45:
27:
36:
339:
214:
758:
884:
563:
788:. As sugar is not generally soluble in solvents other than water, the development of high-yielding reactions has been slow and difficult; hence while furfural has been produced on a large scale since the 1920s, HMF was not produced on a commercial scale until over 90 years later. The first production plant coming online in 2013. Numerous synthetic technologies have been developed, including the use of
639:
861:. In these foods it is also slowly generated during storage. Acid conditions favour generation of HMF. HMF is a well known component of baked goods. Upon toasting bread, the amount increases from 14.8 (5 min.) to 2024.8 mg/kg (60 min). It is also formed during coffee roasting, with up to 769 mg/kg.
1298:
Commission
Implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the
961:
HMF is a natural component in heated food but usually present in low concentrations. The daily intake of HMF may underlie high variations due to individual consumption-patterns. It has been estimated that the intakes range between 4 mg - 30 mg per person per day, while an intake of up to
705:
HMF can form in sugar-containing food, particularly as a result of heating or cooking. Its formation has been the topic of significant study as HMF was regarded as being potentially carcinogenic to humans. However, so far in vivo genotoxicity was negative. No relevance for humans concerning
923:
Higher quantities of HMF are found naturally in coffee and dried fruit. Several types of roasted coffee contained between 300 – 2900 mg/kg HMF. Dried plums were found to contain up to 2200 mg/kg HMF. In dark beer 13.3 mg/kg were found, bakery-products contained between 4.1 –
957:
Depending on production-technology and storage, levels in food vary considerably. To evaluate the contribution of a food to HMF intake, its consumption-pattern has to be considered. Coffee is the food that has a very high relevance in terms of levels of HMF and quantities consumed.
1774:
Sousa, Andreia F.; Vilela, Carla; Fonseca, Ana C.; Matos, Marina; Freire, Carmen S. R.; Gruter, Gert-Jan M.; Coelho, Jorge F. J.; Silvestre, Armando J. D. (2015). "Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: a tribute to furan excellency".
916:-milk. Here, as well as in vinegars, jams, alcoholic products or biscuits, HMF can be used as an indicator for excess heat-treatment. For instance, fresh honey contains less than 15 mg/kg—depending on pH-value and temperature and age, and the
2187:
Husøy, T; Haugen, M; Murkovic, M; Jöbstl, D; Stølen, LH; Bjellaas, T; Rønningborg, C; Glatt, H; Alexander, J (2008). "Dietary exposure to 5-hydroxymethylfurfural from
Norwegian food and correlations with urine metabolites of short-term exposure".
2478:
Kang, Eun-Sil; Hong, Yeon-Woo; Chae, Da Won; Kim, Bora; Kim, Baekjin; Kim, Yong Jin; Cho, Jin Ku; Kim, Young Gyu (13 April 2015). "From
Lignocellulosic Biomass to Furans via 5-Acetoxymethylfurfural as an Alternative to 5-Hydroxymethylfurfural".
1020:
10751–1). Photometric test may be unspecific as they may detect also related substances, leading to higher results than HPLC-measurements. Test-kits for rapid analyses are also available (e.g. Reflectoquant HMF, Merck KGaA).
852:
HMF is practically absent in fresh food, but it is naturally generated in sugar-containing food during heat-treatments like drying or cooking. Along with many other flavor- and color-related substances, HMF is formed in the
571:
543:
1189:
Rosatella, Andreia A.; Simeonov, Svilen P.; Frade, Raquel F. M.; Afonso, Carlos A. M. (2011). "5-Hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications".
1140:
van Putten, Robert-Jan; van der Waal, Jan C.; de Jong, Ed; Rasrendra, Carolus B.; Heeres, Hero J.; de Vries, Johannes G. (2013). "Hydroxymethylfurfural, A Versatile
Platform Chemical Made from Renewable Resources".
983:
studies using transgenic sickle mice showed that orally administered 5HMF inhibits the formation of sickled cells in the blood. Under the development code Aes-103, HMF has been considered for the treatment of
1097:
The
Determination of HMF in Honey with an Evolution Array UV-Visible Spectrophotometer. Nicole Kreuziger Keppy and Michael W. Allen, Ph.D., Application note 51864, Thermo Fisher Scientific, Madison, WI, USA
1949:
Serra-Cayuela, A.; Jourdes, M.; Riu-Aumatell, M.; Buxaderas, S.; Teissedre, P.-L.; López-Tamames, E. (2014). "Kinetics of
Browning, Phenolics, and 5-Hydroxymethylfurfural in Commercial Sparkling Wines".
920:
requires that honey have less than 40 mg/kg HMF to guarantee that the honey has not undergone heating during processing, except for tropical honeys which must be below 80 mg/kg.
1228:
Abraham, Klaus; Gürtler, Rainer; Berg, Katharina; Heinemeyer, Gerhard; Lampen, Alfonso; Appel, Klaus E. (2011-04-04). "Toxicology and risk assessment of 5-Hydroxymethylfurfural in food".
617:
690:
of reducing sugars. It is a white low-melting solid (although commercial samples are often yellow) which is highly soluble in both water and organic solvents. The molecule consists of a
2254:
Abraham, Klaus; Gürtler, Rainer; Berg, Katharina; Heinemeyer, Gerhard; Lampen, Alfonso; Appel, Klaus E. (May 2011). "Toxicology and risk assessment of 5-Hydroxymethylfurfural in food".
