4998:
4995:
4993:
4990:
4988:
4985:
4983:
4980:
4978:
4975:
4973:
4970:
4968:
4965:
4963:
4960:
4958:
4955:
4953:
4952:Lithium metal
4950:
4948:
4945:
4943:
4940:
4938:
4935:
4933:
4930:
4928:
4925:
4923:
4920:
4918:
4915:
4913:
4910:
4908:
4907:Aluminium–air
4905:
4903:
4900:
4899:
4897:
4890:
4885:
4880:
4870:
4867:
4865:
4862:
4860:
4857:
4853:
4850:
4848:
4845:
4844:
4843:
4840:
4838:
4835:
4833:
4832:Galvanic cell
4830:
4829:
4827:
4823:
4819:
4812:
4807:
4805:
4800:
4798:
4793:
4792:
4789:
4783:
4780:
4778:
4774:
4769:
4767:
4764:
4762:
4759:
4758:
4754:
4735:
4729:
4726:
4723:
4719:
4712:
4709:
4704:
4700:
4696:
4692:
4688:
4684:
4676:
4673:
4668:
4664:
4660:
4656:
4649:
4646:
4641:
4637:
4630:
4627:
4622:
4616:
4608:
4604:
4598:
4595:
4585:
4578:
4575:
4570:
4566:
4560:
4557:
4544:
4540:
4537:REDT Energy.
4533:
4530:
4525:
4521:
4517:
4513:
4509:
4505:
4501:
4497:
4493:
4489:
4482:
4479:
4473:
4468:
4464:
4460:
4456:
4452:
4448:
4441:
4438:
4433:
4426:
4423:
4418:
4414:
4409:
4404:
4399:
4394:
4390:
4386:
4382:
4378:
4374:
4367:
4364:
4361:
4355:
4352:
4340:
4334:
4331:
4325:
4322:
4316:
4313:
4307:
4304:
4298:
4295:
4289:
4286:
4280:
4277:
4265:
4261:
4254:
4251:
4239:
4238:
4231:
4228:
4223:
4222:
4217:
4210:
4207:
4202:
4198:
4194:
4190:
4182:
4179:
4173:
4168:
4164:
4160:
4156:
4152:
4148:
4141:
4138:
4133:
4129:
4125:
4121:
4117:
4113:
4109:
4105:
4098:
4095:
4083:
4079:
4072:
4069:
4064:
4060:
4055:
4050:
4046:
4042:
4039:(4): 040801.
4038:
4034:
4030:
4023:
4020:
4015:
4011:
4007:
4003:
3999:
3995:
3991:
3984:
3981:
3976:
3972:
3968:
3964:
3960:
3956:
3952:
3948:
3943:
3938:
3934:
3930:
3923:
3921:
3919:
3915:
3903:
3899:
3895:
3891:
3887:
3883:
3879:
3875:
3870:
3865:
3861:
3857:
3853:
3846:
3843:
3838:
3834:
3830:
3826:
3822:
3818:
3814:
3810:
3803:
3800:
3795:
3791:
3787:
3783:
3779:
3775:
3771:
3767:
3763:
3759:
3751:
3748:
3743:
3739:
3735:
3731:
3727:
3723:
3719:
3715:
3711:
3707:
3699:
3696:
3683:
3679:
3673:
3670:
3658:
3654:
3648:
3645:
3639:
3634:
3630:
3626:
3622:
3618:
3614:
3607:
3605:
3603:
3599:
3594:
3590:
3586:
3582:
3578:
3574:
3570:
3566:
3562:
3558:
3554:
3550:
3546:
3539:
3536:
3524:
3520:
3513:
3510:
3498:
3494:
3487:
3484:
3479:
3475:
3471:
3467:
3463:
3459:
3454:
3449:
3445:
3441:
3437:
3430:
3428:
3424:
