1186:
1226:) decrease PDE4 inhibition activity. Using a carboxylic acid as a starting point, an amide group has similar PDE4 inhibition activity but both groups were shown to be a considerably less potent than a methyl ester group, which had about six-fold increase in PDE4 inhibitory activity. Sulfone group had similar PDE4 inhibition as the methyl ester group. The best PDE4 inhibition was observed when a nitrile group was attached, which has 32 times more PDE4 inhibitory activity than the carboxyl acid. Substituents at Y leading to increasing PDE4 inhibitory activity thus followed the order:
1288:
1215:. Optimal activity is achieved with a methoxy group at the 4-position (X2) and a bigger group, such as cyclopentoxy at the 3-position carbon (X3). However the thalidomide PDE4 inhibitory analogs do not follow the SAR of rolipram analogs directly. For thalidomide analogs, an ethoxy group at X3 and a methoxy group at X2, with X1 being just a hydrogen, gave the highest PDE4 and TNF-α inhibition. Substitutes larger than diethoxy at the X2–X3 position had decreased activity. The effects of these substitutions seem to be mediated by steric effects.
493:
1139:
1197:
1164:
both thalidomide and EM-12 resulted in much more potent inhibition of TNF-α. This also revealed that the amino group needed to be directly opposite the carbonyl group on the isoindolinone ring system for the most potent activity. These analogs do not inhibit PDE4 and therefore do not act by PDE4 inhibition. Other additions of longer and bigger groups at the C4 and C5 position of the phthaloyl ring system of thalidomide, some with an
1708:
1635:
1510:
1110:, is induced in the presence of TNF-α and the adhesion of MM cells to BMSC. In vitro proliferation of MM cell lines and inhibition of Fas-mediated apoptosis is promoted by IL-6. Thalidomide and its analogs directly decrease the up-regulation of IL-6 and indirectly through TNF-α, thereby reducing the secretion of adhesion molecules leading to fewer MM cells adhering to BMSC.
1561:
56:
870:
1317:
418:. This effect is not related to TNF-α inhibition since potent TNF-α inhibitors such as rolipram and pentoxifylline did not inhibit myeloma cell growth nor angiogenesis. Upregulation of interferon gamma, IL-2 and IL-10 have been reported for pomalidomide and may contribute to its anti-angiogenic and anti-myeloma activities.
808:
Findings also support the hypothesis that an increase in the expression of cereblon is an essential element of the anti-myeloma effect of both lenalidomide and pomalidomide. Cereblon expression was three times higher in responding patients compared to non-responders and higher cereblon expression was
381:
of the immune system, and also a mediator of inflammatory response. Thus the drug is effective against some inflammatory diseases such as ENL (6a
Sampaio, Kaplan, Miranda, Nery..... JID 168 (2) 408-414 2008). In 1994 Thalidomide was found to have anti-angiogenic activity and anti-tumor activity which
1302:
ring seems to be required. Different groups were tested in the R position. The substances that had nitrogen salts as the R group showed good activity. The improved angiogenesis inhibitory activity could be due to increased solubility or that the positively charged nitrogen has added interaction with
1013:
and IL-6 appear to be required for endothelial cell migration during angiogenesis. Thalidomide and its analogs are believed to suppress angiogenesis through modulation of the above-mentioned factors where potency in anti-angiogenic activity for lenalidomide and pomalidomide was 2-3 times higher than
1154:
and continued research investigation. The information on SAR of thalidomide and its analogs is still in process so any trends detailed here are observed during individual studies. Research has mainly focused on improving the TNF-α and PDE4 inhibition of thalidomide, as well as the anti-angiogenesis
445:
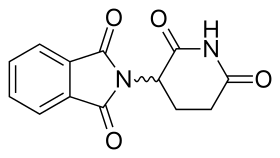
name is 2-(2,6-dioxopiperidin-3-yl)isoindole-1,3-dione and it has one chiral center After thalidomide's selective inhibition of TNF-α had been reported, a renewed effort was put in thalidomide's clinical development. The clinical development led to the discovery of new analogs which strived to have
409:
Pomalidomide (3-aminothalidomide) was the second thalidomide analog to enter the clinic being more potent than both of its predecessors. First reported in 2001, pomalidomide was noted to directly inhibit myeloma cell proliferation and thus inhibiting MM both on the tumor and vascular compartments.
1163:
Research indicated that a substitution at the phthaloyl ring would increase TNF-α inhibition activity (Figure 5). An amino group substitution was tested at various locations on the phthaloyl ring (C4, C5, C6, C7) of thalidomide and EM-12 (previously described). Amino addition at the C4 location on
704:
Pomalidomide was submitted for FDA approval on April 26, 2012 and on 21 June it was announced that the drug would get standard FDA review. A marketing authorization application was filed to EMA 21 June 2012, where a decision could come as soon as early 2013. EMA has already granted pomalidomide an
1250:
Substitutions on the phthaloyl ring have been explored and it was noticed that nitro groups at the C4 or C5 location decreased activity but C4 or C5 amino substitution increased it dramatically. When the substitution at the 4 (Z) location on the phthaloyl ring was examined, hydroxyl and methoxy
397:
group from the phthaloyl ring. Development of lenalidomide began in the late 1990s and clinical trials of lenalidomide began in 2000. In
October 2001 lenalidomide was granted orphan status for the treatment of MM. In mid-2002 it entered phase II and by early 2003 phase III. In February 2003 FDA
3434:
Lepper, Erin R.; Ng, Sylvia S. W.; Gütschow, Michael; Weiss, Michael; Hauschildt, Sunna; Hecker, Thomas K.; Luzzio, Frederick A.; Eger, Kurt; Figg, William D. (1 April 2004). "Comparative
Molecular Field Analysis and Comparative Molecular Similarity Indices Analysis of Thalidomide Analogues as
507:
of the substance because of increased stability. The molecule had been reported to be an even more potent teratogenic agent than thalidomide in rats, rabbits and monkeys. Additionally, these analogs are more potent inhibitors of angiogenesis than thalidomide. As well, the amino-thalidomide and
252:
properties of the drug yielding a new way of fighting cancer as well as some inflammatory diseases after it had been banned in 1961. The problems with thalidomide included teratogenic side effects, high incidence of other adverse reactions, poor solubility in water and poor absorption from the
1083:
The role of angiogenesis in the support of myeloma was first discovered by Vacca in 1994. They discovered increased bone marrow angiogenesis correlates with myeloma growth and supporting stromal cells are a significant source for angiogenic molecules in myeloma. This is believed to be a main
666:
due to low or intermediate-1-risk myelodysplastic syndromes associated with a deletion 5q cytogenetic abnormality with or without additional cytogenetic abnormalities in the U.S., Canada, Switzerland, Australia, New
Zealand, Malaysia, Israel and several Latin American countries, while
3395:
Avila, Carolina
Martins; Romeiro, Nelilma Correia; Sperandio da Silva, Gilberto M.; Sant’Anna, Carlos M.R.; Barreiro, Eliezer J.; Fraga, Carlos A.M. (1 October 2006). "Development of new CoMFA and CoMSIA 3D-QSAR models for anti-inflammatory phthalimide-containing TNFα modulators".
1207:
The common structure for analogs that inhibit TNF-α via inhibition of PDE4 is prepared on the basis of hydrolysing the glutarimide ring of thalidomide. These analogs do not have an acidic chiral hydrogen, unlike thalidomide, and would therefore be expected to be chirally stable.
1394:
1383:
1149:
Since the mechanism of action of thalidomide and its analogs is not fully clear and the bioreceptor for these substances has not been identified, the insight into the relationship between the structure and activity of thalidomide and its analogs are mostly derived from
1463:
1255:-acetylamino group had slightly lower PDE4 inhibitory activity, compared with the methyl group, but increased the compound's TNF-α inhibitory activity to a further extent. Substituents at Z leading to increasing PDE4 inhibitory activity thus followed the order:
2400:
Man, Hon-Wah; Schafer, Peter; Wong, Lu Min; Patterson, Rebecca T.; Corral, Laura G.; Raymon, Heather; Blease, Kate; Leisten, Jim; Shirley, Michael A.; Tang, Yang; Babusis, Darius M.; Chen, Roger; Stirling, Dave; Muller, George W. (26 March 2009). "Discovery of
1404:
Synthesis of thalidomide has usually been performed as seen in scheme 1. This synthesis is a reasonably simplistic three step process. The downside of this process however is that the last step requires a high-temperature melt reaction which demands multiple
5464:
1934:
Mazzoccoli, L; Cadoso, SH; Amarante, GW; de Souza, MV; Domingues, R; Machado, MA; de
Almeida, MV; Teixeira, HC (July 2012). "Novel thalidomide analogues from diamines inhibit pro-inflammatory cytokine production and CD80 expression while enhancing IL-10".
944:
than thalidomide in anti-inflammatory properties and pomalidomide about 10 times more potent than lenalidomide. It is worth noticing however that, when comparing lenalidomide and pomalidomide, clinical relevance of higher in vitro potency is unclear since
671:
is currently being evaluated in a number of other countries. Numerous clinical trials are already in the pipeline or being conducted to explore further use for lenalidomide, alone or in combination with other drugs. Some of these indications include
1024:
activity through the blocking of IL-6, and NF-κB has been shown to be involved in angiogenesis. Inhibition of TNF-α is not the mechanism of thalidomide's inhibition of angiogenesis since numerous other TNF-α inhibitors do not inhibit angiogenesis.
909:, resulting in diminished amounts of this pro-inflammatory cytokine secreted. This explains the effect of thalidomide when given to ENL patients, as they commonly have high levels of TNF-α in their blood and in dermatological lesions. In contrast,
2465:
Muller, George W.; Corral, Laura G.; Shire, Mary G.; Wang, Hua; Moreira, Andre; Kaplan, Gilla; Stirling, David I. (1 January 1996). "Structural
Modifications of Thalidomide Produce Analogs with Enhanced Tumor Necrosis Factor Inhibitory Activity".
422:
5409:
5365:
860:
is not yet fully understood. It is believed that they work through different mechanisms in various diseases. The net effect is probably due to different mechanisms combined. Mechanism of action will be explained in light of today's knowledge.
3545:
Stewart, Scott G.; Spagnolo, Daniel; Polomska, Marta E.; Sin, Melvin; Karimi, Mahdad; Abraham, Lawrence J. (1 November 2007). "Synthesis and TNF expression inhibitory properties of new thalidomide analogues derived via Heck cross coupling".
1501:
and HCl, was performed. The formulated hydrochloride (Compound 3 in Scheme 3) was then reacted with 3-nitrophthalic anhydride in refluxing acetic acid to yield the 4-nitro substituted thalidomide analog and the nitro group then reduced with
473:-effects were seen in was not sensitive to the thalidomide teratogenic effects. Later reports in rabbits, which is a sensitive species, unveiled teratogenic effects from both isomers. Moreover, thalidomide enantiomers have been shown to be
757:
have been proposed. Two of the best-known have been the anti-angiogenesis hypothesis and oxidative stress model hypothesis, with considerable experimental evidence supporting these two hypotheses regarding thalidomide's teratogenicity.
5454:
1251:
groups seem to make the analog a less potent PDE4 inhibitor. An increase in activity was observed with amino and dimethylamino to a similar extent but a methyl group improved the activity further than the aforementioned groups. A 4-
382:
propelled the initiation of clinical trials for cancer including multiple myeloma. The discovery of the anti-inflammatory, anti-angiogenic and anti-tumor activities of thalidomide increased the interest of further research and
5509:
5504:
5434:
1168:
functionality, have been tested with various results. Increased inhibitory effect, compared to thalidomide, was noticed with the groups that had an oxygen atom attached directly to the C5 or C4 olefin.
3510:
Muller, GW; Chen, R; Huang, SY; Corral, LG; Wong, LM; Patterson, RT; Chen, Y; Kaplan, G; Stirling, DI (Jun 7, 1999). "Amino-substituted thalidomide analogs: potent inhibitors of TNF-alpha production".