2412:
Schultheiss, J.; Jensen, D.; Galensa, R. (2000). "Determination of aldehydes in food by high-performance liquid chromatography with biosensor coupling and micromembrane suppressors".
2062:
Ruiz-Matute, AI; Weiss, M; Sammataro, D; Finely, J; Sanz, ML (2010). "Carbohydrate composition of high-fructose corn syrups (HFCS) used for bee feeding: effect on honey composition".
1653:
Zakrzewska, Małgorzata E.; Bogel-Łukasik, Ewa; Bogel-Łukasik, Rafał (2011). "Ionic Liquid-Mediated
Formation of 5-Hydroxymethylfurfural—A Promising Biomass-Derived Building Block".
652:
1906:
Macheiner, Lukas; Schmidt, Anatol; Karpf, Franz; Mayer, Helmut K. (2021). "A novel UHPLC method for determining the degree of coffee roasting by analysis of furans".
1037:
for pentoses, the hydroxymethylfurfural from hexoses may give a muddy-brown or gray solution, but this is easily distinguishable from the green color of pentoses.
706:
carcinogenic and genotoxic effects can be derived. HMF is classified as a food improvement agent and is primarily being used in the food industry in form of a
2225:
Ramírez-Jiménez, A; Garcı́a-Villanova, Belén; Guerra-Hernández, Eduardo (2000). "Hydroxymethylfurfural and methylfurfural content of selected bakery products".
388:
1861:
Arribas-Lorenzo, G; Morales, FJ (2010). "Estimation of dietary intake of 5-hydroxymethylfurfural and related substances from coffee to
Spanish population".
2064:
1532:
Yuriy Román-Leshkov; Juben N. Chheda; James A. Dumesic (2006). "Phase
Modifiers Promote Efficient Production of Hydroxymethylfurfural from Fructose".
2152:
997:
1082:
2449:
Gaspar, Elvira M.S.M.; Lucena, Ana F.F. (2009). "Improved HPLC methodology for food control – furfurals and patulin as markers of quality".
500:
1985:
Pereira, V. (2011). "Evolution of 5-hydroxymethylfurfural (HMF) and furfural (F) in fortified wines submitted to overheating conditions".
1739:
Teong, Siew Ping; Yi, Guangshun; Zhang, Yugen (2014). "Hydroxymethylfurfural production from bioresources: past, present and future".
913:
353:
26:
2527:
812:
HMF itself has few applications. It can however be converted into other more useful compounds. Of these the most important is
1495:"Untersuchungen über Kohlenhydrate. I. Ueber die bei Einwirkung von Schwefelsäure auf Zucker entstehende Säure (Levulinsäure)"
1698:"Highly Selective and Near-Quantitative Conversion of Fructose to 5-Hydroxymethylfurfural Using Mildly Acidic Ionic Liquids"
1299:
Council and repealing
Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC Text with EEA relevance
938:(HFCS), levels around 20 mg/kg HMF were found, increasing during storage or heating. This is a problem for American
659:
2190:
1863:
1000:
with UV-detection is the reference-method (e.g. DIN 10751–3). Classic methods for the quantification of HMF in food use
607:
296:
804:
to either remove the HMF before it reacts further or to otherwise promote its formation and inhibit its decomposition.
710:
as a biomarker as well as a flavoring agent for food products. It is also produced industrially on a modest scale as a
2414:
2227:
1397:
837:
317:
562:
1066:
813:
221:
2451:
2299:"5-hydroxymethyl-2-furfural modifies intracellular sickle haemoglobin and inhibits sickling of red blood cells"
935:
793:
974:
in humans is 5-hydroxymethyl-2-furoic acid (HMFA), also known as Sumiki's acid, which is excreted in urine.
2297:
Abdulmalik, O; Safo, MK; Chen, Q; Yang, J; Brugnara, C; Ohene-Frempong, K; Abraham, DJ; Asakura, T (2005).
1272:
784:
The classical approach tends to suffer from poor yields as HMF continues to react in aqueous acid, forming
209:
743:
2101:"5-Hydroxymethylfurfural (HMF) levels in honey and other food products: effects on bees and human health"
1099:
171:
2150:
Murkovic, M; Pichler, N (2006). "Analysis of 5-hydroxymethylfurfual in coffee, dried fruits and urine".
797:
585:
555:
530:
119:
44:
757:
1823:
1543:
893:
770:
687:
621:
57:
1040:
Acetoxymethyl furfural (AMF) is also bio-derived green platform chemicals as an alternative to HMF.