3419:
3415:
3411:
3407:
3403:
3399:
3391:
3389:
3385:
3380:
3376:
3372:
3368:
3364:
3360:
3353:
3351:
3347:
3335:
3331:
3324:
3321:
3315:
3312:
3307:
3303:
3296:
3293:
3288:
3284:
3278:
3275:
3270:
3263:
3260:
3255:
3249:
3246:
3234:
3230:
3223:
3220:
3215:
3211:
3207:
3203:
3199:
3195:
3188:
3185:
3181:
3175:
3172:
3166:
3161:
3157:
3153:
3149:
3142:
3139:
3134:
3130:
3126:
3122:
3118:
3114:
3110:
3103:
3100:
3095:
3091:
3087:
3083:
3079:
3075:
3071:
3067:
3059:
3056:
3044:
3040:
3033:
3030:
3025:
3021:
3017:
3013:
3009:
3002:
2999:
2994:
2990:
2986:
2982:
2978:
2974:
2970:
2963:
2960:
2955:
2951:
2947:
2943:
2939:
2935:
2931:
2927:
2920:
2917:
2912:
2908:
2904:
2900:
2896:
2892:
2885:
2882:
2869:
2863:
2860:
2855:
2851:
2847:
2843:
2836:
2833:
2828:
2821:
2818:
2810:
2807:
2802:
2798:
2794:
2790:
2786:
2782:
2775:
2768:
2766:
2762:
2757:
2753:
2749:
2745:
2741:
2737:
2733:
2726:
2723:
2718:
2714:
2709:
2704:
2700:
2696:
2692:
2688:
2684:
2677:
2674:
2669:
2665:
2661:
2657:
2653:
2649:
2642:
2639:
2634:
2630:
2626:
2622:
2618:
2614:
2607:
2604:
2599:
2595:
2590:
2589:11577/3217695
2585:
2581:
2577:
2573:
2569:
2562:
2559:
2554:
2550:
2546:
2542:
2538:
2534:
2527:
2524:
2514:
2508:
2505:
2495:
2491:
2485:
2482:
2472:
2466:
2463:
2458:
2454:
2450:
2446:
2442:
2438:
2434:
2430:
2423:
2420:
2414:
2411:
2405:
2402:
2397:
2393:
2389:
2385:
2381:
2377:
2370:
2367:
2362:
2358:
2354:
2350:
2346:
2342:
2335:
2332:
2327:
2323:
2319:
2315:
2311:
2307:
2299:
2296:
2291:
2287:
2283:
2279:
2275:
2271:
2264:
2261:
2257:
2251:
2248:
2243:
2239:
2235:
2231:
2227:
2223:
2216:
2209:
2206:
2201:
2197:
2193:
2189:
2185:
2181:
2177:
2173:
2166:
2163:
2158:
2154:
2150:
2146:
2139:
2136:
2126:
2120:
2117:
2113:
2109:
2106:
2100:
2097:
2084:
2080:
2074:
2071:
2066:
2062:
2058:
2054:
2050:
2046:
2042:
2038:
2034:
2027:
2025:
2021:
2015:
2012:
2005:
2002:
1996:
1993:
1987:
1984:
1981:
1975:
1972:
1969:
1963:
1960:
1954:
1949:
1945:
1941:
1937:
1930:
1927:
1915:
1914:Energy Theory
1911:
1904:
1901:
1896:
1892:
1888:
1884:
1880:
1876:
1869:
1866:
1861:
1854:
1851:
1845:
1844:11577/2682306
1840:
1836:
1832:
1828:
1824:
1820:
1816:
1809:
1806:
1801:
1797:
1792:
1787:
1782:
1777:
1773:
1769:
1765:
1761:
1757:
1750:
1747:
1742:
1738:
1733:
1728:
1724:
1720:
1717:(4): 040801.