5519:
793:) of unknown substrates. Thalidomide has been shown to bind to cereblon, inhibiting the activity of the E3 ubiquitin ligase, resulting in accumulation of the ligase substrates and downregulation of
1520:
Lenalidomide is synthesized in a similar way using compound 3 (3-aminopiperidine-2,6-dione) treated with a nitro-substituted methyl 2-(bromomethyl) benzoate, and hydrogenation of the nitro group.
5439:
2255:
Zeldis, Jerome B.; Knight, Robert; Hussein, Mohamad; Chopra, Rajesh; Muller, George (1 March 2011). "A review of the history, properties, and use of the immunomodulatory compound lenalidomide".
650:
Lenalidomide is approved in nearly 70 countries, in combination with dexamethasone for the treatment of patients with MM who have received at least one prior therapy. Orphan indications include
5524:
5469:
1473:
Both of the amino analogs are prepared from the condensation of 3-aminopiperidine-2,6-dione hydrochloride (Compound 3) which is synthesized in a two step reaction from commercially available
5355:
5533:
5484:
5389:
465:-isomer is devoid of the teratogenic properties but conveys the sedative effects, however this view is highly debated and it has been argued that the animal model that these different
5399:
5345:
5514:
389:
Lenalidomide is the first analog of thalidomide which is marketed. It is considerably more potent than its parent drug with only two differences at a molecular level, with an added
5449:
398:
granted fast-track status to lenalidomide for the treatment of relapsed or refractory MM. In 2006 it was approved for the treatment of MM along with dexamethasone and in 2007 by
94:
508:
amino-EM-12 were potent inhibitors of TNF-α. These two analogs later got the name lenalidomide, which is the EM-12 amino analog, and pomalidomide, the thalidomide amino analog.
503:
One of the analogs of interest was made by isoindolinone replacement of the phthaloyl ring. It was given the name EM-12 (Figure 3). This replacement was thought to increase the
5424:
3470:
Noguchi, Tomomi; Fujimoto, Haruka; Sano, Hiroko; Miyajima, Atsushi; Miyachi, Hiroyuki; Hashimoto, Yuichi (1 December 2005). "Angiogenesis inhibitors derived from thalidomide".
2811:
365:
Four years after thalidomide was withdrawn from the market for its ability to induce severe birth defects, its anti-inflammatory properties were discovered when patients with
5474:
1052:
leading to indirect upregulation of caspase-9 activity. Further anti-tumor activity is mediated through the inhibition of apoptosis protein-2 and pro-survival effects of
5429:
5404:
442:
1918:
5394:
1335:
1454:
for 15–18 hours. During the reflux thalidomide crystallizes out of the mixture. The final step gives 85–93% yield of thalidomide, bringing the total yield to 43–63%.
2683:
5499:
1916:
Reversal of
Fortune: How a Vilified Drug Became a Life-saving Agent in the "War" Against Cancer - Onco'Zine - The International Oncology Network (November 30, 2013)
5494:
5538:
2358:
Lentzsch S, Rogers MS, LeBlanc R, et al. (April 2002). "S-3-Amino-phthalimido-glutarimide inhibits angiogenesis and growth of B-cell neoplasias in mice".
2147:
Bartlett, J. Blake; Dredge, Keith; Dalgleish, Angus G. (1 April 2004). "Timeline: The evolution of thalidomide and its IMiD derivatives as anticancer agents".
5459:
5444:
3833:
5379:
5370:
5350:
2705:
5360:
1177:
addition at C4 or C5 resulted in equal or decreased activity compared to thalidomide. These groups were not compared with lenalidomide or pomalidomide.
2323:
D'Amato, RJ; Lentzsch, S; Anderson, KC; Rogers, MS (December 2001). "Mechanism of action of thalidomide and 3-aminothalidomide in multiple myeloma".
949:
of pomalidomide is 2 mg daily compared to 25 mg for lenalidomide, leading to 10-100 times lower plasma drug concentration of pomalidomide.
4749:
1107:
1374:. Note that these synthesis schemes do not necessarily reflect the organic synthesis strategies used to synthesize these single chemical entities.
480:
due to the acidic chiral hydrogen in the asymmetric center (shown, for the EM-12 analog, in Figure 3), so the plan to administer a purified single
5419:
5414:
639:
2618:
Vallet, S; Witzens-Harig, M; Jaeger, D; Podar, K (March 2012). "Update on immunomodulatory drugs (IMiDs) in hematologic and solid malignancies".
2193:
D'Amato RJ, Lentzsch S, Anderson KC, Rogers MS (December 2001). "Mechanism of action of thalidomide and 3-aminothalidomide in multiple myeloma".
5599:
3654:
1036:
anti-tumor activity of thalidomide is believed to be due to the potent anti-angiogenic effect and also through changes in cytokine expression.
985:. Lenalidomide and pomalidomide are about 100-1000 times more potent in stimulating T-cell clonal proliferation than thalidomide. In addition,
5489:
5275:
3581:
Muller, George W.; Konnecke, William E.; Smith, Alison M.; Khetani, Vikram D. (1 March 1999). "A Concise Two-Step
Synthesis of Thalidomide".
31:
685:
5479:
1091:
Additionally, inflammatory responses within the bone marrow are believed to foster many hematological diseases. The secretion of IL-6 by
369:
used thalidomide as a sedative and it reduced both the clinical signs and symptoms of the disease. Thalidomide was discovered to inhibit
1412:
Scheme 2 is the newer synthesis route which was designed to make the reaction more direct and to produce better yields. This route uses
668:
655:
1006:
830:
119:
2815:
4692:
4062:
1353:
330:
properties were observed. The problems with thalidomide were, aside from the teratogenic side effects, both high incidence of other
2508:
Man, Hon-Wah; Corral, Laura G; Stirling, David I; Muller, George W (1 October 2003). "α-Fluoro-substituted thalidomide analogues".
1915:
3826:
3039:
Sedlarikova, L; Kubiczkova, L; Sevcikova, S; Hajek, R (October 2012). "Mechanism of immunomodulatory drugs in multiple myeloma".
2578:
Sedlarikova, L; Kubiczkova, L; Sevcikova, S; Hajek, R (October 2012). "Mechanism of immunomodulatory drugs in multiple myeloma".
5101:
3295:
Kotla, Venumadhav; Goel, Swati; Nischal, Sangeeta; Heuck, Christoph; Vivek, Kumar; Das, Bhaskar; Verma, Amit (1 January 2009).
2413:-isoindol-4-yl}acetamide (Apremilast), a Potent and Orally Active Phosphodiesterase 4 and Tumor Necrosis Factor-α Inhibitor".
2306:
1185:
2797:
856:
Thalidomide and its analogs, lenalidomide and pomalidomide, are believed to act in a similar fashion even though their exact
3732:"Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma"
2890:
Martiniani, Roberta; Di Loreto, Valentina; Di Sano, Chiara; Lombardo, Alessandra; Liberati, Anna Marina (1 January 2012).
1406:
651:
339:
5579:
3898:
1053:
809:
also associated with partial or full response while lower expression was associated with stable or progressive disease.
370:
257:
5564:
5323:
4007:
3819:
2996:
2709:
901:. The analogs are believed to inhibit the production of TNF-α, where the analogs are up to 50.000 times more potent
3877:
1287:
1485:-glutamine is treated with CDI in refluxing THF to yield Cbz-aminoglutarimide. To remove the Cbz protecting group
929:
is another cytokine both suppressed and enhanced by thalidomide and its analogs. When monocytes are stimulated by
5268:
4555:
611:
399:
74:
3255:
Thomas, Sheeba K.; Richards, Tiffany A.; Weber, Donna M. (1 December 2007). "Lenalidomide in multiple myeloma".
5584:
4935:
2543:
Pan, B; Lentzsch, S (October 2012). "The application and biology of immunomodulatory drugs (IMiDs) in cancer".
1798:
1211:
On the phenyl ring, a 3,4-dialkoxyphenyl moiety (Figure 6) is a known pharmacophore in PDE4 inhibitors such as
1122:
paramount to osteoclast activation, decrease the formation of the cells that form osteoclasts and downregulate
635:
532:
82:
3776:"Phase I Study of an Immunomodulatory Thalidomide Analog, CC-4047, in Relapsed or Refractory Multiple Myeloma"
2775:
5594:
5313:
4930:
1443:
742:
574:
536:
492:
86:
1138:
4735:
4161:
3842:
1490:
1438:-glutamine (4), with 50–70% yield. The substance 4 is then stirred in a mixture with carbonyldiimidazole (
1119:
1044:
in MM cells have been shown, when treated with thalidomide and its analogs, to upregulate the activity of
1005:
Angiogenesis or the growth of new blood vessels has been reported to correspond with MM progression where
946:
818:
802:
738:
673:
273:
245:
111:
2077:"Combination oral antiangiogenic therapy with thalidomide and sulindac inhibits tumour growth in rabbits"
5589:
5569:
5559:
4922:
3206:
Quach, H; Ritchie, D; Stewart, A K; Neeson, P; Harrison, S; Smyth, M J; Prince, H M (12 November 2009).
1808:
1303:
the active site. Tetrafluorination of the phthaloyl ring seems to increase the angiogenesis inhibition.
1196:
905:
than the parent drug thalidomide. The mechanism is believed to be through enhanced degradation of TNF-α
885:
Thalidomide and its immune-modulating analogs alter the production of the inflammatory cytokines TNF-α,
726:
347:
3610:"Thalidomide Pharmacokinetics and Metabolite Formation in Mice, Rabbits, and Multiple Myeloma Patients"
921:
T lymphocytes were stimulated by anti-CD3 which was later confirmed in an early phase trials involving
805:
between the two growth factors, possibly causing both multiple birth defects and anti-myeloma effects.
693:
557:
5261:
3811:
2264:
2029:
886:
817:
Their mechanism of action is not entirely clear, but it is known that they inhibit the production of
710:
659:
586:
403:
373:(TNF-α) in 1991 (5a Sampaio, Sarno, Galilly Cohn and Kaplan, JEM 173 (3) 699–703, 1991) . TNF-α is a
2753:
1218:
For the Y-position, a number of groups have been explored. Substituted amides that were larger than
5574:
4143:
1494:
1439:
1151:
978:
857:
838:
689:
677:
631:
619:
2731:
876:
The mechanism of TLP in multiple myeloma. TLP refers to thalidomide, lenalidomide and pomalidomide
5092:
4193:
3021:
2941:
Quach, H; Ritchie, D; Stewart, AK; Neeson, P; Harrison, S; Smyth, MJ; Prince, HM (January 2010).
2661:
2643:
2288:
2172:
1995:
1371:
930:
521:
499:
Molecular structure of EM-12, an analog of thalidomide. The acidic chiral hydrogen is highlighted
383:
142:
5132:
4990:
2892:"Biological Activity of Lenalidomide and Its Underlying Therapeutic Effects in Multiple Myeloma"
1075:
arrest in leukemia cell lines where the analogs showed 100 times more potency than thalidomide.
977:
type cytokine release of IFN-γ and IL-2 that further stimulates clonal T cell proliferation and
5142:
4828:
4269:
1707:
1634:
5097:
5087:
3888:
3846:
3797:
3753:
3709:
3631:
3563:
3527:
3487:
3452:
3413:
3377:
3328:
3272:
3237:
3185:
3097:
3056:
3013:
2972:
2923:
2863:
2635:
2595:
2560:
2525:
2483:
2430:
2367:
2340:
2280:
2210:
2164:
2106:
2057:
1987:
1952:
1898:
1849:
1803:
1788:
1367:
1127:
934:
777:
and peripheral membrane of cells in numerous parts of the body. It acts as a component of the
714:
265:
249:
106:
5384:
5308:
4893:
4796:
4459:
4355:
3787:
3743:
3699:
3621:
3590:
3555:
3519:
3479:
3444:
3405:
3367:
3359:
3318:
3308:
3264:
3227:
3219:
3177:
3139:
3087:
3048:
3005:
2962:
2954:
2913:
2903:
2853:
2627:
2587:
2552:
2517:
2475:
2422:
2332:
2272:
2237:
2202:
2156:
2096:
2088:
2047:
2037:
1979:
1944:
1888:
1880:
1841:
1758:
842:
826:
778:
730:
547:
541:
366:
331:
261:
78:
753:
Thalidomide's teratogenicity has been a subject of much debate and over the years numerous
17:
5192:
5077:
1447:
1115:
615:
580:
504:
450:
355:
2268:
2033:
1393:
1382:
5318:
5298:
5082:
4950:
4598:
4432:
4411:
4407:
4403:
4031:
3868:
3372:
3363:
3347:
3323:
3296:
3232:
3207:
3092:
3075:
2967:
2942:
2918:
2891:
2101:
2076:
1893:
1868:
1509:
1486:
1462:
926:
898:
894:
790:
786:
562:
99:
3523:
3144:
3127:
2732:"Revlimid (lenalidomide) dosing, indications, interactions, adverse effects, and more"
2521:
2336:
2206:
1970:
Prommer, E. E. (20 October 2009). "Review
Article: Palliative Oncology: Thalidomide".
