318:
311:
304:
160:
153:
146:
253:-2,6-dimethylcyclohexanone are enantiotopic; they are related by an internal plane of symmetry passing through the carbonyl group, but deprotonation on one side of the carbonyl group or on the other will generate compounds that are enantiomers. Similarly, the replacement of one or the other with
245:
Enantiotopic groups are mirror images of each other about an internal plane of symmetry. A chiral environment removes that symmetry. Enantiotopic pairs of NMR-active nuclei are also indistinguishable by NMR and produce a single signal.
534:
448:
70:
when the groups are interchanged with some other atom (such as bromine) while the remaining parts of the molecule stay fixed. Homotopic atoms are always identical, in any environment. Homotopic NMR-active nuclei have the same
353:; no matter its proportion, each enantiomer will generate enantiomeric sets of diastereomers upon substitution of diastereotopic groups (though, as in the case of substitution by bromine in 2-bromobutane,
360:
Diastereotopic groups are not mirror images of one another about any plane. They are always different, in any environment, but may not be distinguishable. For instance, both pairs of CH
564:
The terms enantiotopic and diastereotopic can also be applied to the faces of planar groups (especially carbonyl groups and alkene moieties). See
277:. Diastereotopic groups are often, but not always, identical groups attached to the same atom in a molecule containing at least one chiral center.
576:
Heterotopic groups are those that when substituted are structurally different. They are neither diastereotopic or enantiotopic nor homotopic.
479:
hydrogens in cyclopentanol (Figure 2) are similarly diastereotopic, and this is easily discerned as one of the hydrogens in the pair will be
490:
The term diastereotopic is also applied to identical groups attached to the same end of an alkene moiety which, if replaced, would generate
420:
groups of ipsenol, which are three bonds away from the chiral center, give separate H doublets at 300 MHz and separate C-NMR signals in CDCl
242:, and it gets replaced in the same place during the reverse reaction. The chiral environment needs not be optically pure for this effect.
690:
541:
Diastereotopicity is not limited to organic molecules, nor to groups attached to carbon, nor to molecules with chiral tetrahedral (
475:
carbon will be diastereomers. This kind of relationship is often easier to detect in cyclic molecules. For instance, any pair of CH
412:. Such signals are often complex because of small differences in chemical shift, overlap and an additional strong coupling between
565:
292:)-2,3-dibromobutane. Replacement of the other hydrogen atom (colored red) with a bromine atom will produce the diastereomer (
249:
Enantiotopic groups need not be attached to the same atom. For example, two hydrogen atoms adjacent to the carbonyl group in
349:
In chiral molecules containing diastereotopic groups, such as in 2-bromobutane, there is no requirement for enantiomeric or
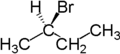
134:)-2-bromobutane. Replacement of the other hydrogen atom (colored red) with a bromine atom will produce the enantiomer (
288:)-2-bromobutane are diastereotopic. Replacement of one hydrogen atom (colored blue) with a bromine atom will produce (
115:
refers to the relationship between two groups in a molecule which, if one or the other were replaced, would generate a
231:
76:
273:
refers to the relationship between two groups in a molecule which, if replaced, would generate compounds that are
685:
639:
Silverstein, R. et al.: Spectrometric
Identification of Organic Compounds, 7th ed., John Wiley & Sons, 2005
471:
hydrogens creates two chiral centers at once, and the two possible hydrogen substitution products at any one CH
408:
group next to the chiral center gives distinct signals from its two hydrogens with the same instrument in CDCl
590:
187:
Enantiotopic groups are identical and indistinguishable except in chiral environments. For instance, the CH
116:
66:
in a chemical compound are equivalent groups. Two groups A and B are homotopic if the molecule remains
440:
Cl), also three bonds away from the chiral center, show barely distinguishable H-NMR signals in DMSO-d
130:
are enantiotopic. Replacement of one hydrogen atom (colored blue) with a bromine atom will produce (
665:
39:
and the structure to which they are attached. Depending on the relationship, such groups can be
491:
648:
624:
609:
266:
212:
108:
88:
28:
455:
Diastereotopic groups also arise in achiral molecules. For instance, any one pair of CH
350:
72:
679:
354:
274:
239:
87:) are homotopic with one another, as are the two hydrogens or the two chlorines in
36:
223:
216:
17:
380:
Cl) are diastereotopic and both give pairs of distinct H-NMR signals in DMSO-d
317:
310:
303:
227:
120:
119:
compound. The two possible compounds resulting from that replacement would be
585:
483:
to the OH group (on the same side of the ring face) while the other will be
254:
159:
152:
145:
610:
300 MHz H-NMR spectrum of ethyl phenylalaninate hydrochloride in DMSO-d
499:
424:, but the diastereotopic hydrogens in ethyl alaninate hydrochloride (CH
413:
219:, or if coordinated to a chiral metal center, or if associated with an
192:
80:
67:
527:
220:
207:) if combined with a chiral center, for instance by conversion to an
127:
126:
For example, the two hydrogen atoms attached to the second carbon in
561:), where the metal center is chiral, are diastereotopic (Figure 2).