1716:
1712:
1708:
1701:
1699:
1695:
1688:
1684:
1681:
1679:
1676:
1674:
1671:
1669:
1666:
1664:
1661:
1660:
1656:
1650:
1645:
1642:
1641:Energy portal
1636:
1631:
1626:
1621:
1618:
1615:
1612:
1609:
1605:
1601:
1597:
1594:
1591:
1588:
1583:
1580:
1576:
1572:
1568:
1564:
1561:
1560:
1558:
1555:
1554:
1553:
1546:
1540:
1533:
1531:
1528:
1525:
1524:
1521:
1519:
1516:
1513:
1511:
1508:
1507:
1504:
1501:
1498:
1496:
1493:
1492:
1488:
1481:
1478:
1475:
1473:
1470:
1469:
1466:
1464:
1462:
1460:
1457:
1456:
1453:
1451:
1449:
1447:
1445:
1442:
1441:
1438:
1436:
1433:
1430:
1428:
1425:
1424:
1420:
1413:
1411:
1409:
1407:
1404:
1403:
1400:
1393:
1390:
1387:
1385:
1382:
1381:
1377:
1370:
1367:
1364:
1361:
1358:Metal-organic
1357:
1356:
1352:
1345:
1342:
1339:
1337:ferrocyanide
1336:
1333:Metal-organic
1332:
1331:
1327:
1324:
1321:
1318:
1315:
1314:
1310:
1303:
1301:
1298:
1295:
1294:
1290:
1283:
1281:
1278:
1275:
1274:
1270:
1263:
1260:
1257:
1254:
1253:
1249:
1242:
1239:
1236:
1233:
1232:
1229:
1227:
1225:< 200
1224:
1221:
1219:
1215:
1214:
1211:
1209:
1207:< 200
1206:
1203:
1201:
1197:
1196:
1193:
1191:
1189:< 200
1188:
1185:
1183:
1179:
1178:
1175:
1173:
1170:
1167:
1165:
1161:
1158:
1157:
1154:
1147:
1144:
1141:
1139:
1135:
1132:
1131:
1128:
1121:
1118:
1115:
1113:
1109:
1106:
1105:
1101:
1098:
1095:
1092:
1089:
1088:
1079:
1077:
1075:
1071:
1067:
1061:
1058:
1056:
1052:
1047:
1044:
1043:nanoparticles
1035:
1028:
1026:
1022:
1019:
1013:
1010:
1002:
1000:
998:
994:
990:
982:
980:
978:
974:
970:
966:
962:
957:
955:
951:
944:
942:
940:
936:
932:
928:
924:
920:
916:
912:
907:
905:
901:
897:
893:
885:
883:
880:
878:
873:
868:
866:
854:
850:
846:
838:
833:
818:
817:power density
814:
788:
787:sulfuric acid
784:
779:
777:
769:
763:Acidic AORFBs
762:
760:
758:
754:
750:
732:
728:
724:
719:
716:
708:
706:
702:
691:
689:
687:
683:
679:
678:metal hydride
671:
669:
600:
597:
593:
589:
581:
579:
576:
539:
538:metal hydride
535:
531:
527:
524:HFBs include
522:
515:
513:
495:
486:
484:
482:
478:
475:(Regenesys),
474:
470:
465:
457:
455:
451:
422:
418:
417:intercalation
414:
410:
406:
402:
394:
392:
390:
385:
383:
379:
371:
368:
365:
361:
360:
359:
356:
353:
347:
344:
340:
337:
334:
331:
328:
324:
321:
320:
319:
313:
311:
309:
304:
302:
301:power density
298:
297:
292:
286:
284:
280:
276:
272:
268:
264:
256:
254:
252:
248:
243:
240:
238:
234:
230:
225:
222:
216:
208:
203:
198:
195:
192:
189:
186:
183:
180:
177:
174:
171:
168:
165:
162:
159:
152:
145:
143:
141:
137:
133:
129:
125:
123:
119:
114:
112:
108:
104:
100:
94:
92:
88:
83:
81:
77:
72:
69:
67:
63:
59:
55:
51:
47:
43:
34:
30:
19:
5443:Flow battery
5442:
5175:Zinc–bromine
4982:Silver oxide
4917:Chromic acid
4889:Primary cell
4869:Voltaic pile
4847:Flow battery
4846:
4740:. Retrieved
4728:
4711:
4686:
4682:
4675:
4658:
4648:
4639:
4629:
4606:
4597:
4577:
4569:the original
4559:
4547:. Retrieved
4543:the original
4532:
4491:
4487:
4481:
4454:
4450:
4440:
4425:
4380:
4376:
4366:
4354:
4342:. Retrieved
4333:
4324:
4315:
4306:
4297:
4288:
4279:
4267:. Retrieved
4263:
4253:
4242:, retrieved
4236:
4230:
4219:
4209:
4192:
4188:
4181:
4154:
4150:
4140:
4107:
4103:
4097:
4087:24 September
4085:. Retrieved
4081:
4071:
4036:
4032:
4022:
3997:
3993:
3983:
3932:
3928:
3905:. Retrieved
3859:
3855:
3845:
3812:
3808:
3802:
3761:
3757:
3750:
3709:
3705:
3698:
3686:. Retrieved
3681:
3672:
3660:. Retrieved
3656:
3647:
3620:
3616:
3552:
3548:
3538:
3526:. Retrieved
3522:
3512:
3500:. Retrieved
3496:
3486:
3443:
3439:
3401:
3397:
3362:
3358:
3337:. Retrieved
3333:
3323:
3314:
3305:
3295:
3286:
3277:
3262:
3248:
3236:. Retrieved
3232:
3222:
3197:
3193:
3187:
3179:
3174:
3155:
3151:
3141:
3116:
3112:
3102:
3069:
3065:
3058:
3046:. Retrieved
3042:
3032:
3007:
3001:
2976:
2972:
2962:
2929:
2925:
2919:
2894:
2890:
2884:
2872:. Retrieved
2862:
2845:
2841:
2835:
2820:
2809:
2784:
2780:
2739:
2735:
2725:
2690:
2686:
2676:
2651:
2647:
2641:
2616:
2612:
2606:
2574:(4): 20–31.