761:
Recently, new findings have emerged that suggest a novel mechanism of teratogenicity.
5553:
5232:
5162:
5072:
4979:
4765:
4465:
4387:
4339:
4319:
4177:
4093:
3944:
3910:
3704:
3687:
2858:
2841:
2662:"Thalomid (Thalidomide) dosing, indications, interactions, adverse effects, and more"
2556:
2276:
2052:
2017:
1922:
1845:
1793:
1503:
1498:
1474:
1420:
1095:
890:
846:
822:
782:
766:
706:
681:
599:
568:
438:
315:
179:
175:
115:
3025:
1999:
5242:
5202:
5187:
5182:
4898:
4848:
4843:
4823:
4703:
4698:
4561:
4546:
4494:
4470:
4417:
4294:
4284:
4103:
4020:
4015:
3973:
3926:
3883:
3850:
2647:
2292:
2176:
1778:
1773:
1681:
1605:
982:
958:
922:
774:
474:
410:
This dual activity of pomalidomide makes it more efficacious than thalidomide both
351:
281:
277:
269:
244:
The development of analogs of thalidomide was precipitated by the discovery of the
195:
191:
3626:
3609:
3052:
2591:
981:
proliferation and activity. This enhances natural and antibody dependent cellular
3181:
2631:
1948:
5303:
5284:
5237:
5177:
5167:
5157:
5057:
5047:
5037:
5032:
4997:
4908:
4903:
4878:
4868:
4858:
4833:
4818:
4813:
4771:
4741:
4727:
4722:
4708:
4651:
4609:
4604:
4590:
4585:
4571:
4551:
4504:
4480:
4438:
4422:
4393:
4377:
4345:
4329:
4324:
4299:
4249:
4239:
4234:
4229:
4218:
4208:
4113:
4025:
3983:
3968:
3962:
3958:
3905:
1768:
1763:
1534:
1299:
1219:
1123:
1092:
957:
Thalidomide and its analogs help with the co-stimulation of T-cells through the
528:). Indications for these agents that have received regulatory approval include:
390:
378:
359:
297:
199:
187:
3748:
3731:
3559:
3483:
3268:
1884:
913:
assay demonstrated that TNF-α is actually enhanced in T-cell activation, where
5227:
5222:
5207:
5147:
5137:
5122:
5117:
5112:
5062:
5052:
5042:
5007:
4985:
4971:
4883:
4873:
4863:
4853:
4838:
4781:
4776:
4755:
4670:
4665:
4623:
4617:
4518:
4475:
4371:
4361:
4314:
4304:
4279:
4274:
4264:
4259:
4254:
4244:
4203:
4188:
4183:
4167:
4108:
4088:
4037:
3997:
3978:
3920:
3409:
2241:
1783:
1111:
1072:
1057:
754:
603:
481:
343:
335:
327:
326:
for morning sickness in pregnant women. The drug was banned in 1961 after its
323:
264:(MM) under strict regulations. This has led to the development of a number of
203:
46:
2991:
1983:
5217:
5212:
5197:
5172:
5127:
5107:
5067:
5002:
4966:
4888:
4684:
4637:
4566:
4532:
4499:
4309:
4289:
4198:
4098:
3938:
3792:
3775:
1413:
1049:
1045:
1041:
770:
607:
598:
Thalidomide has been approved by the FDA for ENL and MM in combination with
421:
136:
3801:
3757:
3713:
3635:
3567:
3531:
3491:
3456:
3417:
3332:
3313:
3276:
3241:
3208:"Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma"
3189:
3101:
3060:
3017:
2976:
2943:"Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma"
2927:
2867:
2639:
2599:
2564:
2529:
2434:
2371:
2344:
2284:
2214:
2168:
2110:
2092:
2042:
1991:
1956:
1902:
1853:
461:
is associated with the infamous teratogenic effects of thalidomide and the
3381:
3076:"What is the functional role of the thalidomide binding protein cereblon?"
2908:
2487:
2061:
1118:
and secretion of various MM survival factors. They decrease the levels of
849:
production. Their teratogenic effects appear to be mediated by binding to
155:
5152:
5027:
4716:
4224:
4134:
4070:
3992:
3223:
2958:
1212:
1069:
1065:
1048:. This causes cross talking of apoptotic signaling between caspase-8 and
1021:
974:
966:
850:
762:
394:
374:
319:
214:
207:
1560:
1370:
thalidomide, lenalidomide, and pomalidomide, as reported from prominent
553:
Off-label indications for which they seem promising treatments include:
441:
and consists of a glutarimide ring and a phthaloyl ring (Figure 5). Its
225:
55:
4645:
1174:
1016:
741:. There may be an increased risk of secondary malignancies, especially
734:
623:
525:
285:
3594:
3448:
3009:
2479:
2426:
231:
4960:
4791:
2228:
Zimmerman, Todd (1 May 2009). "Immunomodulatory agents in oncology".
1832:
Knight, R (August 2005). "IMiDs: a novel class of immunomodulators".
1753:
1451:
1294:
Common structure for thalidomide analogs with angiogenesis inhibition
1170:
1165:
1103:
1099:
834:
663:
627:
517:
458:
2160:
237:
3297:"Mechanism of action of lenalidomide in hematological malignancies"
3168:
Melchert, Magda; List, Alan (1 July 2007). "The thalidomide saga".
869:
4659:
4512:
1508:
1461:
1392:
1381:
1286:
1195:
1184:
1061:
994:
868:
798:
491:
420:
219:
183:
123:
2706:"Celgene Biopharmaceutical - Investor relations - Press Releases"
206:). These drugs may also be referred to as 'Cereblon modulators'.
4631:
4579:
4540:
4526:
4080:
3954:
1010:
962:
906:
794:
428:
Chronological view of the history of thalidomide and its analogs
5257:
3815:
5253:
4488:
3348:"Bone marrow angiogenesis and progression in multiple myeloma"
3126:
Huang, Yen-Ta; Hsu, Chih W.; Chiu, Ted H. (1 September 2008).
1310:
990:
918:
914:
484:
to avoid the teratogenic effects will most likely be in vain.
3688:"Lenalidomide in the treatment of multiple myeloma: a review"
402:(EMA). In 2008, phase II trial observed efficacy in treating
3170:
The International Journal of Biochemistry & Cell Biology
2812:"European Medicines Agency - Search results from your query"
2075:
Verheul HM, Panigrahy D, Yuan J, D'Amato RJ (January 1999).
940:
Lenalidomide is believed to be about 1000 times more potent
833:(which leads to its anti-angiogenic effects), co-stimulates
516:
The primary use of IMiDs in medicine is in the treatment of
3080:
International Journal of Biochemistry and Molecular Biology
602:. EMA has also approved it to treat MM in combination with
5465:
Nucleoside and nucleotide reverse-transcriptase inhibitors
2016:
D'Amato RJ, Loughnan MS, Flynn E, Folkman J (April 1994).
662:. Lenalidomide is also approved for transfusion-dependent
3655:"Summary of product characteristics: Thalidomid Celgene"
3346:
Vacca A, Ribatti D, Roncali L, et al. (July 1994).
1203:
Common structure for PDE4-inhibiting thalidomide analogs
781:, regulating various developmental processes, including
217:
to both "IMD" for "immunomodulatory drug" and the forms
2842:"Deciphering the mystery of thalidomide teratogenicity"
2684:"Thalidomide Celgene (previously Thalidomide Pharmion)"
1331:
210:(CRBN) is the protein targeted by this class of drugs.
437:
The thalidomide molecule is a synthetic derivative of
5410:
Dual serotonin and norepinephrine reuptake inhibitors
973:
data suggests this co-stimulation leads to increased
393:
at position 4 of the phthaloyl ring and removal of a
3686:
Armoiry, X.; Aulagner, G.; Facon, T. (1 June 2008).
1426:
as a starting material and by letting it react with
1192:
Rolipram, highlighting the 3,4-dialkoxyphenyl moiety
1064:. They have also been shown to cause dose dependent
5336:
5291:
5020:
4948:
4921:
4806:
4449:
4151:
4142:
4133:
4126:
4079:
4061:
4052:
4006:
3937:
3867:
3858:
1972:
American Journal of Hospice and Palliative Medicine
1867:Aragon-Ching AB, Li H, Gardner ER, Figg WD (2007).
1326:
may be too technical for most readers to understand
1098:(BMSC) and the secretion of the adhesion molecules
717:and post-essential thrombocythaemia myelofibrosis.
524:(including one that is a response to the infection
284:, which are currently marketed and manufactured by
149:
135:
130:
105:
93:
70:
65:
39:
3128:"Thalidomide and Its Analogs as Anticancer Agents"
2318:
2316:
2011:
2009:
449:Clinically, thalidomide has always been used as a
3725:
3723:
3649:
3647:
3645:
3257:Best Practice & Research Clinical Haematology
1020:assays, Thalidomide has also been shown to block
993:cells into Th1 by enhancing transcription factor
2885:
2883:
2881:
2879:
2877:
1298:For angiogenesis inhibition activity, an intact
1088:by which thalidomide inhibits multiple myeloma.
789:and cell cycle regulation, through degradation (
446:improved activities and decreased side effects.
5455:Non-nucleoside reverse-transcriptase inhibitors
3163:
3161:
3159:
3157:
3155:
2798:"Celgene Submits Pomalidomide For FDA Approval"
2142:
2140:
1827:
1825:
3681:
3679:
3677:
3675:
2138:
2136:
2134:
2132:
2130:
2128:
2126:
2124:
2122:
2120:
1409:and is not compliant with standard equipment.
1400:Newer thalidomide synthesis, two step reaction
622:, primary brain malignancies, AIDS-associated
5269:
3827:
3692:Journal of Clinical Pharmacy and Therapeutics
3505:
3503:
3501:
3429:
3427:
2460:
2458:
2456:
2454:
2452:
2450:
2448:
2446:
2444:
2018:"Thalidomide is an inhibitor of angiogenesis"
256:In 1998 thalidomide was approved by the U.S.
8:
3769:
3767:
3548:Bioorganic & Medicinal Chemistry Letters
3512:Bioorganic & Medicinal Chemistry Letters
3472:Bioorganic & Medicinal Chemistry Letters
3290:
3288:
3286:
3201:
3199:
3121:
3119:
3117:
3115:
3113:
3111:
2992:"Immunomodulatory drugs in multiple myeloma"
2840:Ito, Takumi; Handa, Hiroshi (1 March 2012).