2346:
985:
841:
699:
334:
85:
883:
2532:
2328:
1567:
1378:
917:
888:
613:
131:
1812:"Advances in polymer precursors and bio-based polymers synthesized from 5-hydroxymethylfurfural"
35:
1320:
1122:
2504:
2496:
2431:
2394:
2320:
2279:
2271:
2207:
2169:
2132:
2081:
2039:
1967:
1931:
1923:
1888:
1792:
1756:
1678:
1670:
1635:
1559:
1534:
1514:
1475:
1418:
1370:
1253:
1245:
1207:
1166:
1158:
1078:
1030:
1001:
854:
825:
817:
739:
1063:
Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book)
2488:
2460:
2423:
2384:
2310:
2263:
2236:
2199:
2161:
2122:
2112:
2073:
2031:
2002:
1994:
1959:
1915:
1880:
1872:
1841:
1831:
1784:
1748:
1719:
1709:
1662:
1627:
1598:
1551:
1506:
1465:
1455:
1410:
1360:
1237:
1199:
1150:
1070:
1005:
683:
411:
191:
305:
1013:
865:
240:
151:
1827:
1547:
338:
213:
95:
2127:
2100:
2035:
1444:"Synthesis, chemistry and applications of 5-hydroxymethyl-furfural and its derivatives"
1398:"Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering"
1034:
858:
801:
785:
723:
711:
630:
2427:
2240:
2521:
2315:
2298:
1470:
1382:
1296:
1118:
928:
833:
789:
707:
490:
480:
202:
2332:
1571:
2464:
1919:
873:
285:
1998:
1618:
Brownlee, Harold J.; Miner, Carl S. (1948). "Industrial Development of Furfural".
1427:
1460:
1443:
1004:. The method according to White is a differential UV-photometry with and without
1074:
599:
1846:
828:(DMF), a liquid that is a potential biofuel with a greater energy content than
2203:
2117:
1876:
1402:
971:
939:
869:
829:
821:
726:
from sugar and sulfuric acid. This remains the classical route, with 6-carbon
439:
182:
2500:
2389:
2372:
2275:
1927:
1796:
1760:
1696:
Eminov, Sanan; Wilton-Ely, James D. E. T.; Hallett, Jason P. (2 March 2014).
1674:
1639:
1603:
1586:
1518:
1510:
1479:
1374:
1249:
1211:
1162:
595:
1555:
1009:
947:
840:. Acid-catalysed hydrolysis converts HMF into gamma-hydroxyvaleric acid and
746:
is produced instead of HMF. Similar chemistry is seen with 5-carbon sugars (
2508:
2492:
2435:
2324:
2283:
2267:
2211:
2173:
2165:
2136:
2085:
2043:
1971:
1935:
1892:
1724:
1682:
1563:
1422:
1257:
1241:
1170:
1008:-reduction of HMF. Winkler photometric method is a colour-reaction using p-
1365:
1348:
2398:
2022:
Amerine, Maynard A. (1948). "Hydroxymethylfurfural in California Wines".
2007:
751:
747:
735:
695:
525:
1631:
1884:
1836:
1811:
1788:
1752:
1203:
979:
950:, and HMF is toxic to them. Adding bases such as soda ash or potash to
731:
470:
272:
222:
2077:
1963:
1714:
1697:
1666:
1414:
1154:
722:
HMF was first reported in 1875 as an intermediate in the formation of
591:
943:
942:
because they use HFCS as a source of sugar when there are not enough
877:
727:
162:
1494:
629:
Except where otherwise noted, data are given for materials in their
495:
114 to 116 °C (237 to 241 °F; 387 to 389 K) (1 mbar)
962:
350 mg can result from, e.g., beverages made from dried plums.
260:
909:
905:
882:
756:
691:
142:
118:
108:
1349:"AVA Biochem: commercialising renewable platform chemical 5-HMF"
460:
251:
2373:"Spectrophotometric method for hydroxymethylfurfural in honey"
2099:
Shapla, UM; Solayman, M; Alam, N; Khalil, MI; Gan, SH (2018).
1017:
1135:
1133:
1131:
977:
HMF bind intracellular sickle hemoglobin (HbS). Preliminary
322:
951:
714:
feedstock for the production of fuels and other chemicals.
2377:
Journal of the Association of Official Analytical Chemists
1029:
HMF is an intermediate in the titration of hexoses in the
1396:
Huber, George W.; Iborra, Sara; Corma, Avelino (2006).
1184:
1182:
1180:
647:
485:
30 to 34 °C (86 to 93 °F; 303 to 307 K)
2351:
National Center for Advancing Translational Sciences
1816:
Journal of Polymer Science Part A: Polymer Chemistry
1273:"EU Food Improvement Agents - PubChem Data Source"
1810:Zhang, Daihui; Dumont, Marie-Josée (1 May 2017).
738:undergoing acid catalyzed poly-dehydration. When
362:InChI=1S/C6H6O3/c7-3-5-1-2-6(4-8)9-5/h1-3,8H,4H2
284:
816:, which has been proposed as a replacement for
372:InChI=1/C6H6O3/c7-3-5-1-2-6(4-8)9-5/h1-3,8H,4H2
94:
2057:
2055:
2053:
1587:"Synthesis of 5-(Hydroxymethyl)furfural (HMF)"
1342:
1340:
8:
1493:Grote, A. Freiherrn V.; Tollens, B. (1875).
1702:ACS Sustainable Chemistry & Engineering
1056:
1054:
1052:
876:and those sweetened with grape concentrate
2065:Journal of Agricultural and Food Chemistry
337:
212:
190:
18:
2388:
2314:
2126:
2116:
2006:
1845:
1835:
1723:
1713:
1602:
1469:
1459:
1364:
750:), which react with aqueous acid to form
304:
1437:
1435:
896:, Australia. The fruiting body contains
2256:Molecular Nutrition & Food Research
2153:Molecular Nutrition & Food Research
1230:Molecular Nutrition & Food Research
1114:
1112:
1110:
1108:
1048:
868:time−temperature marker, especially in
393:
358:
333:
1620:Industrial & Engineering Chemistry
203:
954:the HFCS slows the formation of HMF.