2571:
2567:
2561:
2536:
2532:
2526:
2507:
2484:
2465:
2432:
2428:
2422:
2413:
2404:
2379:
2375:
2369:
2344:
2340:
2334:
2309:
2306:ChemPlusChem
2305:
2298:
2273:
2269:
2263:
2250:
2225:
2221:
2208:
2175:
2171:
2165:
2148:
2144:
2138:
2119:
2099:
2087:. Retrieved
2082:
2073:
2040:
2036:
2014:
2004:
1995:
1986:
1974:
1962:
1943:
1939:
1929:
1917:. Retrieved
1913:
1903:
1878:
1868:
1859:
1853:
1818:
1814:
1808:
1763:
1759:
1749:
1714:
1710:
1557:Grid storage
1550:
1547:Applications
1472:Zinc–bromine
1062:
1059:
1048:
1040:
1023:
1014:
1009:laminar flow
1006:
1003:Membraneless
986:
958:
948:
908:
904:ferrocyanide
890:Quinone and
889:
881:
869:
865:Ferrocyanide
849:ferrocyanide
834:
780:
766:
757:ferrocyanide
721:AORFBs used
720:
712:
703:
695:
675:
601:
586:A prototype
585:
526:zinc–bromine
523:
519:
490:
479:(IRFB), and
466:
458:
411:rather than
398:
386:
375:
357:
354:
351:
317:
305:
294:
287:
265:in which an
260:
244:
241:
237:vanadium RFB
226:
217:
199:
196:
193:
190:
187:
184:
181:
178:
175:
172:
169:
166:
163:
160:
157:
128:Cell voltage
126:
115:
95:
84:
73:
70:
66:Ion transfer
45:
42:flow battery
41:
39:
29:
5433:Blue energy
5264:Salt bridge
5249:Electrolyte
5180:Zinc–cerium
5165:Solid state
5150:Silver–zinc
5125:Nickel–zinc
5110:Nickel–iron
5085:Molten salt
5053:Dual carbon
5048:Lithium ion
5043:Lithium–air
5002:Zinc–carbon
4977:Silicon–air
4957:Lithium–air
3815:: 506–518.
3048:23 December
3008:Nano Energy
2874:13 February
2276:: 108–116.
2228:: 119–153.
1821:: 325–335.
1489:> 2,000
1066:lithium-ion
983:Other types
672:Proton flow
530:zinc–cerium
267:electrolyte
80:electrolyte
5562:Fuel cells
5546:Categories
5311:Fuel cells
5217:Cell parts
5208:Solar cell
5190:Other cell
5155:Sodium ion
5026:Automotive
4549:27 January
4157:: 97–106.
3688:6 December
3339:8 December
3238:10 January
2829:. R&D.
2089:12 October
1689:References
1080:Comparison
1016:they form
995:, and the
892:fluorenone
835:Replacing
755:–viologen/
727:an anolyte
592:polyiodide
532:, soluble
421:fuel cells
378:fuel cells
341:Little/no
314:Evaluation
140:electrodes
5254:Half-cell
5244:Electrode
5203:Fuel cell
5080:Metal–air
5031:Lead–acid
4947:Leclanché
4859:Fuel cell
4264:New Atlas
4195:: 91–99.
4063:2166-2746
4014:0013-4686
4000:: 41–50.
3942:1404.0917
3907:20 August
3869:1404.0917
3837:1364-0321
3593:234794555
3577:0036-8075
3523:New Atlas
3497:New Atlas
3478:101469730
3462:0013-4651
3418:2380-8195
3379:1754-5692
3271:. Gizmag.
