419:
310:
measurement. The in-growth method is one way of measuring the decay constant of a system, which involves accumulating daughter nuclides. Unfortunately for nuclides with high decay constants (which are useful for dating very old samples), long periods of time (decades) are required to accumulate enough decay products in a single sample to accurately measure them. A faster method involves using particle counters to determine alpha, beta or gamma activity, and then dividing that by the number of radioactive nuclides. However, it is challenging and expensive to accurately determine the number of radioactive nuclides. Alternatively, decay constants can be determined by comparing isotope data for rocks of known age. This method requires at least one of the isotope systems to be very precisely calibrated, such as the
1766:
1738:
393:, resetting the isotopic "clock" to zero. As the mineral cools, the crystal structure begins to form and diffusion of isotopes is less easy. At a certain temperature, the crystal structure has formed sufficiently to prevent diffusion of isotopes. Thus an igneous or metamorphic rock or melt, which is slowly cooling, does not begin to exhibit measurable radioactive decay until it cools below the closure temperature. The age that can be calculated by radiometric dating is thus the time at which the rock or mineral cooled to closure temperature. This temperature varies for every mineral and isotopic system, so a system can be
145:
3066:
913:, and animals acquire it from consumption of plants and other animals. When an organism dies, it ceases to take in new carbon-14, and the existing isotope decays with a characteristic half-life (5730 years). The proportion of carbon-14 left when the remains of the organism are examined provides an indication of the time elapsed since its death. This makes carbon-14 an ideal dating method to date the age of bones or the remains of an organism. The carbon-14 dating limit lies around 58,000 to 62,000 years.
323:
251:, eventually ending with the formation of a stable (nonradioactive) daughter nuclide; each step in such a chain is characterized by a distinct half-life. In these cases, usually the half-life of interest in radiometric dating is the longest one in the chain, which is the rate-limiting factor in the ultimate transformation of the radioactive nuclide into its stable daughter. Isotopic systems that have been exploited for radiometric dating have half-lives ranging from only about 10 years (e.g.,
1752:
1056:. The radiation causes charge to remain within the grains in structurally unstable "electron traps". Exposure to sunlight or heat releases these charges, effectively "bleaching" the sample and resetting the clock to zero. The trapped charge accumulates over time at a rate determined by the amount of background radiation at the location where the sample was buried. Stimulating these mineral grains using either light (
640:
864:
803:. Closure temperatures are so high that they are not a concern. Rubidium-strontium dating is not as precise as the uranium–lead method, with errors of 30 to 50 million years for a 3-billion-year-old sample. Application of in situ analysis (Laser-Ablation ICP-MS) within single mineral grains in faults have shown that the Rb-Sr method can be used to decipher episodes of fault movement.
955:
338:. Precision is enhanced if measurements are taken on multiple samples from different locations of the rock body. Alternatively, if several different minerals can be dated from the same sample and are assumed to be formed by the same event and were in equilibrium with the reservoir when they formed, they should form an
291:, whose decay rate may be affected by local electron density. For all other nuclides, the proportion of the original nuclide to its decay products changes in a predictable way as the original nuclide decays over time. This predictability allows the relative abundances of related nuclides to be used as a
1156:
At the beginning of the solar system, there were several relatively short-lived radionuclides like Al, Fe, Mn, and I present within the solar nebula. These radionuclides—possibly produced by the explosion of a supernova—are extinct today, but their decay products can be detected in very old material,
388:
The closure temperature or blocking temperature represents the temperature below which the mineral is a closed system for the studied isotopes. If a material that selectively rejects the daughter nuclide is heated above this temperature, any daughter nuclides that have been accumulated over time will
1152:
Absolute radiometric dating requires a measurable fraction of parent nucleus to remain in the sample rock. For rocks dating back to the beginning of the solar system, this requires extremely long-lived parent isotopes, making measurement of such rocks' exact ages imprecise. To be able to distinguish
365:
Accurate radiometric dating generally requires that the parent has a long enough half-life that it will be present in significant amounts at the time of measurement (except as described below under "Dating with short-lived extinct radionuclides"), the half-life of the parent is accurately known, and
1025:
between 1952 and 1958. The residence time of Cl in the atmosphere is about 1 week. Thus, as an event marker of 1950s water in soil and ground water, Cl is also useful for dating waters less than 50 years before the present. Cl has seen use in other areas of the geological sciences, including dating
373:
The precision of a dating method depends in part on the half-life of the radioactive isotope involved. For instance, carbon-14 has a half-life of 5,730 years. After an organism has been dead for 60,000 years, so little carbon-14 is left that accurate dating cannot be established. On the other hand,
1165:
and using isochronplots, it is possible to determine relative ages of different events in the early history of the solar system. Dating methods based on extinct radionuclides can also be calibrated with the U–Pb method to give absolute ages. Thus both the approximate age and a high time resolution
262:
For most radioactive nuclides, the half-life depends solely on nuclear properties and is essentially constant. This is known because decay constants measured by different techniques give consistent values within analytical errors and the ages of the same materials are consistent from one method to
333:
The basic equation of radiometric dating requires that neither the parent nuclide nor the daughter product can enter or leave the material after its formation. The possible confounding effects of contamination of parent and daughter isotopes have to be considered, as do the effects of any loss or
712:
One of its great advantages is that any sample provides two clocks, one based on uranium-235's decay to lead-207 with a half-life of about 700 million years, and one based on uranium-238's decay to lead-206 with a half-life of about 4.5 billion years, providing a built-in crosscheck that allows
309:
The radioactive decay constant, the probability that an atom will decay per year, is the solid foundation of the common measurement of radioactivity. The accuracy and precision of the determination of an age (and a nuclide's half-life) depends on the accuracy and precision of the decay constant
401:
for another. Dating of different minerals and/or isotope systems (with differing closure temperatures) within the same rock can therefore enable the tracking of the thermal history of the rock in question with time, and thus the history of metamorphic events may become known in detail. These
662:
involves using uranium-235 or uranium-238 to date a substance's absolute age. This scheme has been refined to the point that the error margin in dates of rocks can be as low as less than two million years in two-and-a-half billion years. An error margin of 2–5% has been achieved on younger
1067:
These methods can be used to date the age of a sediment layer, as layers deposited on top would prevent the grains from being "bleached" and reset by sunlight. Pottery shards can be dated to the last time they experienced significant heat, generally when they were fired in a kiln.
366:
enough of the daughter product is produced to be accurately measured and distinguished from the initial amount of the daughter present in the material. The procedures used to isolate and analyze the parent and daughter nuclides must be precise and accurate. This normally involves
885:
is also simply called carbon-14 dating. Carbon-14 is a radioactive isotope of carbon, with a half-life of 5,730 years (which is very short compared with the above isotopes), and decays into nitrogen. In other radiometric dating methods, the heavy parent isotopes were produced by
817:
A relatively short-range dating technique is based on the decay of uranium-234 into thorium-230, a substance with a half-life of about 80,000 years. It is accompanied by a sister process, in which uranium-235 decays into protactinium-231, which has a half-life of 32,760 years.
2512:
Manyeruke, Tawanda D.; Thomas G. Blenkinsop; Peter
Buchholz; David Love; Thomas Oberthür; Ulrich K. Vetter; Donald W. Davis (2004). "The age and petrology of the Chimbadzi Hill Intrusion, NW Zimbabwe: first evidence for early Paleoproterozoic magmatism in Zimbabwe".
1008:
which have a variable amount of uranium content. Because the fission tracks are healed by temperatures over about 200 °C the technique has limitations as well as benefits. The technique has potential applications for detailing the thermal history of a deposit.
697:, but strongly reject lead. Zircon has a very high closure temperature, is resistant to mechanical weathering and is very chemically inert. Zircon also forms multiple crystal layers during metamorphic events, which each may record an isotopic age of the event.
2928:
Jacobs, J.; R. J. Thomas (August 2001). "A titanite fission track profile across the southeastern Archæan
Kaapvaal Craton and the Mesoproterozoic Natal Metamorphic Province, South Africa: evidence for differential cryptic Meso- to Neoproterozoic tectonism".