2510:Bioorganic & Medicinal Chemistry Letters
2188:
2186:
933:, IL-12 production is suppressed but during
488:Development of lenalidomide and pomalidomide
2814:. European Medicines Agency. Archived from
2613:
2611:
2609:
1869:"Thalidomide analogues as anticancer drugs"
1114:become highly active during MM, leading to
725:The major toxicities of approved IMiDs are
302:Thalidomide was originally released in the
5276:
5262:
5254:
4148:
4139:
4130:
4058:
3864:
3834:
3820:
3812:
3583:Organic Process Research & Development
2835:
2833:
2503:
2501:
2499:
2497:
2395:
2393:
2391:
2389:
2387:
2385:
2383:
2381:
2257:Annals of the New York Academy of Sciences
1389:Thalidomide synthesis, the older procedure
865:Thalidomide, lenalidomide and pomalidomide
318:). The drug was primarily prescribed as a
54:
3791:
3747:
3703:
3625:
3371:
3322:
3312:
3231:
3143:
3091:
2966:
2917:
2907:
2857:
2100:
2051:
2041:
1892:
1450:) to catalyze the reaction and heated to
1354:Learn how and when to remove this message
1338:, without removing the technical details.
1145:Thalidomide with the ring system outlined
2990:Andhavarapu, S; Roy, V (February 2013).
2754:"Search of: lenalidomide - List Results"
1685:
1609:
1538:
1137:
925:and inflammatory dermatologic diseases.
610:. Orphan indications by the FDA include
322:or hypnotic, but it was also used as an
5415:Selective serotonin reuptake inhibitors
1821:
1442:) with enough 4-dimethylaminopyridine (
640:hematopoietic stem cell transplantation
563:Light chain-associated (AL) amyloidosis
36:
1336:make it understandable to non-experts
1062:TNF-related apoptosis inducing ligand
696:, myelodysplastic syndrome and more.
614:, mycobacterial infection, recurrent
141:
32:immune-mediated inflammatory diseases
30:"IMiD" redirects here. For IMID, see
7:
3398:Bioorganic & Medicinal Chemistry
3301:Journal of Hematology & Oncology
367:erythema nodosum leprosum (ENL)
989:data suggests pomalidomide reverts
669:marketing authorization application
5505:Bcr-Abl tyrosine-kinase inhibitors
3730:Richardson, P. G. (12 July 2002).
3364:10.1111/j.1365-2141.1994.tb08304.x
1873:Recent Pat Anti-Cancer Drug Discov
1669:3.1–4.2 hours in subjects with MM
1007:vascular endothelial growth factor
306:(West Germany) under the label of
25:
5520:Neurokinin 1 receptor antagonists
5395:Dipeptidyl peptidase-4 inhibitors
2620:Expert Opinion on Pharmacotherapy
1937:Biomedicine & Pharmacotherapy
1626:0.6–1.5 hours in healthy subjects
260:(FDA) for use in newly diagnosed
5510:Cannabinoid receptor antagonists
3705:10.1111/j.1365-2710.2008.00920.x
2859:10.1111/j.1741-4520.2011.00351.x
2557:10.1016/j.pharmthera.2012.07.004
2277:10.1111/j.1749-6632.2011.05974.x
1846:10.1053/j.seminoncol.2005.06.018
1706:
1633:
1629:0.5–4 hours in subjects with MM
1559:
1366:Described below are schemes for
1315:
89:and other immunologic conditions
5339:and development of drug classes
3074:Chang, XB; Stewart, AK (2011).
2545:Pharmacology & Therapeutics
1159:TNF-α inhibitors (not via PDE4)
1134:Structure-activity relationship
897:and anti-inflammatory cytokine
705:orphan designation for primary
350:in large majority of patients,
186:group. The IMiD class includes
27:Class of immunomodulatory drugs
3774:Schey, S.A. (15 August 2004).
3608:Chung, F. (1 September 2004).
3437:Journal of Medicinal Chemistry
2468:Journal of Medicinal Chemistry
2415:Journal of Medicinal Chemistry
1555:4–6 hours in subjects with MM
1:
5600:Cereblon E3 ligase modulators
5356:Angiotensin receptor blockers
3627:10.1158/1078-0432.CCR-04-0421
3524:10.1016/s0960-894x(99)00250-4
3145:10.1016/S1016-3190(08)60034-8
3053:10.1016/j.leukres.2012.05.010
2778:. EU Clinical Trials Register
2592:10.1016/j.leukres.2012.05.010
2522:10.1016/S0960-894X(03)00778-9
2337:10.1016/S0093-7754(01)90031-4
2230:Update on Cancer Therapeutics
2207:10.1016/S0093-7754(01)90031-4
1458:Lenalidomide and pomalidomide
1430:-carbethoxyphthalimide gives
686:Waldenström macroglobulinemia
652:diffuse large B-cell lymphoma
164:Cereblon E3 ligase modulators
3899:dihydroorotate dehydrogenase
3780:Journal of Clinical Oncology
3182:10.1016/j.biocel.2007.01.022
2632:10.1517/14656566.2012.656091
2409:-{2--1,3-dioxo-2,3-dihydro-1
2022:Proc. Natl. Acad. Sci. U.S.A
1949:10.1016/j.biopha.2012.05.001
1056:, increasing sensitivity to
937:the production is enhanced.
881:Altering cytokine production
656:chronic lymphocytic leukemia
371:tumour necrosis factor-alpha
346:. Adverse reactions include
258:Food and Drug Administration
168:immunomodulatory imide drugs
40:Cereblon E3 ligase modulator
5534:Melatonin receptor agonists
5485:Thalidomide and its analogs
5440:Memantine and related drugs
5390:Cyclooxygenase 2 inhibitors
3878:purine synthesis inhibitors
3660:. European Medicines Agency
2997:Expert Review of Hematology
2686:. European Medicines Agency
1667:3 hours in healthy subjects
1084:component of the mechanism
1014:for thalidomide in various
965:complex by phosphorylating
544:, a complication of leprosy
535:, a precursor condition to
304:Federal Republic of Germany
18:Immunomodulatory imide drug
5616:
5400:Direct thrombin inhibitors
5337:Case studies of discovery
3749:10.1182/blood-2002-03-0996
3560:10.1016/j.bmcl.2007.08.042
3484:10.1016/j.bmcl.2005.08.086
3435:Angiogenesis Inhibitors".
3269:10.1016/j.beha.2007.09.002
2776:"Clinical Trials Register"
1885:10.2174/157489207780832478
1679:
1603:
1532:
1130:expressed in osteoclasts.
795:fibroblast growth factor 8
745:in those receiving IMiDs.
295:
29:
5515:CCR5 receptor antagonists
4063:IL-1 receptor antagonists
3410:10.1016/j.bmc.2006.06.042
2242:10.1016/j.uct.2009.03.003
1705:
1688:
1657:Has not yet been studied
1632:
1612:
1558:
1541:
1009:(VEGF) and its receptor,
769:protein localized in the
612:graft-versus-host disease
400:European Medicines Agency
154:
75:Erythema nodosum leprosum
53:
44:
5450:Neuraminidase inhibitors
4936:Anti-lymphocyte globulin
3614:Clinical Cancer Research
1984:10.1177/1049909109348981
1799:Immunomodulation therapy
636:myelodysplastic syndrome
533:Myelodysplastic syndrome
83:myelodysplastic syndrome
5425:HIV-protease inhibitors
5346:5α-Reductase inhibitors
4931:Anti-thymocyte globulin
3843:Immunosuppressive drugs
3793:10.1200/JCO.2004.10.052
3132:Tzu Chi Medical Journal
1583:Hydrolized metabolites
1283:Angiogenesis inhibition
1079:Bone marrow environment
1058:FAS mediated cell death
743:acute myeloid leukaemia
575:Acute myeloid leukaemia
537:acute myeloid leukaemia
310:on October 1, 1957, by
87:acute myeloid leukaemia
5475:Proton pump inhibitors
4736:Interleukin-6 receptor
4162:Complement component 5
3314:10.1186/1756-8722-2-36
2896:Advances in Hematology
2093:10.1038/sj.bjc.6690020
2043:10.1073/pnas.91.9.4082
1840:(4 Suppl 5): S24–S30.
1517:
1516:Lenalidomide synthesis
1506:to give pomalidomide.
1470:
1469:Pomalidomide synthesis
1446:) in tetrahydrofuran (
1401:
1390:
1295:
1204:
1193:
1146:
969:on the CD28 receptor.
947:maximum tolerated dose
877:
819:tumour necrosis factor
803:positive feedback loop
739:venous thromboembolism
674:acute myeloid leukemia
500:
429:
404:Non-Hodgkin's lymphoma
176:immunomodulatory drugs
2800:. The myeloma beacon.
2734:. Medscape references
2149:Nature Reviews Cancer
1809:Immunomodulatory drug
1512:
1493:of hydrogen with 10%
1465:
1396:
1385:
1290:
1199:
1188:
1141:
872:
727:peripheral neuropathy
495:
424:
348:peripheral neuropathy
5430:Integrase inhibitors
5405:Direct Xa inhibitors
3224:10.1038/leu.2009.236
2959:10.1038/leu.2009.236
2846:Congenital Anomalies
2664:. MedScape reference
2325:Seminars in Oncology
1921:January 3, 2014, at
1834:Seminars in Oncology
1481:-glutamine. The Cbz-
801:. This disrupts the
660:mantle cell lymphoma
587:renal cell carcinoma
5580:Medicinal chemistry
2909:10.1155/2012/842945
2269:2011NYASA1222...76Z
2034:1994PNAS...91.4082D
1152:molecular modelling
1060:and enhancement of
1029:Anti-tumor activity
979:natural killer cell
931:lipopolysaccharides
858:mechanism of action
813:Mechanism of action
779:E3 ubiquitin ligase
690:lupus erythematosus
678:follicular lymphoma
620:aphthous stomatitis
618:, severe recurrent
522:autoimmune diseases
190:and its analogues (
178:(drugs that adjust
5565:Immunosuppressants
5500:Tubulin inhibitors
5093:Diroximel fumarate
4766:IL-2 receptor/CD25
4194:Certolizumab pegol
3847:Immunosuppressants
3176:(7–8): 1489–1499.
2712:on 19 January 2013
2307:"Vector has moved"
1518:
1471:
1407:recrystallizations
1402:
1391:
1372:primary literature
1296:
1205:
1194:
1147:
1120:adhesion molecules
935:T-cell stimulation
878:
711:systemic sclerosis
694:Hodgkin's lymphoma
558:Hodgkin's lymphoma
501:
430:
386:of safer analogs.
174:), are a class of
5547:
5546:
5495:TRPV1 antagonists
5435:Lipase inhibitors
5251:
5250:
5098:Efgartigimod alfa
5088:Dimethyl fumarate
5016:
5015:
4944:
4943:
4917:
4916:
4122:
4121:
4048:
4047:
3889:Mycophenolic acid
3786:(16): 3269–3276.
3620:(17): 5949–5956.
3595:10.1021/op980201b
3554:(21): 5819–5824.
3478:(24): 5509–5513.
3449:10.1021/jm0304820
3404:(20): 6874–6885.
3047:(10): 1218–1224.
3041:Leukemia Research
3010:10.1586/ehm.12.62
2756:. Clinical Trials
2586:(10): 1218–1224.
2580:Leukemia Research
2516:(20): 3415–3417.
2480:10.1021/jm9603328
2474:(17): 3238–3240.
2427:10.1021/jm900210d
1804:Immunosuppressant
1789:Organic chemistry
1744:
1743:
1673:
1672:
1597:
1596:
1484:
1480:
1437:
1423:
1416:
1364:
1363:
1356:
1128:cysteine protease
1001:Anti-angiogenesis
953:T-cell activation
332:adverse reactions
312:Chemie Grünenthal
250:anti-inflammatory
161:
160:
107:Biological target
66:Class identifiers
16:(Redirected from
5607:
5539:Renin inhibitors
5385:c-Met inhibitors
5309:Drug development
5278:
5271:
5264:
5255:
4894:Telimomab aritox
4797:Zolimomab aritox
4618:CD62L/L-selectin
4356:Immunoglobulin E
4149:
4140:
4131:
4059:
3865:
3836:
3829:
3822:
3813:
3806:
3805:
3795:
3771:
3762:
3761:
3751:
3742:(9): 3063–3067.
3727:
3718:
3717:
3707:
3683:
3670:
3669:
3667:
3665:
3659:
3651:
3640:
3639:
3629:
3605:
3599:
3598:
3578:
3572:
3571:
3542:
3536:
3535:
3507:
3496:
3495:
3467:
3461:
3460:
3443:(9): 2219–2227.