3214:0013-4686
3086:1614-6840
2993:2050-7488
2756:0013-4651
2717:2352-152X
2693:: 69–90.
2192:0009-2665
2083:New Atlas
2057:0009-2665
1895:202210484
1741:2166-2746
1322:> 500
1296:MV-TEMPO
1234:Iron–iron
969:styrofoam
954:cellulose
877:phenazine
783:electrode
753:sulfonate
686:fuel cell
534:lead–acid
405:secondary
263:fuel cell
245:In 2022,
207:US 224404
87:fuel cell
76:electrode
62:dissolved
5531:Glossary
5495:Hydrogen
5234:Catalyst
5095:Nanowire
5090:Nanopore
5036:gel–VRLA
4997:Zinc–air
4902:Alkaline
4703:97853351
4640:Fox News
4615:cite web
4524:53218660
4516:30385552
4417:25309898
4269:9 August
4244:9 August
4132:97634258
3975:14719469
3967:23949161
3935:: 2346.
3902:14719469
3894:23949161
3862:: 2346.
3786:26503039
3734:26503039
3682:phys.org
3585:34016776
3502:18 March
3287:phys.org
3094:97838438
2954:97300070
2801:27853767
2598:28206437
2553:96195780
2457:97891309
2396:97853351
2361:97464125
2326:97168677
2256:preprint
2200:18557655
2108:Archived
1800:25309898
1627:See also
1362:bromine
1325:10 Wh/L
1218:chromium
1200:titanium
1164:hydrogen
1134:Hydrogen
1108:Hydrogen
977:oligomer
961:dialysis
950:Oligomer
945:Oligomer
931:chromium
917:such as
915:chelates
886:Alkaline
872:analytes
768:Quinones
749:Viologen
487:Vanadium
469:vanadium
283:adsorbed
5518:Vehicle
5513:Storage
5508:Station
5503:Economy
5354:By fuel
5239:Cathode
4992:Zamboni
4962:Mercury
4927:Daniell
4777:YouTube
4722:YouTube
4496:Bibcode
4488:Science
4459:Bibcode
4408:4174133
4385:Bibcode
4159:Bibcode
4112:Bibcode
4041:Bibcode
3947:Bibcode
3874:Bibcode
3817:Bibcode
3794:4393601
3766:Bibcode
3742:4393601
3714:Bibcode
3662:27 July
3625:Bibcode
3557:Bibcode
3549:Science
3470:1370440
3121:Bibcode
3012:Bibcode
2934:Bibcode
2899:Bibcode
2695:Bibcode
2656:Bibcode
2621:Bibcode
2437:Bibcode
2278:Bibcode
2230:Bibcode
2065:1799071
1823:Bibcode
1791:4174133
1768:Bibcode
1719:Bibcode
1600:voltage
1250:10,000
1160:Bromine
1102:Cycles
1090:Couple
935:bromine
911:ligands
853:polymer
832:Wh/L).
692:Organic
521:area.
481:uranium
296:in situ
231:at the
146:History
48:(after
5426:Others
5229:Binder
4987:Weston
4912:Bunsen
4742:21 May
4701:
4590:
4522:
4514:
4415:
4405:
4383:: 79.
4344:21 May
4130:
4061:
4012:
3973:
3965:
3900:
3892:
3835:
3792:
3784:
3758:Nature
3740:
3732:
3706:Nature
3591:
3583:
3575:
3528:26 May
3476:
3468:
3460:
3416:
3377:
3212:
3092:
3084:
2991:
2952:
2799:
2754:
2715:
2596:
2551:
2519:
2500:
2492:,
2477:
2455:
2394:
2359:
2324:
2198:
2190:
2131:
2063:
2055:
2009:(1988)
1893:
1798:
1788:
1766:: 79.
1739:
1536:
1502:~1,000
1484:
1479:~1,000
1421:2,000
1416:
1396:
1373:
1368:3,000
1348:
1343:2,000
1328:1,000
1306:
1286:
1266:
1261:13,000
1245:
1150:
1145:10,000
1124:
1119:15,000
991:, the
973:Nafion
896:ketone
875:TEMPO/
863:Wh/L.