713:
accurate determination of the age of the sample even if some of the lead has been lost. This can be seen in the concordia diagram, where the samples plot along an errorchron (straight line) which intersects the concordia curve at the age of the sample.
973:. This causes induced fission of U, as opposed to the spontaneous fission of U. The fission tracks produced by this process are recorded in the plastic film. The uranium content of the material can then be calculated from the number of tracks and the
2476:
Oberthür, Thomas; Davis, Donald W.; Blenkinsop, Thomas G.; Höhndorf, Axel (2002). "Precise U–Pb mineral ages, Rb–Sr and Sm–Nd systematics for the Great Dyke, Zimbabwe—constraints on late
Archean events in the Zimbabwe craton and Limpopo belt".
589:. This is well established for most isotopic systems. However, construction of an isochron does not require information on the original compositions, using merely the present ratios of the parent and daughter isotopes to a standard isotope. An
625:," depending on their mass and level of ionization. On impact in the cups, the ions set up a very weak current that can be measured to determine the rate of impacts and the relative concentrations of different atoms in the beams.
1064:) causes a luminescence signal to be emitted as the stored unstable electron energy is released, the intensity of which varies depending on the amount of radiation absorbed during burial and specific properties of the mineral.
2548:
Li, Xian-hua; Liang, Xi-rong; Sun, Min; Guan, Hong; Malpas, J. G. (2001). "Precise Pb/U age determination on zircons by laser ablation microprobe-inductively coupled plasma-mass spectrometry using continuous linear ablation".
2100:
Begemann, F.; Ludwig, K.R.; Lugmair, G.W.; Min, K.; Nyquist, L.E.; Patchett, P.J.; Renne, P.R.; Shih, C.-Y.; Villa, I.M.; Walker, R.J. (January 2001). "Call for an improved set of decay constants for geochronological use".
1722:, the authors proposed that the terms "parent isotope" and "daughter isotope" be avoided in favor of the more descriptive "precursor isotope" and "product isotope", analogous to "precursor ion" and "product ion" in
1153:
the relative ages of rocks from such old material, and to get a better time resolution than that available from long-lived isotopes, short-lived isotopes that are no longer present in the rock can be used.
418:
2893:
243:, usually given in units of years when discussing dating techniques. After one half-life has elapsed, one half of the atoms of the nuclide in question will have decayed into a "daughter" nuclide or
354:
is used which also decreases the problem of nuclide loss. Finally, correlation between different isotopic dating methods may be required to confirm the age of a sample. For example, the age of the
969:
of uranium-238 impurities. The uranium content of the sample has to be known, but that can be determined by placing a plastic film over the polished slice of the material, and bombarding it with
4284:
916:
The rate of creation of carbon-14 appears to be roughly constant, as cross-checks of carbon-14 dating with other dating methods show it gives consistent results. However, local eruptions of
2136:
Stewart, Kathy; Turner, Simon; Kelley, Simon; Hawkesworth, Chris; Kirstein, Linda; Mantovani, Marta (1996). "3-D, Ar-Ar geochronology in the Paraná continental flood basalt province".
655:. All the samples show loss of lead isotopes, but the intercept of the errorchron (straight line through the sample points) and the concordia (curve) shows the correct age of the rock.
334:
gain of such isotopes since the sample was created. It is therefore essential to have as much information as possible about the material being dated and to check for possible signs of
890:
in supernovas, meaning that any parent isotope with a short half-life should be extinct by now. Carbon-14, though, is continuously created through collisions of neutrons generated by
2439:
Vinyu, M. L.; R. E. Hanson; M. W. Martin; S. A. Bowring; H. A. Jelsma; P. H. G. M. Dirks (2001). "U–Pb zircon ages from a craton-margin archaean orogenic belt in northern
Zimbabwe".
3065:
Application of the authigenic 10 Be/ 9 Be dating method to Late
Miocene–Pliocene sequences in the northern Danube Basin;Michal Šujan – Global and Planetary Change 137 (2016) 35–53;
425:
isochrons plotted of meteorite samples. The age is calculated from the slope of the isochron (line) and the original composition from the intercept of the isochron with the y-axis.
920:
or other events that give off large amounts of carbon dioxide can reduce local concentrations of carbon-14 and give inaccurate dates. The releases of carbon dioxide into the
757:
decay of potassium-40 to argon-40. Potassium-40 has a half-life of 1.3 billion years, so this method is applicable to the oldest rocks. Radioactive potassium-40 is common in
2654:
Mukasa, S. B.; A. H. Wilson; R. W. Carlson (December 1998). "A multielement geochronologic study of the Great Dyke, Zimbabwe: significance of the robust and reset ages".
1944:
Pommé, S.; Stroh, H.; Altzitzoglou, T.; Paepen, J.; Van Ammel, R.; Kossert, K.; Nähle, O.; Keightley, J. D.; Ferreira, K. M.; Verheyen, L.; Bruggeman, M. (1 April 2018).
1823:
1040:
Luminescence dating methods are not radiometric dating methods in that they do not rely on abundances of isotopes to calculate age. Instead, they are a consequence of
2754:
1708:
chronometer gives an estimate of the time period for formation of primitive meteorites of only a few million years (1.4 million years for
Chondrule formation).
1223:. The iodine-xenon chronometer is an isochron technique. Samples are exposed to neutrons in a nuclear reactor. This converts the only stable isotope of iodine (
3987:
3663:
1317:
ratio is observed across several consecutive temperature steps, it can be interpreted as corresponding to a time at which the sample stopped losing xenon.
613:. In the century since then the techniques have been greatly improved and expanded. Dating can now be performed on samples as small as a nanogram using a
362:(billion years ago) using uranium–lead dating and 3.56 ± 0.10 Ga (billion years ago) using lead–lead dating, results that are consistent with each other.
585:
The above equation makes use of information on the composition of parent and daughter isotopes at the time the material being tested cooled below its
1804:
374:
the concentration of carbon-14 falls off so steeply that the age of relatively young remains can be determined precisely to within a few decades.
617:. The mass spectrometer was invented in the 1940s and began to be used in radiometric dating in the 1950s. It operates by generating a beam of
1765:
3403:
3384:
3346:
2985:
2337:
2291:
1928:
3434:
326:
131:
Different methods of radiometric dating vary in the timescale over which they are accurate and the materials to which they can be applied.
659:
621:
from the sample under test. The ions then travel through a magnetic field, which diverts them into different sampling sensors, known as "
204:
and spontaneously transform into a different nuclide. This transformation may be accomplished in a number of different ways, including
3781:
3365:
2894:"Cosmic background reduction in the radiocarbon measurement by scintillation spectrometry at the underground laboratory of Gran Sasso"
235:
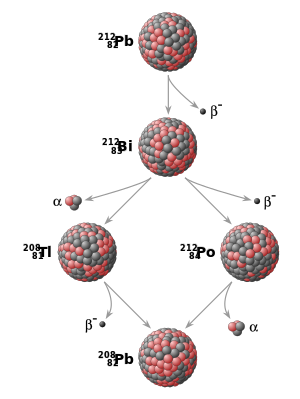
While the moment in time at which a particular nucleus decays is unpredictable, a collection of atoms of a radioactive nuclide decays
928:
have also depressed the proportion of carbon-14 by a few percent; in contrast, the amount of carbon-14 was increased by above-ground
3982:
3578:
3225:
3086:
2441:
2205:
2180:
1057:
3618:
1999:
2584:
Wingate, M.T.D. (2001). "SHRIMP baddeleyite and zircon ages for an
Umkondo dolerite sill, Nyanga Mountains, Eastern Zimbabwe".
2390:
706:
367:
4028:
1320:
Samples of a meteorite called
Shallowater are usually included in the irradiation to monitor the conversion efficiency from
778:
722:
422:
116:. By allowing the establishment of geological timescales, it provides a significant source of information about the ages of
1428:
when they each stopped losing xenon. This in turn corresponds to a difference in age of closure in the early solar system.
735:
of 1.06 x 10 years. Accuracy levels of within twenty million years in ages of two-and-a-half billion years are achievable.