365:Key: NOEGNKMFWQHSLB-UHFFFAOYSA-N
170:
150:
62:5-(Hydroxymethyl)furan-2-carbaldehyde
7:
1223:
1221:
904:HMF can be found in low amounts in
375:Key: NOEGNKMFWQHSLB-UHFFFAOYAB
275:
259:
2036:10.1111/j.1365-2621.1948.tb16621.x
1499:Justus Liebig's Annalen der Chemie
14:
2347:"Aes-103 for Sickle Cell Disease"
1321:"5-(Hydroxymethyl)-2-furaldehyde"
2316:10.1111/j.1365-2141.2004.05332.x
637:
561:
423:
43:
34:
25:
633:(at 25 °C , 100 kPa).
71:5-(Hydroxymethyl)-2-furaldehyde
2465:10.1016/j.foodchem.2008.11.097
2303:British Journal of Haematology
1920:10.1016/j.foodchem.2020.128165
1353:Green Processing and Synthesis
1067:The Royal Society of Chemistry
429:
417:
1:
2428:10.1016/S0021-9673(99)01086-9
2241:10.1016/S0963-9969(00)00102-2
1999:10.1016/j.foodres.2010.11.011
769:, two intermediate stages of
521:Related furan-2-carbaldehydes
2191:Food and Chemical Toxicology
1864:Food and Chemical Toxicology
1461:10.3998/ark.5550190.0002.102
844:, with loss of formic acid.
2415:Journal of Chromatography A
2228:Food Research International
1987:Food Research International
1075:10.1039/9781849733069-FP001
918:codex alimentarius standard
838:2,5-bis(hydroxymethyl)furan
2549:
824:. HMF can be converted to
814:2,5-furandicarboxylic acid
2371:White Jr., J. W. (1979).
2204:10.1016/j.fct.2008.09.048
2118:10.1186/s13065-018-0408-3
1877:10.1016/j.fct.2009.11.046
1585:Simeonov, Svilen (2016).
1471:2027/spo.5550190.0002.102
1123:5-(Hydroxymethyl)furfural
680:5-(hydroxymethyl)furfural
627:
542:
537:
514:
404:
384:
349:
78:
73:5-(Hydroxymethyl)furfural
68:
56:
51:
42:
33:
24:
1604:10.15227/orgsyn.093.0029
1511:10.1002/jlac.18751750113
1347:Kläusli, Thomas (2014).
1325:pubchem.ncbi.nlm.nih.gov
1277:pubchem.ncbi.nlm.nih.gov
936:high-fructose corn syrup
794:liquid-liquid extraction
718:Production and reactions
608:Precautionary statements
455:Low melting white solid
2528:Hydroxymethyl compounds
2024:Journal of Food Science
1556:10.1126/science.1126337
2493:10.1002/cssc.201403252
2390:10.1093/jaoac/62.3.509
2268:10.1002/mnfr.201000564
2166:10.1002/mnfr.200500262
1442:Lewkowski, J. (2001).
1242:10.1002/mnfr.201000564
901:
781:
744:5-chloromethylfurfural
694:ring, containing both
20:Hydroxymethylfurfural
1428:MIT Technology Review
1366:10.1515/gps-2014-0029
1069:. 2014. p. 911.
898:hydroxymethylfurfural
886:
820:in the production of
798:reactive distillation
760:
672:Hydroxymethylfurfural
531:Methoxymethylfurfural
924:151 mg/kg HMF.
894:Cooktown, Queensland
802:solid acid catalysts
58:Preferred IUPAC name
1952:J. Agric. Food Chem
1847:20.500.11794/100964
1828:2017JPoSA..55.1478Z
1632:10.1021/ie50458a005
1548:2006Sci...312.1933R
1542:(5782): 1933–1937.
986:sickle cell disease
927:It can be found in
842:gamma-valerolactone
702:functional groups.
447: g·mol
132:Beilstein Reference
21:
1837:10.1002/pola.28527
1789:10.1039/C5PY00686D
1753:10.1039/c3gc42018c
1204:10.1039/c0gc00401d
902:
889:Phallus indusiatus
857:as well as during
848:Occurrence in food
782:
660:Infobox references
515:Related compounds
19:
2078:10.1021/jf100758x
1964:10.1021/jf403281y
1783:(33): 5961–5983.
1715:10.1021/sc400553q
1667:10.1021/cr100171a
1591:Organic Syntheses
1415:10.1021/cr068360d
1155:10.1021/cr300182k
1119:Sigma-Aldrich Co.
1084:978-0-85404-182-4
1033:. In the related
855:Maillard reaction
826:2,5-dimethylfuran
818:terephthalic acid
765:, fructofuranose
740:hydrochloric acid
678:), also known as
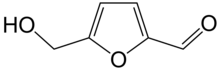
668:Chemical compound
666:
665:
586:Hazard statements
465:Buttery, caramel
318:CompTox Dashboard
120:Interactive image
16:Chemical compound
2540:
2513:
2512:
2487:(7): 1179–1188.