3431:
3422:
3421:
3392:
3386:
3385:
3375:
3343:
3337:
3336:
3326:
3316:
3292:
3281:
3280:
3252:
3246:
3245:
3235:
3203:
3194:
3193:
3165:
3150:
3149:
3147:
3123:
3106:
3105:
3095:
3071:
3065:
3064:
3036:
3030:
3029:
2987:
2981:
2980:
2970:
2938:
2932:
2931:
2921:
2911:
2887:
2872:
2871:
2861:
2837:
2828:
2827:
2825:
2823:
2808:
2802:
2801:
2794:
2788:
2787:
2785:
2783:
2772:
2766:
2765:
2763:
2761:
2750:
2744:
2743:
2741:
2739:
2728:
2722:
2721:
2719:
2717:
2708:. Archived from
2702:
2696:
2695:
2693:
2691:
2680:
2674:
2673:
2671:
2669:
2658:
2652:
2651:
2615:
2604:
2603:
2575:
2569:
2568:
2540:
2534:
2533:
2505:
2492:
2491:
2462:
2439:
2438:
2397:
2376:
2375:
2355:
2349:
2348:
2320:
2311:
2310:
2303:
2297:
2296:
2252:
2246:
2245:
2225:
2219:
2218:
2190:
2181:
2180:
2144:
2115:
2114:
2104:
2072:
2066:
2065:
2055:
2045:
2013:
2004:
2003:
1967:
1961:
1960:
1931:
1925:
1913:
1907:
1906:
1896:
1864:
1858:
1857:
1829:
1759:Multiple myeloma
1710:
1686:
1637:
1610:
1563:
1539:
1524:Pharmacokinetics
1482:
1478:
1435:
1421:
1414:
1359:
1352:
1348:
1345:
1339:
1319:
1318:
1311:
843:interferon gamma
827:immunoglobulin G
731:thrombocytopenia
632:Kaposi's sarcoma
548:Multiple myeloma
542:Erythema nodosum
453:. Generally the
334:along with poor
262:multiple myeloma
213:The name "IMiD"
182:) containing an
180:immune responses
166:, also known as
145:
79:multiple myeloma
58:
37:
21:
5615:
5614:
5610:
5609:
5608:
5606:
5605:
5604:
5585:PDE4 inhibitors
5550:
5549:
5548:
5543:
5528:
5470:PDE5 inhibitors
5460:NS5A inhibitors
5445:mTOR inhibitors
5374:
5338:
5332:
5292:Steps in design
5287:
5282:
5252:
5247:
5193:Rozanolixizumab
5078:Deucravacitinib
5012:
4940:
4913:
4802:
4451:
4445:
4153:
4118:
4075:
4054:
4044:
4002:
3942:
3933:
3869:Antimetabolites
3860:
3854:
3840:
3810:
3809:
3773:
3772:
3765:
3729:
3728:
3721:
3685:
3684:
3673:
3663:
3661:
3657:
3653:
3652:
3643:
3607:
3606:
3602:
3580:
3579:
3575:
3544:
3543:
3539:
3518:(11): 1625–30.
3509:
3508:
3499:
3469:
3468:
3464:
3433:
3432:
3425:
3394:
3393:
3389:
3352:Br. J. Haematol
3345:
3344:
3340:
3294:
3293:
3284:
3254:
3253:
3249:
3205:
3204:
3197:
3167:
3166:
3153:
3125:
3124:
3109:
3073:
3072:
3068:
3038:
3037:
3033:
2989:
2988:
2984:
2940:
2939:
2935:
2889:
2888:
2875:
2839:
2838:
2831:
2821:
2819:
2818:on 5 March 2016
2810:
2809:
2805:
2796:
2795:
2791:
2781:
2779:
2774:
2773:
2769:
2759:
2757:
2752:
2751:
2747:
2737:
2735:
2730:
2729:
2725:
2715:
2713:
2704:
2703:
2699:
2689:
2687:
2682:
2681:
2677:
2667:
2665:
2660:
2659:
2655:
2617:
2616:
2607:
2577:
2576:
2572:
2542:
2541:
2537:
2507:
2506:
2495:
2464:
2463:
2442:
2399:
2398:
2379:
2357:
2356:
2352:
2322:
2321:
2314:
2305:
2304:
2300:
2254:
2253:
2249:
2227:
2226:
2222:
2192:
2191:
2184:
2161:10.1038/nrc1323
2146:
2145:
2118:
2074:
2073:
2069:
2015:
2014:
2007:
1969:
1968:
1964:
1933:
1932:
1928:
1914:
1910:
1866:
1865:
1861:
1831:
1830:
1823:
1818:
1813:
1749:
1716:Protein binding
1703:
1698:
1684:
1678:
1668:
1643:Protein binding
1627:
1622:
1608:
1602:
1569:Protein binding
1556:
1551:
1537:
1531:
1526:
1460:
1380:
1360:
1349:
1343:
1340:
1332:help improve it
1329:
1320:
1316:
1309:
1285:
1278:
1274:
1270:
1266:
1262:
1245:
1241:
1237:
1233:
1225:
1183:
1181:PDE4 inhibitors
1161:
1136:
1126:, an important
1116:bone resorption
1081:
1031:
1003:
955:
883:
867:
815:
751:
723:
721:Adverse effects
702:
648:
616:aphthous ulcers
596:
581:Prostate cancer
514:
505:bioavailability
490:
435:
362:complications.
356:thromboembolism
300:
294:
246:anti-angiogenic
61:
35:
28:
23:
22:
15:
12:
11:
5:
5613:
5611:
5603:
5602:
5597:
5595:TNF inhibitors
5592:
5587:
5582:
5577:
5572:
5567:
5562:
5552:
5551:
5545:
5544:
5542:
5541:
5536:
5531:
5526:
5522:
5517:
5512:
5507:
5502:
5497:
5492:
5487:
5482:
5477:
5472:
5467:
5462:
5457:
5452:
5447:
5442:
5437:
5432:
5427:
5422:
5417:
5412:
5407:
5402:
5397:
5392:
5387:
5382:
5380:Cephalosporins
5377:
5372:
5368:
5363:
5358:
5353:
5351:ACE inhibitors
5348:
5342:
5340:
5334:
5333:
5331:
5330:
5329:
5328:
5327:
5326:
5316:
5306:
5301:
5299:Drug discovery
5295:
5293:
5289:
5288:
5283:
5281:
5280:
5273:
5266:
5258:
5249:
5248:
5246:
5245:
5240:
5235:
5230:
5225:
5220:
5215:
5210:
5205:
5200:
5195:
5190:
5185:
5180:
5175:
5170:
5165:
5160:
5155:
5150:
5145:
5140:
5135:
5130:
5125:
5120:
5115:
5110:
5105:
5102:+hyaluronidase
5095:
5090:
5085:
5083:Deuruxolitinib
5080:
5075:
5070:
5065:
5060:
5055:
5050:
5045:
5040:
5035:
5030:
5024:
5022:
5018:
5017:
5014:
5013:
5011:
5010:
5005:
5000:
4995:
4994:
4993:
4988:
4976:
4975:
4974:
4969:
4956:
4954:
4946:
4945:
4942:
4941:
4939:
4938:
4933:
4927:
4925:
4919:
4918:
4915:
4914:
4912:
4911:
4906:
4901:
4896:
4891:
4886:
4881:
4876:
4871:
4866:
4861:
4856:
4851:
4846:
4841:
4836:
4831:
4826:
4821:
4816:
4810:
4808:
4804:
4803:
4801:
4800:
4787:
4786:
4785:
4784:
4779:
4774:
4761:
4760:
4759:
4758:
4746:
4745:
4744:
4732:
4731:
4730:
4725:
4713:
4712:
4711:
4706:
4701:
4689:
4688:
4687:
4676:
4675:
4674:
4673:
4668:
4656:
4655:
4654:
4642:
4641:
4640:
4628:
4627:
4626:
4614:
4613:
4612:
4607:
4595:
4594:
4593:
4588:
4576:
4575:
4574:
4569:
4564:
4559:
4556:+hyaluronidase
4549:
4537:
4536:
4535:
4523:
4522:
4521:
4509:
4508:
4507:
4502:
4497:
4485:
4484:
4483:
4478:
4473:
4468:
4455:
4453:
4447:
4446:
4444:
4443:
4442:
4441:
4428:
4427:
4426:
4425:
4420:
4399:
4398:
4397:
4396:
4383:
4382:
4381:
4380:
4367:
4366:
4365:
4364:
4351:
4350:
4349:
4348:
4335:
4334:
4333:
4332:
4327:
4322:
4317:
4312:
4307:
4302:
4297:
4292:
4287:
4282:
4277:
4272:
4267:
4262:
4257:
4252:
4247:
4242:
4237:
4232:
4227:
4214:
4213:
4212:
4211:
4206:
4201:
4196:
4191:
4186:
4173:
4172:
4171:
4170:
4157:
4155:
4146:
4137:
4128:
4124:
4123:
4120:
4119:
4117:
4116:
4111:
4106:
4101:
4096:
4091:
4085:
4083:
4077:
4076:
4074:
4073:
4067:
4065:
4056:
4050:
4049:
4046:
4045:
4043:
4042:
4041:
4040:
4032:PDE4 inhibitor
4028:
4023:
4018:
4012:
4010:
4004:
4003:
4001:
4000:
3995:
3989:
3988:
3987:
3986:
3981:
3976:
3971:
3950:
3948:
3935:
3934:
3932:
3931:
3930:
3929:
3916:
3915:
3914:
3913:
3908:
3894:
3893:
3892:
3891:
3886:
3873:
3871:
3862:
3856:
3855:
3841:
3839:
3838:
3831:
3824:
3816:
3808:
3807:
3763:
3719:
3698:(3): 219–226.
3671:
3641:
3600:
3589:(2): 139–140.
3573:
3537:
3497:
3462:
3423:
3387:
3338:
3282:
3263:(4): 717–735.
3247:
3195:
3151:
3138:(3): 188–195.
3107:
3066:
3031:
2982:
2933:
2873:
2829:
2803:
2789:
2767:
2745:
2723:
2697:
2675:
2653:
2626:(4): 473–494.
2605:
2570:
2535:
2493:
2440:
2377:
2350:
2331:(6): 597–601.
2312:
2298:
2247:
2236:(4): 170–181.
2220:
2201:(6): 597–601.
2182:
2155:(4): 314–322.
2116:
2067:
2005:
1978:(3): 198–204.
1962:
1926:
1908:
1879:(2): 167–174.