3776:
965:
This involves inspection of a polished slice of a material to determine the density of "track" markings left in it by the
769:, though the closure temperature is fairly low in these materials, about 350 °C (mica) to 500 °C (hornblende).
3771:
3203:
Alexander N. Krot(2002) Dating the
Earliest Solids in our Solar System, Hawai'i Institute of Geophysics and Planetology
2758:
2279:
398:
1110:
1104:
4279:
3961:
3831:
2404:
Stacey, J. S.; J. D. Kramers (June 1975). "Approximation of terrestrial lead isotope evolution by a two-stage model".
837:, from which their ratios are measured. The scheme has a range of several hundred thousand years. A related method is
144:
2362:
1128:
812:
744:
109:
65:
products, which form at a known constant rate of decay. The use of radiometric dating was first published in 1907 by
1855:"The Ultimate Disintegration Products of the Radio-active Elements. Part II. The disintegration products of uranium"
4171:
4151:
1919:
Bernard-Griffiths, J.; Groan, G. (1989). "The samarium–neodymium method". In Roth, Etienne; Poty, Bernard (eds.).
1122:
838:
4161:
4133:
3745:
1061:
2966:"The Application of Fission-Track Dating to the Depositional and Thermal History of Rocks in Sedimentary Basins"
593:
is used to solve the age equation graphically and calculate the age of the sample and the original composition.
200:. Some nuclides are inherently unstable. That is, at some point in time, an atom of such a nuclide will undergo
57:
were selectively incorporated when they were formed. The method compares the abundance of a naturally occurring
4228:
3427:
2689:
Tillberg, Mikael; Drake, Henrik; Zack, Thomas; Kooijman, Ellen; Whitehouse, Martin J.; Åström, Mats E. (2020).
4116:
3290:
1166:
can be obtained. Generally a shorter half-life leads to a higher time resolution at the expense of timescale.
1086:
898:
and thus remains at a near-constant level on Earth. The carbon-14 ends up as a trace component in atmospheric
644:
634:
347:
113:
2820:
1952:. ICRM 2017 Proceedings of the 21st International Conference on Radionuclide Metrology and its Applications.
1092:
4166:
3903:
3750:
2088:
1789:
1134:
988:(glass fragments from volcanic eruptions), and meteorites are best used. Older materials can be dated using
164:. The final decay product, lead-208 (Pb), is stable and can no longer undergo spontaneous radioactive decay.
152:
from lead-212 (Pb) to lead-208 (Pb) . Each parent nuclide spontaneously decays into a daughter nuclide (the
1080:
4066:
3867:
3613:
3512:
690:
322:
86:
3668:
1743:
693:). Zircon and baddeleyite incorporate uranium atoms into their crystalline structure as substitutes for
304:
4101:
1098:
311:
4042:
4018:
4013:
3326:
3250:
3186:
3145:
3014:
2938:
2839:
2702:
2663:
2593:
2558:
2522:
2486:
2450:
2413:
2233:
2145:
2110:
2049:
2008:
1957:
1866:
1784:
1718:
1041:
980:
This scheme has application over a wide range of geologic dates. For dates up to a few million years
949:
355:
42:
295:
to measure the time from the incorporation of the original nuclides into a material to the present.
4128:
4023:
3966:
3941:
3931:
3706:
3603:
3593:
3420:
1907:
1035:
966:
586:
383:
229:
101:
4253:
4233:
4111:
4081:
4003:
3802:
3766:
3568:
3309:
3268:
3048:
2965:
2801:
2736:
2636:
2369:
2067:
1882:
1278:
1053:
1045:
882:
858:
648:
105:
4061:
3857:
3852:
3696:
3532:
3399:
3380:
3361:
3342:
3221:
3082:
3040:
2981:
2728:
2343:
2333:
2297:
2287:
2201:
2176:
1975:
1924:
1757:
1751:
1723:
1519:
1162:
1021:(half-life ~300ky) were produced by irradiation of seawater during atmospheric detonations of
925:
614:
606:
351:
236:
201:
62:
3305:
4258:
4207:
4123:
4071:
3847:
3825:
3691:
3588:
3334:
3301:
3258:
3153:
3149:
3114:
3030:
3022:
2973:
2946:
2908:
2847:
2793:
2718:
2710:
2671:
2667:
2628:
2601:
2566:
2530:
2494:
2458:
2421:
2417:
2251:
2241:
2153:
2149:
2118:
2057:
2016:
1965:
1874:
1837:
970:
895:
796:
750:
610:
602:
407:
225:
173:
66:
4106:
1116:
4191:
4156:
4143:
4053:
3911:
3806:
3740:
3701:
3538:
3375:
McSween, Harry Y; Richardson, Steven Mcafee; Uhle, Maria E; Uhle, Professor Maria (2003).
2619:
Ireland, Trevor (December 1999). "Isotope
Geochemistry: New Tools for Isotopic Analysis".
2276:
Principles and applications of geochemistry: a comprehensive textbook for geology students
887:
590:
339:
279:. The only exceptions are nuclides that decay by the process of electron capture, such as
70:
2867:
940:
above the current value would depress the amount of carbon-14 created in the atmosphere.
867:
3330:
3254:
3190:
3174:
3018:
2942:
2843:
2706:
2597:
2562:
2526:
2490:
2454:
2237:
2114:
2053:
2021:
2012:
1994:
1961:
1870:
909:
A carbon-based life form acquires carbon during its lifetime. Plants acquire it through
4091:
3889:
3877:
3821:
3816:
3810:
3735:
3658:
3583:
3158:
3133:
3099:
3035:
3002:
2723:
2690:
1901:
Radiometric Dating and the Geological Time Scale: Circular Reasoning or Reliable Tools?
1771:
1022:
937:
910:
899:
846:
530:
276:
272:
209:
185:
46:
3204:
2950:
2675:
2570:
2498:
2462:
2122:
639:
4273:
4008:
3926:
3921:
3872:
3862:
3623:
3502:
3313:
3272:
2805:
2740:
2640:
2534:
2425:
2386:
2283:
2157:
1886:
1794:
1779:
878:
were dated at 56 CE using the carbon-14 method on organic material found at the site.
560:
394:
343:
244:
177:
153:
97:
3263:
3238:
2781:
1833:
1518:
of 720 000 years. The dating is simply a question of finding the deviation from the
574:
is known to high precision, and one has accurate and precise measurements of D* and
4086:
3884:
3598:
3573:
3558:
3524:
3484:
3052:
2071:
1970:
1945:
1438:
974:
929:
830:
792:
788:
335:
288:
284:
256:
93:
82:
78:
58:
2632:
486:
is number of atoms of the daughter isotope in the original or initial composition,
2977:
1392:
ratios of the sample and Shallowater then corresponds to the different ratios of
3786:
3683:
3645:
3628:
3563:
3544:
3477:
3457:
1828:
1048:
is absorbed by mineral grains in sediments and archaeological materials such as
1018:
891:
784:
728:
682:
622:
280:
264:
248:
205:
157:
149:
125:
3118:
3003:"Ancient biomolecules from deep ice cores reveal a forested southern Greenland"
2714:
863:
429:
The mathematical expression that relates radioactive decay to geologic time is
17:
3553:
3548:
3443:
2913:
2852:
2821:"The ~2400-year cycle in atmospheric radiocarbon concentration: Bispectrum of
2797:
2246:
2221:
1903:
1733:
1175:
933:
766:
541:
The equation is most conveniently expressed in terms of the measured quantity
213:
161:
2892:
Plastino, Wolfango; Lauri Kaihola; Paolo Bartolomei; Francesco Bella (2001).