2475:
2469:
2468:
2446:
2440:
2439:
2409:
2403:
2402:
2392:
2368:
2362:
2361:
2359:
2358:
2343:
2337:
2336:
2318:
2294:
2288:
2287:
2251:
2245:
2244:
2222:
2216:
2215:
2198:(12): 3697–702.
2184:
2178:
2177:
2147:
2141:
2140:
2130:
2120:
2096:
2090:
2089:
2059:
2048:
2047:
2019:
2013:
2012:
2010:
1982:
1976:
1975:
1958:(5): 1159–1166.
1946:
1940:
1939:
1914:(Pt 1): 128165.
1903:
1897:
1896:
1858:
1852:
1851:
1849:
1839:
1822:(9): 1478–1492.
1807:
1801:
1800:
1771:
1765:
1764:
1736:
1730:
1729:
1727:
1717:
1693:
1687:
1686:
1655:Chemical Reviews
1650:
1644:
1643:
1615:
1609:
1608:
1606:
1582:
1576:
1575:
1529:
1523:
1522:
1505:(1–2): 181–204.
1490:
1484:
1483:
1473:
1463:
1439:
1430:
1426:
1393:
1387:
1386:
1368:
1344:
1335:
1334:
1332:
1331:
1316:
1310:
1309:
1308:
1307:
1293:
1287:
1286:
1284:
1283:
1268:
1262:
1261:
1225:
1216:
1215:
1186:
1175:
1174:
1149:(3): 1499–1597.
1143:Chemical Reviews
1137:
1126:
1116:
1103:
1095:
1089:
1088:
1061:"Front Matter".
1058:
1006:sodium bisulfite
946:sources to feed
934:HMF can form in
777:and finally HMF
684:organic compound
650:
644:
641:
640:
623:
619:
615:
601:
597:
593:
565:
446:
431:
425:
419:
412:Chemical formula
342:
341:
326:
324:
308:
288:
277:
263:
241:Gmelin Reference
224:
216:
205:
194:
174:
154:
122:
98:
47:
38:
29:
22:
2548:
2547:
2543:
2542:
2541:
2539:
2538:
2537:
2518:
2517:
2516:
2477:
2476:
2472:
2448:
2447:
2443:
2422:(1–2): 233–42.
2411:
2410:
2406:
2370:
2369:
2365:
2356:
2354:
2345:
2344:
2340:
2296:
2295:
2291:
2253:
2252:
2248:
2224:
2223:
2219:
2186:
2185:
2181:
2149:
2148:
2144:
2098:
2097:
2093:
2072:(12): 7317–22.
2061:
2060:
2051:
2021:
2020:
2016:
1984:
1983:
1979:
1948:
1947:
1943:
1905:
1904:
1900:
1860:
1859:
1855:
1809:
1808:
1804:
1773:
1772:
1768:
1741:Green Chemistry
1738:
1737:
1733:
1695:
1694:
1690:
1652:
1651:
1647:
1617:
1616:
1612:
1584:
1583:
1579:
1531:
1530:
1526:
1492:
1491:
1487:
1441:
1440:
1433:
1395:
1394:
1390:
1346:
1345:
1338:
1329:
1327:
1318:
1317:
1313:
1305:
1303:
1295:
1294:
1290:
1281:
1279:
1270:
1269:
1265:
1227:
1226:
1219:
1192:Green Chemistry
1188:
1187:
1178:
1139:
1138:
1129:
1117:
1106:
1096:
1092:
1085:
1060:
1059:
1050:
1046:
1027:
1014:barbituric acid
994:
968:
850:
810:
773:(not isolated)
761:fructopyranose
720:
669:
662:
657:
656:
655: ?)
646:
642:
638:
634:
610:
588:
574:
558:
528:
522:
506:
444:
434:
428:
422:
414:
400:
397:
392:
391:
380:
377:
376:
373:
367:
366:
363:
357:
356:
345:
327:
320:
311:
291:
278:
266:
243:
234:
197:
177:
157:
134:
125:
112:
101:
88:
74:
72:
64:
63:
17:
12:
11:
5:
2546:
2544:
2536:
2535:
2530:
2520:
2519:
2515:
2514:
2470:
2452:Food Chemistry
2441:
2404:
2383:(3): 509–514.
2363:
2338:
2289:
2262:(5): 667–678.
2246:
2217:
2179:
2142:
2091:
2049:
2030:(3): 264–269.
2014:
1977:
1941:
1908:Food Chemistry
1898:
1853:
1802:
1766:
1731:
1708:(4): 978–981.
1688:
1661:(2): 397–417.
1645:
1626:(2): 201–204.
1610:
1577:
1524:
1485:
1431:
1409:(9): 4044–98.
1388:
1359:(3): 235–236.
1336:
1311:
1288:
1263:
1236:(5): 667–678.