861:
857:
847:) and
841:
830:
826:
745:
740:
735:
684:(PEM)
516:Hybrid
257:Design
247:Dalian
212:
97:lower
56:where
5224:Anode
4942:Grove
4922:Clark
4825:Types
4737:(PDF)
4699:S2CID
4520:S2CID
4451:Joule
4128:S2CID
3971:S2CID
3937:arXiv
3898:S2CID
3864:arXiv
3790:S2CID
3738:S2CID
3617:Joule
3589:S2CID
3474:S2CID
3113:Joule
3090:S2CID
2950:S2CID
2777:(PDF)
2594:S2CID
2549:S2CID
2453:S2CID
2392:S2CID
2357:S2CID
2322:S2CID
2218:(PDF)
1919:3 May
1891:S2CID
1579:solar
1570:cost.
1486:Wh/kg
1375:Wh/L
1365:2.13
1350:Wh/L
1340:1.62
1308:Wh/L
1299:1.25
1216:Iron–
1198:Iron–
1180:Iron–
1171:7,950
1152:Wh/kg
1126:Wh/kg
1049:In a
813:redox
401:redox
251:China
136:volts
44:, or
5259:Ions
4744:2023
4621:link
4551:2014
4512:PMID
4413:PMID
4346:2023
4271:2022
4246:2022
4089:2014
4059:ISSN
4010:ISSN
3963:PMID
3909:2013
3890:PMID
3833:ISSN
3782:PMID
3730:PMID
3690:2015
3664:2020
3581:PMID
3573:ISSN
3530:2021
3504:2022
3466:OSTI
3458:ISSN
3414:ISSN
3375:ISSN
3341:2015
3240:2014
3210:ISSN
3082:ISSN
3050:2015
2989:ISSN
2876:2014
2797:PMID
2752:ISSN
2713:ISSN
2196:PMID
2188:ISSN
2091:2022
2061:OSTI
2053:ISSN
1921:2024
1796:PMID
1737:ISSN
1575:wind
1567:grid
1538:Wh/L
1514:2.43
1499:1.82
1476:1.85
1434:~800
1431:1.54
1418:Wh/L
1398:Wh/L
1391:~800
1346:21.7
1319:0.9
1311:100
1291:100
1288:Wh/L
1268:Wh/L
1264:21.4
1247:Wh/L
1237:1.21
1222:1.07
1204:0.43
1186:0.62
1168:1.07
1148:1400
919:EDTA
773:PbSO
729:and
715:NaCl
588:zinc
552:and
542:VOSO
477:iron
450:NASA
399:The
362:Low
221:NASA
200:The
4932:Dry
4775:on
4720:on
4691:doi
4663:doi
4581:in
4504:doi
4492:362
4467:doi
4403:PMC
4393:doi
4197:doi
4193:228
4167:doi
4155:323
4120:doi
4049:doi
4002:doi
3998:267
3955:doi
3882:doi
3825:doi
3774:doi
3762:527
3722:doi
3710:527
3633:doi
3565:doi
3553:372
3448:doi
3444:163
3406:doi
3367:doi
3202:doi
3160:doi
3129:doi
3074:doi
3020:doi
2981:doi
2942:doi
2907:doi
2850:doi
2789:doi
2744:doi
2740:163
2703:doi
2664:doi
2652:196
2629:doi
2584:hdl
2576:doi
2541:doi
2445:doi
2384:doi
2349:doi
2314:doi
2286:doi
2238:doi
2180:doi
2176:108
2153:doi
2045:doi
2041:120
1948:doi
1883:doi
1839:hdl
1831:doi
1786:PMC
1776:doi
1727:doi
1590:UPS
1577:or
1529:1.2
1394:25
1388:1.4
1378:10
1353:75
1304:8.4
1284:7.1
1279:1.2
1271:10
1258:0.8
1182:tin
1142:1.4
1122:750
1116:1.1
845:KOH
785:in
771:Pb/
743:8.4
725:as
575:KOH
452:'s
415:or
277:to
5548::
4697:.
4687:19
4685:.
4657:.
4638:.
4617:}}
4613:{{
4605:.
4518:.
4510:.
4502:.
4490:.
4465:.
4453:.
4449:.
4411:.
4401:.
4391:.
4379:.
4375:.
4262:.
4218:.
4191:.
4165:.
4153:.
4149:.
4126:.
4118:.
4106:.
4080:.
4057:.
4047:.
4037:35
4035:.
4031:.
4008:.
3996:.
3969:.
3961:.
3953:.
3945:.
3931:.
3917:^
3896:.
3888:.
3880:.
3872:.