533:
459:
hydrogens in 3-pentanol (Figure 1) are diastereotopic, as the two CH
447:
545:-hybridized) centers: for instance, the pair of hydrogens in any CH
532:
446:
208:
649:
300 MHz H-NMR spectrum of ethyl alaninate hydrochloride in DMSO-d
537:
Figure 2. Diastereotopic hydrogens in a chiral metal complex.
494:(also falling in the category of diastereomers). Thus, the CH
203:
OH) are normally enantiotopic, but can be made different (
384:
at 300 MHz, but in the similar ethyl 2-nitrobutanoate (CH
625:
300 MHz H-NMR spectrum of ethyl 2-nitrobutanoate in CDCl
451:
Figure 1. Diastereotopic hydrogens in achiral compounds.
364:
hydrogens in ethyl phenylalaninate hydrochloride (PhCH
79:spectrum. For example, the four hydrogen atoms of
514:to it, and replacement of one or the other with CH
357:isomers have, strictly speaking, no enantiomer).
234:, one specific hydrogen is removed from the CH
280:For example, the two hydrogen atoms of the CH
8:
35:is the stereochemical relationship between
557:(ethylenediamine)chromium(III) ion (Cr(en)
226:, since enzymes are constituted of chiral
238:group during the oxidation of ethanol to
467:. Substitution of any one of the four CH
416:hydrogens. On the other hand, the two CH
230:. Indeed, in the presence of the enzyme
602:
7:
204:
25:
464:
298:
140:
566:Cahn-Ingold-Prelog priority rule
316:
309:
302:
158:
151:
144:
502:are diastereotopic, one being
487:to it (on the opposite side).
1:
324:
301:
166:
510:group, and the other being
257:will generate enantiomers.
707:
691:Nuclear magnetic resonance
668:. University of Wisconsin.
666:"Symmetry in NMR Spectra"
591:conformational analysis
653:from Sigma-Aldrich Co.
629:from Sigma-Aldrich Co.
614:from Sigma-Aldrich Co.
538:
452:
536:
450:
296:)-2,3-dibromobutane.
343:)-2,3-dibromobutane
336:)-2,3-dibromobutane
539:
453:
492:geometric isomers
347:
346:
185:
184:
138:)-2-bromobutane.
16:(Redirected from
698:
670:
669:
661:
655:
646:
640:
637:
631:
622:
616:
607:
320:
313:
306:
299:
181:)-2-bromobutane
162:
155:
148:
141:
64:Homotopic groups
21:
18:Homotopic groups
706:
705:
701:
700:
699:
697:
696:
695:
686:Stereochemistry
676:
675:
674:
673:
664:Hans J. Reich.