1859:
1820:
1819:
1817:
1814:
1812:
1811:
1806:
1801:
1796:
1791:
1786:
1781:
1776:
1771:
1766:
1761:
1756:
1750:
1748:
1745:
1742:
1741:
1740:6.2–7.9 hours
1738:
1732:
1731:
1728:
1722:
1721:
1718:
1712:
1711:
1704:
1700:
1696:
1691:
1690:
1680:Main article:
1677:
1674:
1671:
1670:
1665:
1659:
1658:
1655:
1649:
1648:
1645:
1639:
1638:
1631:
1624:
1620:
1615:
1614:
1604:Main article:
1601:
1598:
1595:
1594:
1593:5.5–7.6 hours
1591:
1585:
1584:
1581:
1575:
1574:
1571:
1565:
1564:
1557:
1553:
1549:
1544:
1543:
1533:Main article:
1530:
1527:
1525:
1522:
1489:, under 50–60
1487:hydrogenolysis
1459:
1456:
1424:-glutamic acid
1379:
1376:
1362:
1361:
1323:
1321:
1314:
1308:
1305:
1284:
1281:
1280:
1279:
1276:
1272:
1268:
1264:
1260:
1248:
1247:
1243:
1239:
1235:
1231:
1223:
1182:
1179:
1160:
1157:
1135:
1132:
1080:
1077:
1030:
1027:
1002:
999:
954:
951:
882:
879:
866:
863:
841:and increases
814:
811:
791:ubiquitination
787:carcinogenesis
750:
749:Teratogenicity
747:
722:
719:
701:
698:
647:
644:
595:
592:
591:
590:
583:
578:
572:
565:
560:
551:
550:
545:
539:
513:
510:
489:
486:
475:interconversed
434:
431:
360:dermatological
296:Main article:
293:
290:
276:which include
272:and increased
159:
158:
152:
151:
147:
146:
139:
133:
132:
128:
127:
109:
103:
102:
97:
91:
90:
72:
68:
67:
63:
62:
59:
51:
50:
42:
41:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
5612:
5601:
5598:
5596:
5593:
5591:
5588:
5586:
5583:
5581:
5578:
5576:
5573:
5571:
5568:
5566:
5563:
5561:
5558:
5557:
5555:
5540:
5537:
5535:
5532:
5530:
5523:
5521:
5518:
5516:
5513:
5511:
5508:
5506:
5503:
5501:
5498:
5496:
5493:
5491:
5488:
5486:
5483:
5481:
5478:
5476:
5473:
5471:
5468:
5466:
5463:
5461:
5458:
5456:
5453:
5451:
5448:
5446:
5443:
5441:
5438:
5436:
5433:
5431:
5428:
5426:
5423:
5421:
5418:
5416:
5413:
5411:
5408:
5406:
5403:
5401:
5398:
5396:
5393:
5391:
5388:
5386:
5383:
5381:
5378:
5376:
5369:
5367:
5366:Beta-blockers
5364:
5362:
5361:Antiandrogens
5359:
5357:
5354:
5352:
5349:
5347:
5344:
5343:
5341:
5335:
5325:
5322:
5321:
5320:
5317:
5315:
5312:
5311:
5310:
5307:
5305:
5302:
5300:
5297:
5296:
5294:
5290:
5286:
5279:
5274:
5272:
5267:
5265:
5260:
5259:
5256:
5244:
5241:
5239:
5236:
5234:
5233:Tildrakizumab
5231:
5229:
5226:
5224:
5221:
5219:
5216:
5214:
5211:
5209:
5206:
5204:
5201:
5199:
5196:
5194:
5191:
5189:
5186:
5184:
5181:
5179:
5176:
5174:
5171:
5169:
5166:
5164:
5163:Pegcetacoplan
5161:
5159:
5156:
5154:
5151:
5149:
5146:
5144:
5141:
5139:
5136:
5134:
5131:
5129:
5126:
5124:
5121:
5119:
5116:
5114:
5111:
5109:
5106:
5103:
5099:
5096:
5094:
5091:
5089:
5086:
5084:
5081:
5079:
5076:
5074:
5073:Darvadstrocel
5071:
5069:
5066:
5064:
5061:
5059:
5056:
5054:
5051:
5049:
5046:
5044:
5041:
5039:
5036:
5034:
5031:
5029:
5026:
5025:
5023:
5019:
5009:
5006:
5004:
5001:
4999:
4996:
4992:
4989:
4987:
4984:
4983:
4982:
4981:
4980:TNF inhibitor
4977:
4973:
4970:
4968:
4965:
4964:
4963:
4962:
4958:
4957:
4955:
4952:
4947:
4937:
4934:
4932:
4929:
4928:
4926:
4924:
4920:
4910:
4907:
4905:
4902:
4900:
4897:
4895:
4892:
4890:
4887:
4885:
4882:
4880:
4877:
4875:
4872:
4870:
4867:
4865:
4862:
4860:
4857:
4855:
4852:
4850:
4847:
4845:
4842:
4840:
4837:
4835:
4832:
4830:
4827:
4825:
4822:
4820:
4817:
4815:
4812:
4811:
4809:
4805:
4798:
4794:
4793:
4789:
4788:
4783:
4780:
4778:
4775:
4773:
4770:
4769:
4768:
4767:
4763:
4762:
4757:
4754:
4753:
4752:
4751:
4747:
4743:
4740:
4739:
4738:
4737:
4733:
4729:
4726:
4724:
4721:
4720:
4719:
4718:
4714:
4710:
4707:
4705:
4702:
4700:
4697:
4696:
4695:
4694:
4690:
4686:
4683:
4682:
4681:
4678:
4677:
4672:
4669:
4667:
4664:
4663:
4662:
4661:
4657:
4653:
4650:
4649:
4648:
4647:
4646:CD147/Basigin
4643:
4639:
4636:
4635:
4634:
4633:
4629:
4625:
4622:
4621:
4620:
4619:
4615:
4611:
4608:
4606:
4603:
4602:
4601:
4600:
4596:
4592:
4589:
4587:
4584:
4583:
4582:
4581:
4577:
4573:
4570:
4568:
4565:
4563:
4560:
4557:
4553:
4550:
4548:
4545:
4544:
4543:
4542:
4538:
4534:
4531:
4530:
4529:
4528:
4524:
4520:
4517:
4516:
4515:
4514:
4510:
4506:
4503:
4501:
4498:
4496:
4493:
4492:
4491:
4490:
4486:
4482:
4479:
4477:
4474:
4472:
4469:
4467:
4466:Muromonab-CD3
4464:
4463:
4462:
4461:
4457:
4456:
4454:
4448:
4440:
4437:
4436:
4435:
4434:
4430:
4429:
4424:
4421:
4419:
4416:
4415:
4414:
4413:
4409:
4405:
4401:
4400:
4395:
4392:
4391:
4390:
4389:
4385:
4384:
4379:
4376:
4375:
4374:
4373:
4369:
4368:
4363:
4360:
4359:
4358:
4357:
4353:
4352:
4347:
4344:
4343:
4342:
4341:
4340:Interleukin 5
4337:
4336:
4331:
4328:
4326:
4323:
4321:
4320:Tildrakizumab
4318:
4316:
4313:
4311:
4308:
4306:
4303:
4301:
4298:
4296:
4293:
4291:
4288:
4286:
4283:
4281:
4278:
4276:
4273:
4271:
4268:
4266:
4263:
4261:
4258:
4256:
4253:
4251:
4248:
4246:
4243:
4241:
4238:
4236:
4233:
4231:
4228:
4226:
4223:
4222:
4221:
4220:
4216:
4215:
4210:
4207:
4205:
4202:
4200:
4197:
4195:
4192:
4190:
4187:
4185:
4182:
4181:
4180:
4179:
4175:
4174:
4169:
4166:
4165:
4164:
4163:
4159:
4158:
4156:
4154:(noncellular)
4150:
4147:
4145:
4141:
4138:
4136:
4132:
4129:
4127:Extracellular
4125:
4115:
4112:
4110:
4107:
4105:
4102:
4100:
4097:
4095:
4094:Ridaforolimus
4092:
4090:
4087:
4086:
4084:
4082:
4078:
4072:
4069:
4068:
4066:
4064:
4060:
4057:
4053:Intracellular
4051:
4039:
4036:
4035:
4034:
4033:
4029:
4027:
4024:
4022:
4019:
4017:
4014:
4013:
4011:
4009:
4005:
3999:
3996:
3994:
3991:
3990:
3985:
3982:
3980:
3977:
3975:
3972:
3970:
3967:
3966:
3965:
3964:
3960:
3956:
3952:
3951:
3949:
3946:
3940:
3936:
3928:
3925:
3924:
3923:
3922:
3918:
3917:
3912:
3911:Teriflunomide
3909:
3907:
3904:
3903:
3901:
3900:
3896:
3895:
3890:
3887:
3885:
3882:
3881:
3880:
3879:
3875:
3874:
3872:
3870:
3866:
3863:
3859:Intracellular
3857:
3852:
3848:
3844:
3837:
3832:
3830:
3825:
3823:
3818:
3817:
3814:
3803:
3799:
3794:
3789:
3785:
3781:
3777:
3770:
3768:
3764:
3759:
3755:
3750:
3745:
3741:
3737:
3733:
3726:
3724:
3720:
3715:
3711:
3706:
3701:
3697:
3693:
3689:
3682:
3680:
3678:
3676:
3672:
3656:
3650:
3648:
3646:
3642:
3637:
3633:
3628:
3623:
3619:
3615:
3611:
3604:
3601:
3596:
3592:
3588:
3584:
3577:
3574:
3569:
3565:
3561:
3557:
3553:
3549:
3541:
3538:
3533:
3529:
3525:
3521:
3517:
3513:
3506:
3504:
3502:
3498:
3493:
3489:
3485:
3481:
3477:
3473:
3466:
3463:
3458:
3454:
3450:
3446:
3442:
3438:
3430:
3428:
3424:
3419:
3415:
3411:
3407:
3403:
3399:
3391:
3388:
3383:
3379:
3374:
3369:
3365:
3361:
3357:
3353:
3349:
3342:
3339:
3334:
3330:
3325:
3320:
3315:
3310:
3306:
3302:
3298:
3291:
3289:
3287:
3283:
3278:
3274:
3270:
3266:
3262:
3258:
3251:
3248:
3243:
3239:
3234:
3229:
3225:
3221:
3217:
3213:
3209:
3202:
3200:
3196:
3191:
3187:
3183:
3179:
3175:
3171:
3164:
3162:
3160:
3158:
3156:
3152:
3146:
3141:
3137:
3133:
3129:
3122:
3120:
3118:
3116:
3114:
3112:
3108:
3103:
3099:
3094:
3089:
3086:(3): 287–94.
3085:
3081:
3077:
3070:
3067:
3062:
3058:
3054:
3050:
3046:
3042:
3035:
3032:
3027:
3023:
3019:
3015:
3011:
3007:
3003:
2999:
2998:
2993:
2986:
2983:
2978:
2974:
2969:
2964:
2960:
2956:
2952:
2948:
2944:
2937:
2934:
2929:
2925:
2920:
2915:
2910:
2905:
2901:
2897:
2893:
2886:
2884:
2882:
2880:
2878:
2874:
2869:
2865:
2860:
2855:
2851:
2847:
2843:
2836:
2834:
2830:
2817:
2813:
2807:
2804:
2799:
2793:
2790:
2777:
2771:
2768:
2755:
2749:
2746:
2733:
2727:
2724:
2711:
2707:
2701:
2698:
2685:
2679:
2676:
2663:
2657:
2654:
2649:
2645:
2641:
2637:
2633:
2629:
2625:
2621:
2614:
2612:
2610:
2606:
2601:
2597:
2593:
2589:
2585:
2581:
2574:
2571:
2566:
2562:
2558:
2554:
2550:
2546:
2539:
2536:
2531:
2527:
2523:
2519:
2515:
2511:
2504:
2502:
2500:
2498:
2494:
2489:
2485:
2481:
2477:
2473:
2469:
2461:
2459:
2457:
2455:
2453:
2451:
2449:
2447:
2445:
2441:
2436:
2432:
2428:
2424:
2421:(6): 1522–4.
2420:
2416:
2412:
2408:
2404:
2396:
2394:
2392:
2390:
2388:
2386:
2384:
2382:
2378:
2373:
2369:
2366:(8): 2300–5.
2365:
2361:
2354:
2351:
2346:
2342:
2338:
2334:
2330:
2326:
2319:
2317:
2313:
2308:
2302:
2299:
2294:
2290:
2286:
2282:
2278:
2274:
2270:
2266:
2262:
2258:
2251:
2248:
2243:
2239:
2235:
2231:
2224:
2221:
2216:
2212:
2208:
2204:
2200:
2196:
2189:
2187:
2183:
2178:
2174:
2170:
2166:
2162:
2158:
2154:
2150:
2143:
2141:
2139:
2137:
2135:
2133:
2131:
2129:
2127:
2125:
2123:
2121:
2117:
2112:
2108:
2103:
2098:
2094:
2090:
2086:
2082:
2081:Br. J. Cancer
2078:
2071:
2068:
2063:
2059:
2054:
2049:
2044:
2039:
2035:
2031:
2028:(9): 4082–5.