1217:
1176:
1127:
1104:
1090:
1083:
1047:
1045:
1042:
1031:Molisch's test
1026:
1023:
993:
992:Quantification
990:
967:
964:
859:caramelization
849:
846:
809:
806:
786:levulinic acid
724:levulinic acid
719:
716:
712:carbon-neutral
686:formed by the
667:
664:
663:
658:
636:
635:
631:standard state
628:
625:
624:
618:P305+P351+P338
611:
606:
603:
602:
589:
584:
581:
580:
575:
570:
567:
566:
559:
554:
551:
550:
540:
539:
535:
534:
523:
520:
517:
516:
512:
511:
508:
504:
497:
496:
493:
487:
486:
483:
477:
476:
473:
467:
466:
463:
457:
456:
453:
449:
448:
442:
436:
435:
432:
426:
420:
415:
410:
407:
406:
402:
401:
399:
398:
396:c1cc(oc1CO)C=O
395:
387:
386:
385:
382:
381:
379:
378:
374:
371:
370:
368:
364:
361:
360:
352:
351:
350:
347:
346:
344:
343:
330:
328:
316:
313:
312:
310:
309:
301:
299:
293:
292:
290:
289:
281:
279:
271:
268:
267:
265:
264:
256:
254:
248:
247:
244:
239:
236:
235:
233:
232:
228:
226:
218:
217:
207:
199:
198:
196:
195:
187:
185:
179:
178:
176:
175:
167:
165:
159:
158:
156:
155:
147:
145:
139:
138:
135:
130:
127:
126:
124:
123:
115:
113:
106:
103:
102:
100:
99:
91:
89:
84:
81:
80:
76:
75:
70:
66:
65:
61:
60:
54:
53:
49:
48:
40:
39:
31:
30:
15:
13:
10:
9:
6:
4:
3:
2:
2545:
2534:
2531:
2529:
2526:
2525:
2523:
2510:
2506:
2502:
2498:
2494:
2490:
2486:
2482:
2474:
2471:
2466:
2462:
2458:
2454:
2453:
2445:
2442:
2437:
2433:
2429:
2425:
2421:
2417:
2416:
2408:
2405:
2400:
2396:
2391:
2386:
2382:
2378:
2374:
2367:
2364:
2352:
2348:
2342:
2339:
2334:
2330:
2326:
2322:
2317:
2312:
2309:(4): 552–61.
2308:
2304:
2300:
2293:
2290:
2285:
2281:
2277:
2273:
2269:
2265:
2261:
2257:
2250:
2247:
2242:
2238:
2234:
2230:
2229:
2221:
2218:
2213:
2209:
2205:
2201:
2197:
2193:
2192:
2183:
2180:
2175:
2171:
2167:
2163:
2159:
2155:
2154:
2146:
2143:
2138:
2134:
2129:
2124:
2119:
2114:
2110:
2106:
2102:
2095:
2092:
2087:
2083:
2079:
2075:
2071:
2067:
2066:
2058:
2056:
2054:
2050:
2045:
2041:
2037:
2033:
2029:
2025:
2018:
2015:
2009:
2008:10400.14/7635
2004:
2000:
1996:
1992:
1988:
1981:
1978:
1973:
1969:
1965:
1961:
1957:
1953:
1945:
1942:
1937:
1933:
1929:
1925:
1921:
1917:
1913:
1909:
1902:
1899:
1894:
1890:
1886:
1882:
1878:
1874:
1870:
1866:
1865:
1857:
1854:
1848:
1843:
1838:
1833:
1829:
1825:
1821:
1817:
1813:
1806:
1803:
1798:
1794:
1790:
1786:
1782:
1778:
1770:
1767:
1762:
1758:
1754:
1750:
1746:
1742:
1735:
1732:
1726:
1725:10044/1/31478
1721:
1716:
1711:
1707:
1703:
1699:
1692:
1689:
1684:
1680:
1676:
1672:
1668:
1664:
1660:
1656:
1649:
1646:
1641:
1637:
1633:
1629:
1625:
1621:
1614:
1611:
1605:
1600:
1596:
1592:
1588:
1581:
1578:
1573:
1569:
1565:
1561:
1557:
1553:
1549:
1545:
1541:
1537:
1536:
1528:
1525:
1520:
1516:
1512:
1508:
1504:
1500:
1496:
1489:
1486:
1481:
1477:
1472:
1467:
1462:
1457:
1453:
1449:
1445:
1438:
1436:
1432:
1429:
1424:
1420:
1416:
1412:
1408:
1405:
1404:
1399:
1392:
1389:
1384:
1380:
1376:
1372:
1367:
1362:
1358:
1354:
1350:
1343:
1341:
1337:
1326:
1322:
1315:
1312:
1301:
1300:
1292:
1289:
1278:
1274:
1267:
1264:
1259:
1255:
1251:
1247:
1243:
1239:
1235:
1231:
1224:
1222:
1218:
1213:
1209:
1205:
1201:
1197:
1193:
1185:
1183:
1181:
1177:
1172:
1168:
1164:
1160:
1156:
1152:
1148:
1144:
1136:
1134:
1132:
1128:
1124:
1120:
1115:
1113:
1111:
1109:
1105:
1101:
1094:
1091:
1086:
1080:
1076:
1072:
1068:
1065:. Cambridge:
1064:
1057:
1055:
1053:
1049:
1043:
1041:
1038:
1036:
1032:
1024:
1022:
1019:
1015:
1011:
1007:
1003:
999:
991:
989:
987:
982:
981:
975:
973:
965:
963:
959:
955:
953:
949:
945:
941:
937:
932:
930:
929:glucose syrup
925:
921:
919:
915:
911:
907:
899:
895:
891:
890:
885:
881:
879:
875:
871:
867:
864:It is a good
862:
860:
856:
847:
845:
843:
839:
836:of HMF gives
835:
834:Hydrogenation
831:
827:
823:
819:
815:
807:
805:
803:
799:
795:
792:, continuous
791:
790:ionic liquids
787:
780:
776:
772:
768:
764:
759:
755:
753:
749:
745:
741:
737:
733:
729:
725:
717:
715:
713:
709:
708:food additive
703:
701:
697:
693:
689:
685:
681:
677:
673:
661:
654:
649:
632:
626:
612:
609:
605:
604:
590:
587:
583:
582:
579:
576:
573:
569:
568:
564:
560:
557:
553:
552:
548:
546:
541:
536:
533:
532:
527:
524:
519:
518:
513:
509:
502:
499:
498:
494:
492:
491:Boiling point
489:
488:
484:
482:
481:Melting point
479:
478:
474:
472:
469:
468:
464:
462:
459:
458:
454:
451:
450:
443:
441:
438:
437:
416:
413:
409:
408:
403:
394:
390:
383:
369:
359:
355:
348:
340:
336:
335:DTXSID3030428
332:
331:
329:
319:
315:
314:
307:
303:
302:
300:
298:
295:
294:
287:
283:
282:
280:
274:
270:
269:
262:
258:
257:
255:
253:
250:
249:
245:
242:
238:
237:
230:
229:
227:
225:
220:
219:
215:
211:
208:
206:
204:ECHA InfoCard
201:
200:
193:
189:
188:
186:
184:
181:
180:
173:
169:
168:
166:
164:
161:
160:
153:
149:
148:
146:
144:
141:
140:
136:
133:
129:
128:
121:
117:
116:
114:
110:
105:
104:
97:
93:
92:
90:
87:
83:
82:
77:
67:
59:
55:
50:
46:
41:
37:
32:
28:
23:
2484:
2480:
2473:
2456:
2450:
2444:
2419:
2413:
2407:
2380:
2376:
2366:
2355:. Retrieved
2353:. 2015-03-18
2350:
2341:
2306:
2302:
2292:
2259:
2255:
2249:
2232:
2226:
2220:
2195:
2189:
2182:
2160:(9): 842–6.
2157:
2151:
2145:
2108:
2104:
2094:
2069:
2063:
2027:
2023:
2017:
1990:
1986:
1980:
1955:
1951:
1944:
1911:
1907:
1901:
1871:(2): 644–9.
1868:
1862:
1856:
1819:
1815:
1805:
1780:
1776:
1769:
1744:
1740:
1734:
1705:
1701:
1691:
1658:
1654:
1648:
1623:
1619:
1613:
1594:
1590:
1580:
1539:
1533:
1527:
1502:
1498:
1488:
1451:
1447:
1406:
1401:
1391:
1356:
1352:
1328:. Retrieved
1324:
1314:
1304:, retrieved
1302:, 2012-10-02
1297:
1291:
1280:. Retrieved
1276:
1266:
1233:
1229:
1195:
1191:
1146:
1142:
1093:
1062:
1039:
1028:
995:
978:
976:
969:
960:
956:
933:
926:
922:
903:
897:
887:
866:wine storage
863:
851:
811:
783:
778:
774:
766:
762:
721:
704:
679:
675:
671:
670:
577:
544:
529:
172:ChEMBL185885
152:CHEBI:412516
79:Identifiers
69:Other names
2481:ChemSusChem
2459:(4): 1576.
2235:(10): 833.
2105:Chem Cent J
1885:10261/82147
1777:Polym. Chem
1747:(4): 2015.
1035:Bial's test
870:sweet wines
808:Derivatives
771:dehydration
688:dehydration
572:Signal word
452:Appearance
405:Properties
210:100.000.595
2522:Categories
2357:2022-01-20
1403:Chem. Rev.
1330:2018-06-25
1306:2018-06-25
1282:2018-06-25
1198:(4): 754.
1044:References
1002:photometry
972:metabolite
966:Biomedical
952:neutralize
940:beekeepers
830:bioethanol
822:polyesters
734:) such as
556:Pictograms
475:1.29 g/cm
440:Molar mass
306:70ETD81LF0
183:ChemSpider
107:3D model (
86:CAS Number
2533:Furfurals
2501:1864-564X
2276:1613-4133
2111:(1): 35.
1993:: 71–76.
1928:0308-8146
1797:1759-9954
1761:1463-9262
1675:0009-2665
1640:0019-7866
1597:: 29–36.
1519:0075-4617
1480:1424-6376
1454:: 17–54.
1383:100848139
1375:2191-9550
1319:Pubchem.
1271:PubChem.