1878:
1832:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
4243:
4223:
3956:
3916:
3653:
3462:
3338:
3026:
2347:
2301:
1841:
1799:
1515:
1475:
1277:). After irradiation, samples are heated in a series of steps and the xenon
1158:
1140:
954:
921:
800:
732:
694:
534:
403:
390:
359:
247:. In many cases, the daughter nuclide itself is radioactive, resulting in a
240:
121:
3044:
2732:
2691:"In situ Rb-Sr dating of slickenfibres in deep crystalline basement faults"
1979:
3179:
Press Abstracts from the Nineteenth Lunar and Planetary Science Conference
4238:
4076:
3720:
3633:
997:
932:
tests that were conducted into the early 1960s. Also, an increase in the
834:
762:
754:
686:
664:
652:
268:
221:
217:
196:
in the nucleus. A particular isotope of a particular element is called a
54:
3132:
Gilmour, J. D.; O. V Pravdivtseva; A. Busfield; C. M. Hohenberg (2006).
791:, with a half-life of 50 billion years. This scheme is used to date old
4248:
3472:
3467:
2329:
1001:
993:
985:
958:
917:
826:
822:
671:
252:
197:
193:
189:
74:
2757:. The Swedish National Heritage Board. 11 October 2006. Archived from
2256:
2605:
2325:
2062:
2037:
1049:
1005:
989:
875:
842:
702:
674:
181:
169:
117:
50:
1854:
475:
is number of atoms of the radiogenic daughter isotope in the sample,
3098:
Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021).
2222:"INTCAL04 Terrestrial Radiocarbon Age Calibration, 0–26 Cal Kyr BP"
833:
are not, and so they are selectively precipitated into ocean-floor
563:(neither parent nor daughter isotopes have been lost from system),
3673:
3173:
Hutcheon, I. D.; Hutchison, R.; Wasserburg, G. J. (1 March 1988).
2200:(2nd ed.). Cambridge: Cambridge Univ. Press. pp. 15–49.
1436:
Another example of short-lived extinct radionuclide dating is the
1161:. By measuring the decay products of extinct radionuclides with a
953:
871:
862:
638:
417:
321:
292:
143:
85:
itself, and can also be used to date a wide range of natural and
3607:
3489:
2322:
Using geochemical data: evaluation, presentation, interpretation
1474:
chronometer, which can be used to estimate the relative ages of
981:
758:
3416:
2038:"Direct test of the constancy of fundamental nuclear constants"
1281:
of the gas evolved in each step is analysed. When a consistent
1148:
Dating with decay products of short-lived extinct radionuclides
601:
Radiometric dating has been carried out since 1905 when it was
533:
of the parent isotope, equal to the inverse of the radioactive
499:
is number of atoms of the parent isotope in the sample at time
3507:
618:
3412:
3321:
Magill, Joseph; Galy, Jean (2005). "Archaeology and Dating".
192:, with each isotope of an element differing in the number of
3175:"Evidence of In-situ Decay of 26Al in a Semarkona Chondrule"
1558:
decay) in comparison with the ratio of the stable isotopes
570:
either must be negligible or can be accurately estimated,
406:
using a high-temperature furnace. This field is known as
402:
temperatures are experimentally determined in the lab by
69:
and is now the principal source of information about the
2964:
Naeser, Nancy; Naeser, Charles; McCulloh, Thane (1989).
263:
another. It is not affected by external factors such as
27:
Technique used to date materials such as rocks or carbon
559:
To calculate the age, it is assumed that the system is
537:
of the parent isotope times the natural logarithm of 2.
1060:
or infrared stimulated luminescence dating) or heat (
681:), though it can be used on other materials, such as
3394:
Harry y. Mcsween, Jr; Huss, Gary R (29 April 2010).
4216:
4200:
4184:
4142:
4052:
4041:
3996:
3988:
Global Boundary Stratotype Section and Point (GSSP)
3975:
3949:
3940:
3902:
3840:
3795:
3759:
3728:
3719:
3682:
3644:
3523:
3498:
3450:
3205:
http://www.psrd.hawaii.edu/Sept02/isotopicAges.html
3237:Pourret, Olivier; Johannesson, Karen (July 2022).
870:at Kåseberga, around ten kilometres south east of
4285:Conservation and restoration of cultural heritage
3134:"The I-Xe Chronometer and the Early Solar System"
3100:"The NUBASE2020 evaluation of nuclear properties"
961:crystals are widely used in fission track dating.
3325:. Springer Berlin Heidelberg. pp. 105–115.
1904:Radiometric Dating and the Geological Time Scale
239:at a rate described by a parameter known as the
188:. Additionally, elements may exist in different
124:change. Radiometric dating is also used to date
1669:ratio to that of other Solar System materials.
1259:via neutron capture followed by beta decay (of
3220:, page 322. Cambridge University Press, 2001.
3081:, page 321. Cambridge University Press, 2001.
701:micro-beam analysis can be achieved via laser
670:Uranium–lead dating is often performed on the
3428:
609:as a method by which one might determine the
8:
2968:. In Naeser, Nancy; McCulloh, Thane (eds.).
2363:"Basics of Radioactive Isotope Geochemistry"
2315:
2313:
2311:
61:within the material to the abundance of its
3291:"Radioactivity: A Tool to Explore the Past"
2782:"A calibration curve for radiocarbon dates"
4049:
3946:
3725:
3520:
3435:
3421:
3413:
3239:"Radiogenic isotope: Not just about words"
2175:. Stanford, Calif.: Stanford Univ. Press.
3379:(2 ed.). Columbia University Press.
3262:
3157:
3034:
2912:
2851:
2819:Vasiliev, S. S.; V. A. Dergachev (2002).
2722:
2255:
2245:
2061:
2020:
1969:
549:) rather than the constant initial value
271:, chemical environment, or presence of a
96:, radiometric dating methods are used in
3983:Global Standard Stratigraphic Age (GSSA)
3306:10.1524/ract.1995.7071.special-issue.305
2269:
2267:
1923:. Springer Netherlands. pp. 53–72.
1805:Sensitive high-resolution ion microprobe
128:materials, including ancient artifacts.
2972:. Springer New York. pp. 157–180.
2387:"Geologic Time: Radiometric Time Scale"
2381:
2379:
1816:
356:Amitsoq gneisses from western Greenland
1356:. The difference between the measured
1176:Iodine-129 § Meteorite age dating
404:artificially resetting sample minerals
104:. Among the best-known techniques are
3356:Allègre, Claude J (4 December 2008).
3323:Radioactivity Radionuclides Radiation
2970:Thermal History of Sedimentary Basins
1995:"Perturbation of Nuclear Decay Rates"
7:
3377:Geochemistry: Pathways and Processes
3216:Imke de Pater and Jack J. Lissauer:
3077:Imke de Pater and Jack J. Lissauer:
1716:In a July 2022 paper in the journal
327:Thermal ionization mass spectrometer
2656:Earth and Planetary Science Letters
2406:Earth and Planetary Science Letters
2138:Earth and Planetary Science Letters
2022:10.1146/annurev.ns.22.120172.001121
783:This is based on the beta decay of
255:) to over 100 billion years (e.g.,
3782:Adoption of the Gregorian calendar
3159:10.1111/j.1945-5100.2006.tb00190.x
1829:Compendium of Chemical Terminology
25:
3138:Meteoritics and Planetary Science
2931:Journal of African Earth Sciences
2515:Journal of African Earth Sciences
2442:Journal of African Earth Sciences
2086:How to Change Nuclear Decay Rates
1058:optically stimulated luminescence
799:, and has also been used to date
358:was determined to be 3.60 ± 0.05
342:. This can reduce the problem of
2586:South African Journal of Geology
2535:10.1016/j.jafrearsci.2004.12.003
2000:Annual Review of Nuclear Science
1764:
1750:
1736:
1044:on certain minerals. Over time,
1017:Large amounts of otherwise rare
845:(thorium-230) to thorium-232 in
773:Rubidium–strontium dating method
717:Samarium–neodymium dating method
41:is a technique which is used to
3664:English and British regnal year
3264:10.1016/j.apgeochem.2022.105348
2391:United States Geological Survey
2103:Geochimica et Cosmochimica Acta
1157:such as that which constitutes
660:Uranium–lead radiometric dating
643:A concordia diagram as used in
368:isotope-ratio mass spectrometry
3398:. Cambridge University Press.
3360:. Cambridge University Press.