2027:
2023:
2019:
2012:
2010:
2006:
2001:
1997:
1993:
1989:
1985:
1981:
1977:
1973:
1966:
1963:
1958:
1954:
1950:
1946:
1942:
1938:
1930:
1927:
1924:
1923:archive.today
1920:
1917:
1912:
1909:
1904:
1900:
1895:
1890:
1886:
1882:
1878:
1874:
1870:
1863:
1860:
1855:
1851:
1847:
1843:
1839:
1835:
1828:
1826:
1822:
1815:
1810:
1807:
1805:
1802:
1800:
1797:
1795:
1794:Health crisis
1792:
1790:
1787:
1785:
1782:
1780:
1777:
1775:
1772:
1770:
1767:
1765:
1762:
1760:
1757:
1755:
1752:
1751:
1746:
1739:
1737:
1734:
1733:
1729:
1727:
1724:
1723:
1719:
1717:
1714:
1713:
1709:
1701:
1699:
1693:
1692:
1689:Pomalidomide
1687:
1683:
1675:
1666:
1664:
1661:
1660:
1656:
1654:
1651:
1650:
1646:
1644:
1641:
1640:
1636:
1630:
1625:
1623:
1617:
1616:
1613:Lenalidomide
1611:
1607:
1599:
1592:
1590:
1587:
1586:
1582:
1580:
1577:
1576:
1572:
1570:
1567:
1566:
1562:
1554:
1552:
1546:
1545:
1540:
1536:
1528:
1523:
1521:
1515:
1511:
1507:
1505:
1504:hydrogenation
1500:
1499:ethyl acetate
1496:
1492:
1488:
1476:
1468:
1464:
1457:
1455:
1453:
1449:
1445:
1441:
1433:
1429:
1425:
1418:
1410:
1408:
1399:
1395:
1388:
1384:
1377:
1375:
1373:
1369:
1358:
1355:
1347:
1337:
1333:
1327:
1324:This section
1322:
1313:
1312:
1306:
1304:
1301:
1293:
1289:
1282:
1271:< NHC(O)CH
1258:
1257:
1256:
1254:
1229:
1228:
1227:
1221:
1216:
1214:
1209:
1202:
1198:
1191:
1187:
1180:
1178:
1176:
1172:
1167:
1158:
1156:
1153:
1144:
1140:
1133:
1131:
1129:
1125:
1121:
1117:
1113:
1109:
1105:
1101:
1097:
1096:stromal cells
1094:
1089:
1087:
1078:
1076:
1074:
1071:
1067:
1063:
1059:
1055:
1051:
1047:
1043:
1039:
1035:
1028:
1026:
1023:
1019:
1018:
1012:
1008:
1000:
998:
996:
992:
988:
984:
980:
976:
972:
968:
964:
960:
952:
950:
948:
943:
938:
936:
932:
928:
924:
920:
916:
912:
908:
904:
900:
896:
892:
888:
880:
875:
871:
864:
862:
859:
854:
852:
848:
847:interleukin 2
844:
840:
836:
832:
828:
824:
823:interleukin 6
820:
812:
810:
806:
804:
800:
796:
792:
788:
784:
783:embryogenesis
780:
776:
772:
768:
764:
759:
756:
748:
746:
744:
740:
736:
732:
728:
720:
718:
716:
715:polycythaemia
712:
708:
707:myelofibrosis
699:
697:
695:
691:
687:
683:
682:MALT lymphoma
679:
675:
670:
665:
661:
657:
653:
645:
643:
641:
637:
633:
629:
625:
621:
617:
613:
609:
605:
601:
600:dexamethasone
593:
588:
584:
582:
579:
576:
573:
570:
569:myelofibrosis
566:
564:
561:
559:
556:
555:
554:
549:
546:
543:
540:
538:
534:
531:
530:
529:
527:
523:
519:
511:
509:
506:
498:
494:
487:
485:
483:
479:
476:
472:
468:
464:
460:
456:
452:
447:
444:
440:
439:glutamic acid
432:
427:
423:
419:
417:
413:
407:
405:
401:
396:
392:
387:
385:
380:
376:
372:
368:
363:
361:
357:
353:
349:
345:
341:
338:in water and
337:
333:
329:
325:
321:
317:
313:
309:
305:
299:
291:
289:
287:
283:
279:
275:
271:
267:
263:
259:
254:
251:
247:
242:
240:
239:
234:
233:
228:
227:
222:
221:
216:
211:
209:
205:
201:
197:
193:
189:
185:
181:
177:
173:
169:
165:
157:
153:
148:
144:
140:
138:
134:
131:Clinical data
129:
125:
121:
117:
113:
110:
108:
104:
101:
98:
96:
92:
88:
84:
80:
76:
73:
69:
64:
57:
52:
49:
48:
43:
38:
33:
19:
5590:Orphan drugs
5570:Phthalimides
5560:Glutarimides
5243:Upadacitinib
5203:Satralizumab
5188:Ritlecitinib
5183:Risankizumab
4978:
4959:
4899:Teprotumumab
4849:Inebilizumab
4844:Fontolizumab
4824:Atorolimumab
4792:T-lymphocyte
4790:
4764:
4748:
4734:
4715:
4704:Lerdelimumab
4699:Bertilimumab
4691:
4679:
4658:
4644:
4630:
4616:
4597:
4578:
4562:Pascolizumab
4547:Obinutuzumab
4539:
4525:
4511:
4495:Clenoliximab
4487:
4471:Otelixizumab
4458:
4431:
4418:Lebrikizumab
4402:
4386:
4370:
4354:
4338:
4295:Satralizumab
4285:Risankizumab
4217:
4176:
4160:
4152:Serum target
4104:Temsirolimus
4030:
4021:Pomalidomide
4016:Lenalidomide
3974:Pimecrolimus
3953:
3927:Methotrexate
3919:
3897:
3884:Azathioprine
3876:
3861:(initiation)
3783:
3779:
3739:
3735:
3695:
3691:
3664:23 September
3662:. Retrieved
3617:
3613:
3603:
3586:
3582:
3576:
3551:
3547:
3540:
3515:
3511:
3475:
3471:
3465:
3440:
3436:
3401:
3397:
3390:
3358:(3): 503–8.
3355:
3351:
3341:
3304:
3300:
3260:
3256:
3250:
3218:(1): 22–32.
3215:
3211:
3173:
3169:
3135:
3131:
3083:
3079:
3069:
3044:
3040:
3034:
3004:(1): 69–82.
3001:
2995:
2985:
2953:(1): 22–32.
2950:
2946:
2936:
2899:
2895:
2849:
2845:
2822:18 September
2820:. Retrieved
2816:the original
2806:
2792:
2782:18 September
2780:. Retrieved
2770:
2760:18 September
2758:. Retrieved
2748:
2738:18 September
2736:. Retrieved
2726:
2716:18 September
2714:. Retrieved
2710:the original
2700:
2690:18 September
2688:. Retrieved
2678:
2668:18 September
2666:. Retrieved
2656:
2623:
2619:
2583:
2579:
2573:
2551:(1): 56–68.
2548:
2544:
2538:
2513:
2509:
2471:
2467:
2418:
2414:
2410:
2406:
2402:
2363:
2359:
2353:
2328:
2324:
2301:
2263:(1): 76–82.
2260:
2256:
2250:
2233:
2229:
2223:
2198:
2195:Semin. Oncol
2194:
2152:
2148:
2087:(1): 114–8.
2084:
2080:
2070:
2025:
2021:
1975:
1971:
1965:
1943:(5): 323–9.
1940:
1936:
1929:
1911:
1876:
1872:
1862:
1837:
1833:
1779:Pomalidomide
1774:Lenalidomide
1735:
1725:
1715:
1694:
1682:Pomalidomide
1676:Pomalidomide
1662:
1652:
1642:
1628:
1618:
1606:Lenalidomide
1600:Lenalidomide
1588:
1578:
1568:
1547:
1542:Thalidomide
1519:
1513:
1472:
1466:
1431:
1427:
1419:rather than
1411:
1403:
1397:
1386:
1368:synthesizing
1365:
1350:
1341:
1325:
1297:
1291:
1252:
1249:
1217:
1210:
1206:
1200:
1189:
1162:
1148:
1142:
1090:
1085:
1082:
1037:
1033:
1032:
1015:
1004:
986:
983:cytotoxicity
970:
956:
941:
939:
923:solid tumors
910:
902:
884:
873:
855:
816:
807:
760:
752:
724:
703:
700:Pomalidomide
649:
646:Lenalidomide
597:
552:
515:
502:
496:
477:
470:
466:
462:
454:
448:
436:
425:
415:
411:
408:
388:
377:produced by
364:
352:constipation
311:
307:
303:
301:
282:pomalidomide
278:lenalidomide
270:side effects
255:
253:intestines.
243:
236:
230:
224:
218:
212:
196:pomalidomide
192:lenalidomide
171:
167:
163:
162:
150:Legal status
143:Drug Classes
45:
5529:antagonists
5314:Preclinical
5304:Hit to lead
5285:Drug design
5238:Tofacitinib
5178:Ravulizumab
5168:Pirfenidone
5158:Peficitinib
5058:Canakinumab
5048:Briakinumab
5038:Bimekizumab
5033:Baricitinib
4998:Aflibercept
4909:Vepalimomab
4904:Vapaliximab
4879:Rovelizumab
4869:Pexelizumab
4859:Morolimumab
4834:Cedelizumab
4819:Anifrolumab
4814:Alemtuzumab
4772:Basiliximab
4742:Tocilizumab
4728:Vedolizumab
4723:Natalizumab
4709:Metelimumab
4652:Gavilimomab
4610:Toralizumab
4605:Teneliximab
4591:Lumiliximab
4586:Gomiliximab
4572:Ublituximab
4552:Ocrelizumab
4505:Zanolimumab
4481:Visilizumab
4439:Secukinumab
4423:Ustekinumab
4394:Elsilimomab
4378:Faralimomab
4346:Mepolizumab
4330:Ustekinumab
4325:Tocilizumab
4300:Secukinumab
4250:Canakinumab
4240:Briakinumab
4235:Bimekizumab
4230:Basiliximab
4219:Interleukin
4209:Nerelimomab
4114:Zotarolimus
4055:(reception)
4026:Thalidomide
3984:Voclosporin
3969:Ciclosporin
3963:Calcineurin
3959:Cyclophilin
3906:Leflunomide
3902:inhibitors
1769:Thalidomide
1764:Drug design
1726:Metabolites
1702:0.5–8 hours
1653:Metabolites
1579:Metabolites
1535:Thalidomide
1529:Thalidomide
1497:mixed with
1434:-phthaloyl-
1378:Thalidomide
1300:glutarimide
1230:COOH ≤ CONH
1220:methylamide
1124:cathepsin K
1112:Osteoclasts
1093:bone marrow
797:(FGF8) and
594:Thalidomide
585:Metastatic
512:Medical use
433:Development
391:amino group
379:macrophages
358:along with
328:teratogenic
298:Thalidomide
268:with fewer
200:mezigdomide
188:thalidomide
156:In Wikidata
60:Thalidomide
5575:Teratogens
5554:Categories
5420:Gliflozins
5228:Sutimlimab
5223:Spesolimab
5208:Siltuximab
5148:Olokizumab
5138:Ixekizumab
5133:Itacitinib
5123:Guselkumab
5118:Fingolimod
5113:Filgotinib
5063:Crovalimab
5053:Brodalumab
5043:Blisibimod
5008:Rilonacept
4991:Opinercept
4986:Etanercept
4972:Belatacept
4923:Polyclonal
4884:Siplizumab
4874:Reslizumab
4864:Ofatumumab
4854:Maslimomab
4839:Emapalumab
4782:Inolimomab
4777:Daclizumab
4756:Odulimomab
4671:Ruplizumab
4666:Frexalimab
4624:Aselizumab
4519:Efalizumab
4476:Teplizumab
4372:Interferon
4362:Omalizumab
4315:Spesolimab
4305:Siltuximab
4280:Rilonacept
4275:Olokizumab
4265:Ixekizumab
4260:Guselkumab
4255:Daclizumab
4245:Brodalumab
4204:Infliximab
4189:Afelimomab
4184:Adalimumab
4168:Eculizumab
4144:Monoclonal
4135:Antibodies
4109:Umirolimus
4089:Everolimus
4038:Apremilast
3998:Gusperimus
3979:Tacrolimus
3947:inhibitors
3939:Macrolides
3921:antifolate
2902:: 842945.