663:
662:
658:
652:
647:
643:
638:
634:
628:
623:
619:
613:
608:
604:
599:
582:
574:
560:
552:
548:
518:would generate
517:
509:
497:
478:
474:
470:
462:
458:
443:
439:
435:
431:
427:
423:
419:
411:
407:
403:
399:
395:
391:
387:
383:
379:
375:
371:
367:
363:
329:)-2-bromobutane
283:
263:
237:
213:carboxylic acid
202:
198:
190:
174:)-2-bromobutane
105:
98:
94:
89:dichloromethane
86:
61:
29:stereochemistry
23:
22:
15:
12:
11:
5:
704:
702:
694:
693:
688:
678:
677:
672:
671:
656:
650:
641:
632:
626:
617:
611:
601:
600:
598:
595:
594:
593:
588:
581:
578:
573:
570:
558:
550:
546:
515:
507:
495:
476:
472:
468:
460:
456:
441:
437:
433:
429:
425:
421:
417:
409:
405:
404:), only the CH
401:
397:
393:
389:
385:
381:
377:
373:
369:
365:
361:
351:optical purity
345:
344:
337:
330:
322:
321:
314:
307:
281:
271:diastereotopic
267:stereochemical
262:
261:Diastereotopic
259:
235:
205:diastereotopic
200:
196:
188:
183:
182:
175:
168:
164:
163:
156:
149:
109:stereochemical
104:
101:
96:
92:
84:
73:chemical shift
60:
57:
53:diastereotopic
24:
14:
13:
10:
9:
6:
4:
3:
2:
703:
692:
689:
687:
684:
683:
681:
667:
660:
657:
654:
645:
642:
636:
633:
630:
621:
618:
615:
606:
603:
596:
592:
589:
587:
584:
583:
579:
577:
571:
569:
567:
562:
556:
544:
535:
531:
529:
525:
521:
513:
505:
501:
498:hydrogens of
493:
488:
486:
482:
466:
449:
445:
415:
358:
356:
352:
342:
338:
335:
331:
328:
323:
319:
315:
312:
308:
305:
300:
297:
295:
291:
287:
278:
276:
275:diastereomers
272:
268:
260:
258:
256:
252:
247:
243:
241:
233:
229:
225:
222:
218:
214:
210:
206:
194:
191:hydrogens in
180:
176:
173:
169:
165:
161:
157:
154:
150:
147:
143:
142:
139:
137:
133:
129:
124:
122:
118:
114:
110:
102:
100:
90:
82:
78:
74:
69:
65:
58:
56:
54:
50:
46:
42:
38:
34:
30:
19:
659:
644:
635:
620:
605:
575:
563:
554:
542:
540:
523:
519:
511:
503:
489:
484:
480:
465:enantiotopic
463:carbons are
454:
359:
348:
340:
333:
326:
293:
289:
285:
279:
270:
264:
250:
248:
244:
240:acetaldehyde
211:of a chiral
186:
178:
171:
135:
131:
125:
113:enantiotopic
112:
106:
103:Enantiotopic
63:
62:
52:
49:enantiotopic
48:
44:
40:
37:substituents
32:
26:
572:Heterotopic
284:moiety in (
228:amino acids
224:active site
217:lactic acid
121:enantiomers
41:heterotopic
680:Categories
597:References
586:Prochiral
553:group in
506:to the CH
255:deuterium
59:Homotopic
45:homotopic
580:See also
215:such as
33:topicity
500:propene
414:geminal
193:ethanol
81:methane
68:achiral
528:butene
524:trans-
432:)COOCH
396:)COOCH
372:)COOCH
221:enzyme
167:Butane
128:butane
117:chiral
75:in an
549:or NH
512:trans
485:trans
428:CH(NH
392:CH(NO
368:CH(NH
341:2S,3S
334:2S,3R
294:2S,3S
290:2S,3R
269:term
209:ester
111:term
51:, or
555:tris
520:cis-
355:meso
265:The
232:LADH
107:The
526:-2-
522:or
504:cis
481:cis
251:cis
195:(CH
99:).
91:(CH
83:(CH
77:NMR
27:In
682::
568:.
543:sp
530:.
444:.
436:CH
400:CH
388:CH
376:CH
199:CH
123:.
95:Cl
55:.
47:,
43:,
31:,
651:6
627:3
612:6
559:3
551:2
547:2
516:3
508:3
496:2
477:2
473:2
469:2
461:2
457:2
442:6
438:3
434:2
430:3
426:3
422:3
418:3
410:3
406:2
402:3
398:2
394:2
390:2
386:3
382:6
378:3
374:2
370:3
366:2
362:2
339:(
332:(
327:S
325:(
286:S
282:2
236:2
201:2
197:3
189:2
179:S
177:(
172:R
170:(
136:S
132:R
97:2
93:2
85:4
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.