2825:data over the last 8000 years"
1971:10.1016/j.apradiso.2017.09.002
1950:Applied Radiation and Isotopes
841:, which measures the ratio of
318:Accuracy of radiometric dating
172:is made up of combinations of
1:
3777:Old Style and New Style dates
2951:10.1016/S0899-5362(01)80066-X
2676:10.1016/S0012-821X(98)00228-3
2633:10.1126/science.286.5448.2289
2571:10.1016/S0009-2541(00)00394-6
2499:10.1016/S0301-9268(01)00215-7
2463:10.1016/S0899-5362(01)90021-1
2123:10.1016/s0016-7037(00)00512-3
1633:*) is found by comparing the
807:Uranium–thorium dating method
739:Potassium–argon dating method
53:, in which trace radioactive
3729:Pre-Julian / Julian
3289:Gunten, Hans R. von (1995).
2978:10.1007/978-1-4612-3492-0_10
2426:10.1016/0012-821X(75)90088-6
2280:Englewood Cliffs, New Jersey
2171:Dalrymple, G. Brent (1994).
2158:10.1016/0012-821X(96)00132-X
299:Decay constant determination
3962:Geological history of Earth
3832:Astronomical year numbering
2320:Rollinson, Hugh R. (1993).
1859:American Journal of Science
1030:Luminescence dating methods
944:Fission track dating method
329:used in radiometric dating.
232:into two or more nuclides.
180:, indicating the number of
4301:
2715:10.1038/s41598-019-57262-5
2198:Radiogenic isotope geology
2036:Shlyakhter, A. I. (1976).
1853:Boltwood, Bertram (1907).
1173:
1033:
947:
856:
810:
776:
742:
720:
632:
629:Uranium–lead dating method
381:
302:
228:). Another possibility is
4134:Thermoluminescence dating
4029:Samarium–neodymium dating
2914:10.1017/S0033822200037954
2853:10.5194/angeo-20-115-2002
2798:10.1017/S0003598X00070277
2247:10.1017/S0033822200032999
1921:Nuclear Methods of Dating
1062:thermoluminescence dating
1013:Chlorine-36 dating method
853:Radiocarbon dating method
779:Rubidium–strontium dating
723:Samarium–neodymium dating
148:Example of a radioactive
120:and the deduced rates of
3848:Chinese sexagenary cycle
3119:10.1088/1674-1137/abddae
2196:Dickin, Alan P. (2008).
1879:10.2475/ajs.s4-23.134.78
503:(the present), given by
94:stratigraphic principles
4062:Amino acid racemisation
3339:10.1007/3-540-26881-2_6
3300:. 70–71 (s1): 305–413.
3150:2006M&PS...41...19G
3027:10.1126/science.1141758
3001:Willerslev, E. (2007).
2668:1998E&PSL.164..353M
2418:1975E&PSL..26..207S
2150:1996E&PSL.143...95S
1842:10.1351/goldbook.R05082
1790:Paleopedological record
1432:The Al – Mg chronometer
1221:0.12 million years
1111:Hafnium–tungsten dating
1076:Other methods include:
77:, including the age of
4067:Archaeomagnetic dating
3579:Era of Caesar (Iberia)
2274:Faure, Gunter (1998).
2232:(3): 1029–1058. 2004.
1170:The I – Xe chronometer
962:
879:
813:Uranium–thorium dating
745:Potassium–argon dating
691:monazite geochronology
656:
426:
410:or thermochronometry.
330:
165:
110:potassium–argon dating
3967:Geological time units
2780:Clark, R. M. (1975).
2361:White, W. M. (2003).
1744:Earth sciences portal
957:
894:with nitrogen in the
866:
839:ionium–thorium dating
647:, with data from the
642:
597:Modern dating methods
466:is age of the sample,
421:
325:
305:Radioactive decay law
147:
79:fossilized life forms
4019:Law of superposition
4014:Isotope geochemistry
3243:Applied Geochemistry
2479:Precambrian Research
2173:The age of the earth
2084:Johnson, B. (1993).
1946:"Is decay constant?"
1785:Isotope geochemistry
1719:Applied Geochemistry
1215:with a half-life of
1042:background radiation
950:fission track dating
924:as a consequence of
397:for one mineral but
176:, each with its own
4152:Fluorine absorption
4129:Luminescence dating
4024:Luminescence dating
3932:Milankovitch cycles
3772:Proleptic Gregorian
3604:Hindu units of time
3331:2005rrr..book.....M
3255:2022ApGC..14205348P
3191:1988LPICo.650...14H
3019:2007Sci...317..111W
2943:2001JAfES..33..323J
2844:2002AnGeo..20..115V
2832:Annales Geophysicae
2707:2020NatSR..10..562T
2627:(5448): 2289–2290.
2598:2001SAJG..104...13W
2563:2001ChGeo.175..209L
2527:2004JAfES..40..281M
2491:2002PreR..113..293O
2455:2001JAfES..32..103V
2238:2004Radcb..46.1029.
2115:2001GeCoA..65..111B
2054:1976Natur.264..340S
2013:1972ARNPS..22..165E
1993:Emery, G T (1972).
1962:2018AppRI.134....6P
1908:TalkOrigins Archive
1871:1907AmJS...23...78B
1712:A terminology issue
1036:Luminescence dating
1026:ice and sediments.
967:spontaneous fission
731:of Sm to Nd with a
645:uranium–lead dating
635:Uranium–lead dating
587:closure temperature
384:Closure temperature
378:Closure temperature
348:uranium–lead dating
230:spontaneous fission
114:uranium–lead dating
102:geologic time scale
75:geological features
73:of rocks and other
59:radioactive isotope
39:radioisotope dating
4280:Radiometric dating
4254:Terminus post quem
4234:Synchronoptic view
4201:Linguistic methods
4162:Obsidian hydration
4097:Radiometric dating
4082:Incremental dating
4004:Chronostratigraphy
3218:Planetary Sciences
3079:Planetary Sciences
2868:"Carbon-14 Dating"
2695:Scientific Reports
2370:Cornell University
2089:Usenet Physics FAQ
1834:radioactive dating
1615:(often designated
1279:isotopic signature
1054:potassium feldspar
1046:ionizing radiation
963:
883:Radiocarbon dating
880:
859:Radiocarbon dating
825:is water-soluble,
727:This involves the
657:
427:
331:
166:
106:radiocarbon dating
87:man-made materials
45:materials such as
35:radioactive dating
31:Radiometric dating
4267:
4266:
4180:
4179:
4037:
4036:
3898:
3897:
3853:Geologic Calendar
3715:
3714:
3405:978-0-521-87862-3
3386:978-0-231-12440-9
3348:978-3-540-26881-9
3298:Radiochimica Acta
3107:Chinese Physics C
3013:(5834): 111–114.
2987:978-1-4612-8124-5
2872:www.chem.uwec.edu
2339:978-0-582-06701-1
2293:978-0-02-336450-1
1930:978-0-7923-0188-2
1758:Geophysics portal
1724:mass spectrometry
1520:natural abundance
1163:mass spectrometer
1117:Potassium–calcium
926:industrialization
797:metamorphic rocks
615:mass spectrometer
607:Ernest Rutherford
352:concordia diagram
202:radioactive decay
174:chemical elements
140:Radioactive decay
100:to establish the
16:(Redirected from
4292:
4259:ASPRO chronology
4208:Glottochronology
4124:Tephrochronology
4072:Dendrochronology
4050:
3947:
3746:Proleptic Julian
3736:Pre-Julian Roman
3726:
3521:
3437:
3430:
3423:
3414:
3409:
3390:
3371:
3352:
3317:
3295:
3277:
3276:
3266:
3234:
3228:
3214:
3208:
3201:
3195:
3194:
3170:
3164:
3163:
3161:
3129:
3123:
3122:
3104:
3095:
3089:
3075:
3069:
3063:
3057:
3056:
3038:
2998:
2992:
2991:
2961:
2955:
2954:
2925:
2919:
2918:
2916:
2898:
2889:
2883:
2882:
2880:
2878:
2864:
2858:
2857:
2855:
2829:
2816:
2810:
2809:
2792:(196): 251–266.