2852:(1): 1–7.
2360:Cancer Res
1816:References
1784:Apremilast
1736:Half-life
1663:Half-life
1589:Half-life
1417:-glutamine
1344:April 2017
1155:activity.
1073:cell cycle
1040:assays on
755:hypotheses
626:syndrome,
604:prednisone
482:enantiomer
344:intestines
340:absorption
336:solubility
324:antiemetic
316:Grünenthal
204:iberdomide
47:Drug class
5218:Sirukumab
5213:Siponimod
5198:Sarilumab
5173:Ponesimod
5143:Netakimab
5128:Iptacopan
5108:Etrasimod
5068:Danicopan
5003:Alefacept
4967:Abatacept
4889:Talizumab
4829:Begelomab
4685:Belimumab
4638:Galiximab
4567:Rituximab
4533:Erlizumab
4500:Keliximab
4310:Sirukumab
4290:Sarilumab
4270:Netakimab
4199:Golimumab
4099:Sirolimus
3307:(1): 36.
1514:Scheme 4:
1467:Scheme 3:
1398:Scheme 2:
1387:Scheme 1:
1307:Synthesis
1292:Figure 8:
1201:Figure 7:
1190:Figure 6:
1143:Figure 5:
1050:caspase-9
1046:caspase-8
1042:apoptosis
874:Figure 2:
771:cytoplasm
765:is a 51 k
630:disease,
608:melphalan
497:Figure 3:
426:Figure 1:
384:synthesis
342:from the
308:Contergan
137:Drugs.com
5490:Triptans
5375:agonists
5319:Clinical
5153:Ozanimod
5028:Avacopan
5021:Unsorted
4807:Unsorted
4717:Integrin
4450:Cellular
4225:Anakinra
4071:Anakinra
3993:Abetimus
3845: /
3802:15249589
3758:12384400
3714:18452408
3636:15355928
3568:17851074
3532:10386948
3492:16183272
3457:15084120
3418:16843662
3333:19674465
3277:18070715
3242:19907437
3212:Leukemia
3190:17369076
3102:22003441
3061:22727252
3026:12782141
3018:23373782
2977:19907437
2947:Leukemia
2928:22919394
2868:22348778
2640:22324734
2600:22727252
2565:22796518
2530:14505639
2435:19256507
2372:11956087
2345:11740816
2285:21434945
2215:11740816
2169:15057291
2111:10408702
2000:24167431
1992:19843880
1957:22770990
1919:Archived
1903:17975653
1854:16085014
1747:See also
1730:Unknown
1720:Unknown
1213:rolipram
1038:In vitro
987:in vitro
971:In vitro
967:tyrosine
942:in vitro
911:in vitro
903:in vitro
851:cereblon
839:NK cells
763:Cereblon
567:Primary
451:racemate
412:in vitro
395:carbonyl
375:cytokine
320:sedative
208:Cereblon
95:ATC code
5480:Statins
4949:-cept (
3382:7527645
3373:3301416
3324:2736171
3233:3922408
3093:3193296
2968:3922408
2919:3417169
2648:7981368
2488:8765505
2293:5336195
2265:Bibcode
2177:7293027
2102:2362163
2062:7513432
2030:Bibcode
1894:2048745
1573:55–65%
1330:Please
1275:< CH
1246:< CN
1234:≤ COOCH
1222:(CONHCH
1175:bromine
1086:in vivo
1034:In vivo
1017:in vivo
835:T cells
775:nucleus
735:anaemia
713:, post-
628:Crohn's
624:wasting
606:and/or
526:leprosy
518:cancers
478:in vivo
416:in vivo
292:History
286:Celgene
274:potency
266:analogs
215:alludes
5324:Phases
4961:CTLA-4
4951:Fusion
4452:target
4433:IL-17A
4410:, and
3943:other
3800:
3756:
3712:
3634:
3566:
3530:
3490:
3455:
3416:
3380:
3370:
3331:
3321:
3275:
3240:
3230:
3188:
3100:
3090:
3059:
3024:
3016:
2975:
2965:
2926:
2916:
2866:
2646:
2638:
2598:
2563:
2528:
2486:
2433:
2370:
2343:
2291:
2283:
2213:
2175:
2167:
2109:
2099:
2060:
2050:
1998:
1990:
1955:
1901:
1891:
1852:
1754:Cancer
1452:reflux
1171:Iodine
1166:olefin
1104:ICAM-1
1100:VCAM-1
709:, MM,
664:anemia
589:(mRCC)
469:- and
459:isomer
235:, and
226:imido-
126:, etc.
4750:LFA-1
4660:CD154
4513:CD11a
4412:IL-23
4408:IL-13
4404:IL-12
4008:IMiDs
3736:Blood
3658:(PDF)
3022:S2CID
2644:S2CID
2289:S2CID
2173:S2CID
2053:43727
1996:S2CID
1647:~30%
1054:IGF-1
1022:NF-κB
995:T-bet
927:IL-12
899:IL-10
895:IL-12
799:FGF10
577:(AML)
571:(PMF)
443:IUPAC
314:(now
232:imid-
220:imide
184:imide
172:IMiDs
124:NF-kB
100:L04AX
5525:5-HT
5371:Beta
4680:BLyS
4632:CD80
4599:CD40
4580:CD23
4541:CD20
4527:CD18
4388:IL-6
4081:mTOR
3955:FKBP
3945:IL-2
3798:PMID
3754:PMID
3710:PMID
3666:2012
3632:PMID
3564:PMID
3528:PMID
3488:PMID
3453:PMID
3414:PMID
3378:PMID
3329:PMID
3273:PMID
3238:PMID
3186:PMID
3098:PMID
3057:PMID
3014:PMID
2973:PMID
2924:PMID
2900:2012
2864:PMID
2824:2012
2784:2012
2762:2012
2740:2012
2718:2012
2692:2012
2670:2012
2636:PMID
2596:PMID
2561:PMID
2526:PMID
2484:PMID
2431:PMID
2368:PMID
2341:PMID
2281:PMID
2261:1222
2211:PMID
2165:PMID
2107:PMID
2058:PMID
1988:PMID
1953:PMID
1899:PMID
1850:PMID
1495:Pd/C
1444:DMAP
1267:≤ NH
1259:N(CH
1238:≤ SO
1173:and
1106:and
1011:bFGF
963:CD28
919:CD8+
917:and
915:CD4+
907:mRNA
891:IL-6
887:IL-1
845:and
837:and
831:VEGF
829:and
825:and
737:and
658:and
638:and
520:and
414:and
280:and
248:and
238:imid
202:and
120:VEGF
116:IL-6
4693:CAT
4489:CD4
4460:CD3
4178:TNF
3851:L04
3788:doi
3744:doi
3740:100
3700:doi
3622:doi
3591:doi
3556:doi
3520:doi
3480:doi
3445:doi
3406:doi
3368:PMC
3360:doi
3319:PMC
3309:doi
3265:doi
3228:PMC
3220:doi
3178:doi
3140:doi
3088:PMC
3049:doi
3006:doi
2963:PMC
2955:doi
2914:PMC
2904:doi
2854:doi
2628:doi
2588:doi
2553:doi
2549:136
2518:doi
2476:doi
2423:doi
2333:doi
2273:doi
2238:doi
2203:doi
2157:doi
2097:PMC
2089:doi
2048:PMC
2038:doi
1980:doi
1945:doi
1889:PMC
1881:doi
1842:doi
1697:max
1621:max
1550:max
1491:psi
1475:Cbz
1448:THF
1440:CDI
1334:to
1108:LFA
991:Th2
975:Th1
112:TNF
71:Use
5556::
4406:,
3796:.
3784:22
3782:.
3778:.
3766:^
3752:.
3738:.
3734:.
3722:^
3708:.
3696:33
3694:.
3690:.
3674:^
3644:^
3630:.
3618:10
3616:.
3612:.
3585:.
3562:.
3552:17
3550:.
3526:.
3514:.
3500:^
3486:.
3476:15
3474:.
3451:.
3441:47
3439:.
3426:^
3412:.
3402:14
3400:.
3376:.
3366:.
3356:87
3354:.
3350:.
3327:.
3317:.
3303:.
3299:.
3285:^
3271:.
3261:20
3259:.
3236:.
3226:.
3216:24
3214:.
3210:.
3198:^
3184:.
3174:39
3172:.
3154:^
3136:20
3134:.
3130:.
3110:^
3096:.
3082:.
3078:.
3055:.
3045:36
3043:.
3020:.
3012:.
3000:.
2994:.
2971:.
2961:.
2951:24
2949:.
2945:.
2922:.
2912:.
2898:.
2894:.
2876:^
2862:.
2850:52
2848:.
2844:.
2832:^
2642:.
2634:.
2624:13
2622:.
2608:^
2594:.
2584:36
2582:.
2559:.
2547:.
2524:.
2514:13
2512:.
2496:^
2482:.
2472:39
2470:.
2443:^
2429:.
2419:52
2417:.
2405:)-
2380:^
2364:62
2362:.
2339:.
2329:28
2327:.
2315:^
2287:.
2279:.
2271:.
2259:.
2232:.
2209:.
2199:28
2197:.
2185:^
2171:.
2163:.
2151:.
2119:^
2105:.
2095:.
2085:79
2083:.
2079:.
2056:.
2046:.
2036:.
2026:91
2024:.
2020:.
2008:^
1994:.
1986:.
1976:27
1974:.
1951:.
1941:66
1939:.
1897:.
1887:.
1875:.
1871:.
1848:.
1838:32
1836:.
1824:^
1242:CH
1102:,
1070:G1
1066:G0
997:.
959:B7
893:,
889:,
853:.
821:,
785:,
773:,
767:Da
733:,
729:,
692:,
688:,
684:,
680:,
676:,
654:,
642:.
634:,
406:.
354:,
288:.
241:.
229:,
223:,
198:,
194:,
122:,
118:,
114:,
85:,
81:,
77:,
5527:3
5373:2
5277:e
5270:t
5263:v
5104:)
5100:(
4953:)
4799:)
4795:(
4558:)
4554:(
3961:/
3957:/
3941:/
3853:)
3849:(
3835:e
3828:t
3821:v
3804:.
3790::
3760:.
3746::
3716:.
3702::
3668:.
3638:.
3624::
3597:.
3593::
3587:3
3570:.
3558::
3534:.
3522::
3516:9
3494:.
3482::
3459:.
3447::
3420:.
3408::
3384:.
3362::
3335:.
3311::
3305:2
3279:.
3267::
3244:.
3222::
3192:.
3180::
3148:.
3142::
3104:.
3084:2
3063:.
3051::
3028:.
3008::
3002:6
2979:.
2957::
2930:.
2906::
2870:.
2856::
2826:.
2786:.
2764:.
2742:.
2720:.
2694:.
2672:.
2650:.
2630::
2602:.
2590::
2567:.
2555::
2532:.
2520::
2490:.
2478::
2437:.
2425::
2411:H
2407:N
2403:S
2401:(
2374:.
2347:.
2335::
2309:.
2295:.
2275::
2267::
2244:.
2240::
2234:3
2217:.
2205::
2179:.
2159::
2153:4
2113:.
2091::
2064:.
2040::
2032::
2002:.
1982::
1959:.
1947::
1905:.
1883::
1877:2
1856:.
1844::
1695:T
1619:T
1548:T
1483:L
1479:L
1477:-
1436:L
1432:N
1428:N
1422:L
1415:L
1357:)
1351:(
1346:)
1342:(
1328:.
1277:3
1273:3
1269:2
1265:2
1263:)
1261:3
1253:N
1244:3
1240:2
1236:3
1232:2
1224:3
1068:/
961:-
471:S
467:R
463:R
457:-
455:S
170:(
34:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.