1250:1613-4125
1212:1463-9262
1163:0009-2665
1010:toluidine
948:honeybees
547:labelling
231:200-654-9
223:EC Number
2509:25619448
2436:10890522
2333:22342114
2325:15686467
2284:21462333
2212:18929614
2174:16917810
2137:29619623
2086:20491475
2044:18870652
1972:24444020
1936:33038777
1893:20005914
1683:20973468
1572:38432592
1564:16809536
1423:16967928
1258:21462333
1171:23394139
970:A major
908:, fruit-
872:such as
752:furfural
748:pentoses
742:is used
736:fructose
696:aldehyde
682:, is an
538:Hazards
526:Furfural
2128:5884753
1824:Bibcode
1544:Bibcode
1535:Science
1448:Arkivoc
1100:article
996:Today,
980:in vivo
874:Madeira
732:hexoses
700:alcohol
653:what is
651: (
578:Warning
510:284 nm
471:Density
445:126.111
273:PubChem
246:278693
137:110889
96:67-47-0
2507:
2499:
2434:
2399:479072
2397:
2331:
2323:
2282:
2274:
2210:
2172:
2135:
2125:
2084:
2042:
1970:
1934:
1926:
1891:
1795:
1759:
1681:
1673:
1638:
1570:
1562:
1517:
1478:
1421:
1381:
1373:
1256:
1248:
1210:
1169:
1161:
1081:
944:nectar
910:juices
878:arrope
728:sugars
648:verify
645:
501:UV-vis
389:SMILES
286:237332
261:C11101
192:207215
163:ChEMBL
52:Names
2329:S2CID
1568:S2CID
1379:S2CID
1025:Other
906:honey
692:furan
354:InChI
143:ChEBI
109:JSmol
2505:PMID
2497:ISSN
2432:PMID
2395:PMID
2321:PMID
2280:PMID
2272:ISSN
2208:PMID
2170:PMID
2133:PMID
2082:PMID
2040:PMID
1968:PMID
1932:PMID
1924:ISSN
1889:PMID
1793:ISSN
1757:ISSN
1679:PMID
1671:ISSN
1636:ISSN
1560:PMID
1515:ISSN
1476:ISSN
1419:PMID
1371:ISSN
1254:PMID
1246:ISSN
1208:ISSN
1167:PMID
1159:ISSN
1079:ISBN
1012:and
998:HPLC
912:and
800:and
698:and
622:P310
614:P261
600:H335
596:H319
592:H315
461:Odor
297:UNII
252:KEGG
2489:doi
2461:doi
2457:114
2424:doi
2420:880
2385:doi
2311:doi
2307:128
2264:doi
2237:doi
2200:doi
2162:doi
2123:PMC
2113:doi
2074:doi
2032:doi
2003:hdl
1995:doi
1960:doi
1916:doi
1912:341
1881:hdl
1873:doi
1842:hdl
1832:doi
1785:doi
1749:doi
1720:hdl
1710:doi
1663:doi
1659:111
1628:doi
1599:doi
1552:doi
1540:312
1507:doi
1503:175
1466:hdl
1456:doi
1411:doi
1407:106
1361:doi
1238:doi
1200:doi
1151:doi
1147:113
1071:doi
1018:DIN
914:UHT
775:3,4
676:HMF
545:GHS
505:max
323:EPA
276:CID
2524::
2503:.
2495:.
2483:.
2455:.
2430:.
2418:.
2393:.
2381:62
2379:.
2375:.
2349:.
2327:.
2319:.
2305:.
2301:.
2278:.
2270:.
2260:55
2258:.
2233:33
2231:.
2206:.
2196:46
2194:.
2168:.
2158:50
2156:.
2131:.
2121:.
2109:12
2107:.
2103:.
2080:.
2070:58
2068:.
2052:^
2038:.
2028:13
2026:.
2001:.
1991:44
1989:.
1966:.
1956:62
1954:.
1930:.
1922:.
1910:.
1887:.
1879:.
1869:48
1867:.
1840:.
1830:.
1820:55
1818:.
1814:.
1791:.
1779:.
1755:.
1745:16
1743:.
1718:.
1704:.
1700:.
1677:.
1669:.
1657:.
1634:.
1624:40
1622:.
1595:93
1593:.
1589:.
1566:.
1558:.
1550:.
1538:.
1513:.
1501:.
1497:.
1474:.
1464:.
1450:.
1446:.
1434:^
1417:.
1400:.
1377:.
1369:.
1355:.
1351:.
1339:^
1323:.
1275:.
1252:.
1244:.
1234:55
1232:.
1220:^
1206:.
1196:13
1194:.
1179:^
1165:.
1157:.
1145:.
1130:^
1125:.
1121:,
1107:^
1077:.
1051:^
988:.
931:.
892:.
880:.
832:.
796:,
754:.
620:,
616:,
598:,
594:,
549::
507:)
503:(λ
2511:.
2491::
2485:8
2467:.
2463::
2438:.
2426::
2401:.
2387::
2360:.
2335:.
2313::
2286:.
2266::
2243:.
2239::
2214:.
2202::
2176:.
2164::
2139:.
2115::
2088:.
2076::
2046:.
2034::
2011:.
2005::
1997::
1974:.
1962::
1938:.
1918::
1895:.
1883::
1875::
1850:.
1844::
1834::
1826::
1799:.
1787::
1781:6
1763:.
1751::
1728:.
1722::
1712::
1706:2
1685:.
1665::
1642:.
1630::
1607:.
1601::
1574:.
1554::
1546::
1521:.
1509::
1482:.
1468::
1458::
1452:1
1425:.
1413::
1385:.
1363::
1357:3
1333:.
1285:.
1260:.
1240::
1214:.
1202::
1173:.
1153::
1102:)
1098:(
1087:.
1073::
1016:(
900:.
779:5
767:2
763:1
730:(
674:(
643:Y
433:3
430:O
427:6
424:H
421:6
418:C
325:)
321:(
111:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.