2777:
2771:
2770:
2768:
2766:
2761:on 31 March 2009
2751:
2745:
2744:
2726:
2686:
2680:
2679:
2662:(1–2): 353–369.
2651:
2645:
2644:
2616:
2610:
2609:
2606:10.2113/104.1.13
2581:
2575:
2574:
2557:(3–4): 209–219.
2551:Chemical Geology
2545:
2539:
2538:
2509:
2503:
2502:
2485:(3–4): 293–306.
2473:
2467:
2466:
2436:
2430:
2429:
2401:
2395:
2394:
2383:
2374:
2373:
2367:
2358:
2352:
2351:
2317:
2306:
2305:
2278:(2nd ed.).
2271:
2262:
2261:
2259:
2249:
2218:
2212:
2211:
2193:
2187:
2186:
2168:
2162:
2161:
2133:
2127:
2126:
2097:
2091:
2082:
2076:
2075:
2065:
2063:10.1038/264340a0
2033:
2027:
2026:
2024:
1990:
1984:
1983:
1973:
1941:
1935:
1934:
1916:
1910:
1899:McRae, A. 1998.
1897:
1891:
1890:
1850:
1844:
1821:
1774:
1769:
1768:
1760:
1755:
1754:
1746:
1741:
1740:
1739:
1707:
1706:
1705:
1698:
1697:
1689:
1688:
1687:
1680:
1679:
1668:
1667:
1666:
1659:
1658:
1650:
1649:
1648:
1641:
1640:
1632:
1631:
1630:
1623:
1622:
1614:
1613:
1612:
1605:
1604:
1593:
1592:
1591:
1584:
1583:
1575:
1574:
1573:
1566:
1565:
1557:
1556:
1555:
1548:
1547:
1540:(the product of
1539:
1538:
1537:
1530:
1529:
1513:
1512:
1511:
1504:
1503:
1495:
1494:
1493:
1486:
1485:
1473:
1472:
1471:
1464:
1463:
1455:
1453:
1452:
1445:
1444:
1427:
1426:
1425:
1418:
1417:
1409:
1408:
1407:
1400:
1399:
1391:
1390:
1389:
1382:
1381:
1373:
1372:
1371:
1364:
1363:
1355:
1354:
1353:
1346:
1345:
1337:
1336:
1335:
1328:
1327:
1316:
1315:
1314:
1307:
1306:
1298:
1297:
1296:
1289:
1288:
1276:
1275:
1274:
1267:
1266:
1258:
1257:
1256:
1249:
1248:
1240:
1239:
1238:
1231:
1230:
1222:
1220:
1214:
1213:
1212:
1205:
1204:
1196:
1195:
1194:
1187:
1186:
1105:Lutetium–hafnium
1093:Lanthanum–barium
896:upper atmosphere
751:electron capture
611:age of the Earth
528:
522:
498:
485:
474:
465:
456:
414:The age equation
408:thermochronology
389:be lost through
226:electron capture
67:Bertram Boltwood
21:
4300:
4299:
4295:
4294:
4293:
4291:
4290:
4289:
4270:
4269:
4268:
4263:
4212:
4196:
4192:Molecular clock
4185:Genetic methods
4176:
4157:Nitrogen dating
4144:Relative dating
4138:
4107:Potassium–argon
4054:Absolute dating
4044:
4033:
3992:
3971:
3936:
3912:Cosmic Calendar
3904:Astronomic time
3894:
3836:
3791:
3755:
3741:Original Julian
3711:
3678:
3640:
3539:Ab urbe condita
3517:
3494:
3446:
3441:
3406:
3393:
3387:
3374:
3368:
3358:Isotope Geology
3355:
3349:
3320:
3293:
3288:
3285:
3283:Further reading
3280:
3236:
3235:
3231:
3215:
3211:
3202:
3198:
3172:
3171:
3167:
3131:
3130:
3126:
3102:
3097:
3096:
3092:
3076:
3072:
3064:
3060:
3000:
2999:
2995:
2988:
2963:
2962:
2958:
2927:
2926:
2922:
2907:(2A): 157–161.
2896:
2891:
2890:
2886:
2876:
2874:
2866:
2865:
2861:
2827:
2818:
2817:
2813:
2779:
2778:
2774:
2764:
2762:
2753:
2752:
2748:
2688:
2687:
2683:
2653:
2652:
2648:
2618:
2617:
2613:
2583:
2582:
2578:
2547:
2546:
2542:
2511:
2510:
2506:
2475:
2474:
2470:
2438:
2437:
2433:
2403:
2402:
2398:
2393:. 16 June 2001.
2385:
2384:
2377:
2365:
2360:
2359:
2355:
2340:
2319:
2318:
2309:
2294:
2273:
2272:
2265:
2220:
2219:
2215:
2208:
2195:
2194:
2190:
2183:
2170:
2169:
2165:
2144:(1–4): 95–109.
2135:
2134:
2130:
2099:
2098:
2094:
2083:
2079:
2035:
2034:
2030:
1992:
1991:
1987:
1943:
1942:
1938:
1931:
1918:
1917:
1913:
1898:
1894:
1852:
1851:
1847:
1822:
1818:
1814:
1770:
1763:
1756:
1749:
1742:
1737:
1735:
1732:
1714:
1704:
1702:
1701:
1700:
1696:
1694:
1693:
1692:
1691:
1686:
1684:
1683:
1682:
1678:
1676:
1675:
1674:
1673:
1665:
1663:
1662:
1661:
1657:
1655:
1654:
1653:
1652:
1647:
1645:
1644:
1643:
1639:
1637:
1636:
1635:
1634:
1629:
1627:
1626:
1625:
1621:
1619:
1618:
1617:
1616:
1611:
1609:
1608:
1607:
1603:
1601:
1600:
1599:
1598:
1590:
1588:
1587:
1586:
1582:
1580:
1579:
1578:
1577:
1572:
1570:
1569:
1568:
1564:
1562:
1561:
1560:
1559:
1554:
1552:
1551:
1550:
1546:
1544:
1543:
1542:
1541:
1536:
1534:
1533:
1532:
1528:
1526:
1525:
1524:
1523:
1510:
1508:
1507:
1506:
1502:
1500:
1499:
1498:
1497:
1492:
1490:
1489:
1488:
1484:
1482:
1481:
1480:
1479:
1470:
1468:
1467:
1466:
1462:
1460:
1459:
1458:
1457:
1451:
1449:
1448:
1447:
1443:
1441:
1440:
1439:
1437:
1434:
1424:
1422:
1421:
1420:
1416:
1414:
1413:
1412:
1411:
1406:
1404:
1403:
1402:
1398:
1396:
1395:
1394:
1393:
1388:
1386:
1385:
1384:
1380:
1378:
1377:
1376:
1375:
1370:
1368:
1367:
1366:
1362:
1360:
1359:
1358:
1357:
1352:
1350:
1349:
1348:
1344:
1342:
1341:
1340:
1339:
1334:
1332:
1331:
1330:
1326:
1324:
1323:
1322:
1321:
1313:
1311:
1310:
1309:
1305:
1303:
1302:
1301:
1300:
1295:
1293:
1292:
1291:
1287:
1285:
1284:
1283:
1282:
1273:
1271:
1270:
1269:
1265:
1263:
1262:
1261:
1260:
1255:
1253:
1252:
1251:
1247:
1245:
1244:
1243:
1242:
1237:
1235:
1234:
1233:
1229:
1227:
1226:
1225:
1224:
1218:
1216:
1211:
1209:
1208:
1207:
1203:
1201:
1200:
1199:
1198:
1197:beta-decays to
1193:
1191:
1190:
1189:
1185:
1183:
1182:
1181:
1180:
1178:
1172:
1150:
1135:Krypton–krypton
1129:Uranium–uranium
1074:
1038:
1032:
1023:nuclear weapons
1015:
952:
946:
936:or the Earth's
905:
888:nucleosynthesis
861:
855:
815:
809:
781:
775:
747:
741:
725:
719:
680:
637:
631:
599:
569:
554:
526:
518:
504:
502:
489:
484:
478:
469:
463:
457:
442:
432:
416:
386:
380:
320:
307:
301:
210:alpha particles
142:
137:
28:
23:
22:
18:Isotopic dating
15:
12:
11:
5:
4298:
4296:
4288:
4287:
4282:
4272:
4271:
4265:
4264:
4262:
4261:
4256:
4251:
4246:
4241:
4236:
4231:
4229:New Chronology
4226:
4220:
4218:
4217:Related topics
4214:
4213:
4211:
4210:
4204:
4202:
4198:
4197:
4195:
4194:
4188:
4186:
4182:
4181:
4178:
4177:
4175:
4174:
4169:
4164:
4159:
4154:
4148:
4146:
4140:
4139:
4137:
4136:
4131:
4126:
4121:
4120:
4119:
4114:
4109:
4104:
4094:
4092:Paleomagnetism
4089:
4084:
4079:
4074:
4069:
4064:
4058:
4056:
4047:
4039:
4038:
4035:
4034:
4032:
4031:
4026:
4021:
4016:
4011:
4006:
4000:
3998:
3994:
3993:
3991:
3990:
3985:
3979:
3977:
3973:
3972:
3970:
3969:
3964:
3959:
3953:
3951:
3944:
3938:
3937:
3935:
3934:
3929:
3924:
3919:
3914:
3908:
3906:
3900:
3899:
3896:
3895:
3893:
3892:
3890:New Earth Time
3887:
3882:
3881:
3880:
3875:
3865:
3860:
3855:
3850:
3844:
3842:
3838:
3837:
3835:
3834:
3829:
3819:
3814:
3799:
3797:
3793:
3792:
3790:
3789:
3784:
3779:
3774:
3769:
3763:
3761:
3757:
3756:
3754:
3753:
3751:Revised Julian
3748:
3743:
3738:
3732:
3730:
3723:
3717:
3716:
3713:
3712:
3710:
3709:
3704:
3699:
3694:
3688:
3686:
3680:
3679:
3677:
3676:
3671:
3669:Lists of kings
3666:
3661:
3659:Canon of Kings
3656:
3650:
3648:
3642:
3641:
3639:
3638:
3637:
3636:
3631:
3626:
3621:
3611:
3601:
3596:
3591:
3586:
3584:Before present
3581:
3576:
3571:
3566:
3561:
3556:
3551:
3542:
3535:
3529:
3527:
3518:
3516:
3515:
3510:
3505:
3499:
3496:
3495:
3493:
3492:
3487:
3482:
3481:
3480:
3470:
3465:
3460:
3454:
3452:
3448:
3447:
3442:
3440:
3439:
3432:
3425:
3417:
3411:
3410:
3404:
3396:Cosmochemistry
3391:
3385:
3372:
3367:978-0521862288
3366:
3353:
3347:
3318:
3284:
3281:
3279:
3278:
3229:
3209:
3196:
3165:
3124:
3090:
3070:
3058:
2993:
2986:
2956:
2937:(2): 323–333.
2920:
2884:
2859:
2838:(1): 115–120.
2811:
2772:
2746:
2681:
2646:
2611:
2576:
2540:
2521:(5): 281–292.
2504:
2468:
2449:(1): 103–114.
2431:
2412:(2): 207–221.
2396:
2375:
2353:
2338:
2307:
2292:
2263:
2213:
2206:
2188:
2181:
2163:
2128:
2109:(1): 111–121.
2092:
2077:
2028:
2007:(1): 165–202.
1985:
1936:
1929:
1911:
1892:
1865:(134): 77–88.
1845:
1815:
1813:
1810:
1809:
1808:
1802:
1797:
1792:
1787:
1782:
1776:
1775:
1772:Physics portal
1761:
1747:
1731:
1728:
1713:
1710:
1703:
1695:
1685:
1677:
1664:
1656:
1646:
1638:
1628:
1620:
1610:
1602:
1597:The excess of
1589:
1581:
1571:
1563:
1553:
1545:
1535:
1527:
1509:
1501:
1491:
1483:
1469:
1461:
1450:
1442:
1433:
1430:
1423:
1415:
1405:
1397:
1387:
1379:
1369:
1361:
1351:
1343:
1333:
1325:
1312:
1304:
1294:
1286:
1272:
1264:
1254:
1246:
1236:
1228:
1210:
1202:
1192:
1184:
1171:
1168:
1149:
1146:
1145:
1144:
1138:
1132:
1126:
1123:Rhenium–osmium
1120:
1114:
1108:
1102:
1096:
1090:
1084:
1073:
1070:
1034:Main article:
1031:
1028:
1014:
1011:
948:Main article:
945:
942:
938:magnetic field
911:photosynthesis
903:
900:carbon dioxide
857:Main article:
854:
851:
847:ocean sediment
811:Main article:
808:
805:
777:Main article:
774:
771:
749:This involves
743:Main article:
740:
737:
721:Main article:
718:
715:
678:
633:Main article:
630:
627:
598:
595:
567:
552:
539:
538:
531:decay constant
524:
516:
500:
487:
482:
476:
467:
440:
431:
415:
412:
382:Main article:
379:
376:
319:
316:
300:
297:
277:electric field
186:atomic nucleus
141:
138:
136:
133:
126:archaeological
92:Together with
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
4297:
4286:
4283:
4281:
4278:
4277:
4275:
4260:
4257:
4255:
4252:
4250:
4247:
4245:
4242:
4240:
4237:
4235:
4232:
4230:
4227:
4225:
4222:
4221:
4219:
4215:
4209:
4206:
4205:
4203:
4199:
4193:
4190:
4189:
4187:
4183:
4173:
4170:
4168:
4165:
4163:
4160:
4158:
4155:
4153:
4150:
4149:
4147:
4145:
4141:
4135:
4132:
4130:
4127:
4125:
4122:
4118:
4115:
4113:
4110:
4108:
4105:
4103:
4100:
4099:
4098:
4095:
4093:
4090:
4088:
4085:
4083:
4080:
4078:
4075:
4073:
4070:
4068:
4065:
4063:
4060:
4059:
4057:
4055:
4051:
4048:
4046:
4043:Chronological
4040:
4030:
4027:
4025:
4022:
4020:
4017:
4015:
4012:
4010:
4009:Geochronology
4007:
4005:
4002:
4001:
3999:
3995:
3989:
3986:
3984:
3981:
3980:
3978:
3974:
3968:
3965:
3963:
3960:
3958:
3955:
3954:
3952:
3948:
3945:
3943:
3942:Geologic time
3939:
3933:
3930:
3928:
3927:Metonic cycle
3925:
3923:
3922:Galactic year
3920:
3918:
3915:
3913:
3910:
3909:
3907:
3905:
3901:
3891:
3888:
3886:
3883:
3879:
3876:
3874:
3871:
3870:
3869:
3866:
3864:
3863:ISO week date
3861:
3859:
3856:
3854:
3851:
3849:
3846:
3845:
3843:
3839:
3833:
3830:
3827:
3823:
3820:
3818:
3815:
3812:
3808:
3804:
3801:
3800:
3798:
3794:
3788:
3785:
3783:
3780:
3778:
3775:
3773:
3770:
3768:
3765:
3764:
3762:
3758:
3752:
3749:
3747:
3744:
3742:
3739:
3737:
3734:
3733:
3731:
3727:
3724:
3722:
3718:
3708:
3705:
3703:
3700:
3698:
3695:
3693:
3690:
3689:
3687:
3685:
3681:
3675:
3672:
3670:
3667:
3665:
3662:
3660:
3657:
3655:
3652:
3651:
3649:
3647:
3643:
3635:
3632:
3630:
3627:
3625:
3622:
3620:
3617:
3616:
3615:
3612:
3609:
3605:
3602:
3600:
3597:
3595:
3592:
3590:
3587:
3585:
3582:
3580:
3577:
3575:
3572:
3570:
3569:Byzantine era
3567:
3565:
3562:
3560:
3557:
3555:
3552:
3550:
3546:
3543:
3541:
3540:
3536:
3534:
3531:
3530:
3528:
3526:
3525:Calendar eras
3522:
3519:
3514:
3511:
3509:
3506:
3504:
3501:
3500:
3497:
3491:
3488:
3486:
3483:
3479:
3476:
3475:
3474:
3471:
3469:
3466:
3464:
3461:
3459:
3456:
3455:
3453:
3449:
3445:
3438:
3433:
3431:
3426:
3424:
3419:
3418:
3415:
3407:
3401:
3397:
3392:
3388:
3382:
3378:
3373:
3369:
3363:
3359:
3354:
3350:
3344:
3340:
3336:
3332:
3328:
3324:
3319:
3315:
3311:
3307:
3303:
3299:
3292:
3287:
3286:
3282:
3274:
3270:
3265:
3260:
3256:
3252:
3248:
3244:
3240:
3233:
3230:
3227:
3226:0-521-48219-4
3223:
3219:
3213:
3210:
3206:
3200:
3197:
3192:
3188:
3184:
3180:
3176:
3169:
3166:
3160:
3155:
3151:
3147:
3143:
3139:
3135:
3128:
3125:
3120:
3116:
3113:(3): 030001.