3858:.
3854:.
3831:.
3823:.
3813:70
3811:.
3788:.
3780:.
3772:.
3760:.
3736:.
3728:.
3720:.
3708:.
3680:.
3655:.
3631:.
3619:.
3615:.
3601:^
3587:.
3579:.
3571:.
3563:.
3551:.
3547:.
3521:.
3495:.
3472:.
3464:.
3456:.
3442:.
3438:.
3426:^
3412:.
3400:.
3387:^
3373:.
3361:.
3349:^
3332:.
3304:.
3285:.
3231:.
3208:.
3198:55
3196:.
3154:.
3150:.
3127:.
3115:.
3111:.
3088:.
3080:.
3068:.
3041:.
3018:.
2987:.
2975:.
2948:.
2940:.
2928:.
2905:.
2895:39
2893:.
2846:10
2844:.
2795:.
2785:52
2783:.
2779:.
2764:^
2750:.
2738:.
2734:.
2711:.
2701:.
2691:15
2689:.
2685:.
2662:.
2650:.
2627:.
2617:27
2615:.
2592:.
2582:.
2572:10
2570:.
2547:.
2537:15
2535:.
2451:.
2443:.
2433:37
2431:.
2390:.
2380:19
2378:.
2355:.
2345:50
2343:.
2320:.
2310:80
2308:.
2284:.
2274:16
2272:.
2258:).
2236:.
2226:11
2224:.
2220:.
2194:.
2186:.
2174:.
2147:.
2081:.
2059:.
2051:.
2039:.
2035:.
2023:^
1944:12
1942:.
1938:.
1912:.
1889:.
1877:.
1837:.
1829:.
1819:29
1817:.
1794:.
1784:.
1774:.
1762:.
1758:.
1735:.
1725:.
1715:35
1713:.
1709:.
1697:^
1534:60
1482:75
1414:50
1371:35
1243:20
1076:.
999:.
843:M
803:Br
791:Br
659:Br
638:Br
604:Zn
563:SO
528:,
471:,
384:.
310:.
249:,
142:.
82:.
40:A
5303:e
5296:t
5289:v
4810:e
4803:t
4796:v
4746:.
4705:.
4693::
4665::
4642:.
4623:)
4553:.
4526:.
4506::
4498::
4475:.
4469::
4461::
4455:1
4434:.
4419:.
4395::
4387::
4381:2
4348:.
4273:.
4224:.
4203:.
4199::
4175:.
4169::
4161::
4134:.
4122::
4114::
4108:1
4091:.
4065:.
4051::
4043::
4016:.
4004::
3977:.
3957::
3949::
3939::
3933:4
3911:.
3884::
3876::
3866::
3860:4
3839:.
3827::
3819::
3796:.
3776::
3768::
3744:.
3724::
3716::
3692:.
3666:.
3641:.
3635::
3627::
3621:3
3595:.
3567::
3559::
3532:.
3506:.
3480:.
3450::
3420:.
3408::
3402:1
3381:.
3369::
3363:9
3343:.
3308:.
3289:.
3242:.
3216:.
3204::
3168:.
3162::
3156:8
3135:.
3131::
3123::
3117:4
3096:.
3076::
3070:6
3052:.
3026:.
3022::
3014::
2995:.
2983::
2977:5
2956:.
2944::
2936::
2930:2
2913:.
2909::
2901::
2878:.
2856:.
2852::
2803:.
2791::
2758:.
2746::
2719:.
2705::
2697::
2670:.
2666::
2658::
2635:.
2631::
2623::
2600:.
2586::
2578::
2555:.
2543::
2459:.
2447::
2439::
2398:.
2386::
2363:.
2351::
2328:.
2316::
2292:.
2288::
2280::
2244:.
2240::
2232::
2202:.
2182::
2159:.
2155::
2149:2
2093:.
2067:.
2047::
1956:.
1950::
1923:.
1897:.
1885::
1847:.
1841::
1833::
1825::
1802:.
1778::
1770::
1764:2
1743:.
1729::
1721::
1360:–
1335:–
1162:–
1136:–
1110:–
822:2
801:/
796:2
775:4
698:2
655:2
650:I
643:–
631:3
626:I
615:I
590:–
568:4
559:2
554:H
547:4
510:5
508:O
506:2
502:5
500:O
498:2
462:2
443:2
438:O
436:/
431:2
426:H
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.