3112:
3108:
3101:
3094:
3091:
3088:
3087:0-521-48219-4
3084:
3080:
3074:
3071:
3068:
3062:
3059:
3054:
3050:
3046:
3042:
3037:
3032:
3028:
3024:
3020:
3016:
3012:
3008:
3004:
2997:
2994:
2989:
2983:
2979:
2975:
2971:
2967:
2960:
2957:
2952:
2948:
2944:
2940:
2936:
2932:
2924:
2921:
2915:
2910:
2906:
2902:
2895:
2888:
2885:
2873:
2869:
2863:
2860:
2854:
2849:
2845:
2841:
2837:
2833:
2826:
2824:
2815:
2812:
2807:
2803:
2799:
2795:
2791:
2787:
2783:
2776:
2773:
2760:
2756:
2755:"Ales stenar"
2750:
2747:
2742:
2738:
2734:
2730:
2725:
2720:
2716:
2712:
2708:
2704:
2700:
2696:
2692:
2685:
2682:
2677:
2673:
2669:
2665:
2661:
2657:
2650:
2647:
2642:
2638:
2634:
2630:
2626:
2622:
2615:
2612:
2607:
2603:
2599:
2595:
2591:
2587:
2580:
2577:
2572:
2568:
2564:
2560:
2556:
2552:
2544:
2541:
2536:
2532:
2528:
2524:
2520:
2516:
2508:
2505:
2500:
2496:
2492:
2488:
2484:
2480:
2472:
2469:
2464:
2460:
2456:
2452:
2448:
2444:
2443:
2435:
2432:
2427:
2423:
2419:
2415:
2411:
2407:
2400:
2397:
2392:
2388:
2382:
2380:
2376:
2371:
2364:
2357:
2354:
2349:
2345:
2341:
2335:
2331:
2327:
2323:
2316:
2314:
2312:
2308:
2303:
2299:
2295:
2289:
2285:
2284:Prentice Hall
2281:
2277:
2270:
2268:
2264:
2258:
2253:
2248:
2243:
2239:
2235:
2231:
2227:
2223:
2217:
2214:
2209:
2207:9780521530170
2203:
2199:
2192:
2189:
2184:
2182:9780804723312
2178:
2174:
2167:
2164:
2159:
2155:
2151:
2147:
2143:
2139:
2132:
2129:
2124:
2120:
2116:
2112:
2108:
2104:
2096:
2093:
2090:
2087:
2081:
2078:
2073:
2069:
2064:
2059:
2055:
2051:
2048:(5584): 340.
2047:
2043:
2039:
2032:
2029:
2023:
2018:
2014:
2010:
2006:
2002:
2001:
1996:
1989:
1986:
1981:
1977:
1972:
1967:
1963:
1959:
1955:
1951:
1947:
1940:
1937:
1932:
1926:
1922:
1915:
1912:
1909:
1905:
1902:
1896:
1893:
1888:
1884:
1880:
1876:
1872:
1868:
1864:
1860:
1856:
1849:
1846:
1843:
1839:
1835:
1831:
1830:
1825:
1820:
1817:
1811:
1806:
1803:
1801:
1798:
1796:
1795:Radioactivity
1793:
1791:
1788:
1786:
1783:
1781:
1780:Hadean zircon
1778:
1777:
1773:
1767:
1762:
1759:
1753:
1748:
1745:
1734:
1729:
1727:
1725:
1721:
1720:
1711:
1709:
1670:
1595:
1521:
1517:
1477:
1454:
1431:
1429:
1318:
1280:
1177:
1169:
1167:
1164:
1160:
1154:
1147:
1142:
1139:
1136:
1133:
1130:
1127:
1124:
1121:
1118:
1115:
1112:
1109:
1106:
1103:
1100:
1097:
1094:
1091:
1088:
1085:
1082:
1079:
1078:
1077:
1072:Other methods
1071:
1069:
1065:
1063:
1059:
1055:
1051:
1047:
1043:
1037:
1029:
1027:
1024:
1020:
1012:
1010:
1007:
1003:
999:
995:
991:
987:
983:
978:
976:
972:
971:slow neutrons
968:
960:
956:
951:
943:
941:
939:
935:
931:
927:
923:
919:
914:
912:
907:
901:
897:
893:
889:
884:
877:
873:
869:
865:
860:
852:
850:
848:
844:
840:
836:
832:
828:
824:
819:
814:
806:
804:
802:
801:lunar samples
798:
794:
790:
786:
780:
772:
770:
768:
764:
760:
756:
752:
746:
738:
736:
734:
730:
724:
716:
714:
710:
708:
704:
700:
696:
692:
688:
684:
676:
673:
668:
666:
661:
654:
650:
646:
641:
636:
628:
626:
624:
620:
619:ionized atoms
616:
612:
608:
604:
596:
594:
592:
591:isochron plot
588:
583:
581:
577:
573:
566:
562:
557:
555:
548:
544:
536:
532:
525:
521:
515:
511:
507:
496:
492:
488:
481:
477:
472:
468:
462:
461:
460:
454:
450:
446:
439:
435:
430:
424:
420:
413:
411:
409:
405:
400:
396:
392:
385:
377:
375:
371:
369:
363:
361:
357:
353:
349:
345:
344:contamination
341:
337:
328:
324:
317:
315:
313:
306:
298:
296:
294:
290:
286:
282:
278:
274:
270:
266:
260:
258:
254:
250:
246:
245:decay product
242:
238:
237:exponentially
233:
231:
227:
224:emission, or
223:
219:
215:
211:
208:(emission of
207:
203:
199:
195:
191:
187:
183:
179:
178:atomic number
175:
171:
168:All ordinary
163:
159:
155:
154:decay product
151:
146:
139:
134:
132:
129:
127:
123:
119:
115:
111:
107:
103:
99:
98:geochronology
95:
90:
88:
84:
80:
76:
72:
68:
64:
60:
56:
52:
48:
44:
40:
36:
32:
19:
4172:Stratigraphy
4117:Uranium–lead
4096:
4087:Lichenometry
3885:Winter count
3868:Mesoamerican
3796:Astronomical
3614:Mesoamerican
3599:Sothic cycle
3574:Seleucid era
3559:Bosporan era
3547: /
3537:
3485:Paleontology
3395:
3376:
3357:
3322:
3297:
3246:
3242:
3232:
3217:
3212:
3199:
3182:
3178:
3168:
3144:(1): 19–31.
3141:
3137:
3127:
3110:
3106:
3093:
3078:
3073:
3061:
3010:
3006:
2996:
2969:
2959:
2934:
2930:
2923:
2904:
2900:
2887:
2875:. Retrieved
2871:
2862:
2835:
2831:
2822:
2814:
2789:
2785:
2775:
2763:. Retrieved
2759:the original
2749:
2698:
2694:
2684:
2659:
2655:
2649:
2624:
2620:
2614:
2592:(1): 13–22.
2589:
2585:
2579:
2554:
2550:
2543:
2518:
2514:
2507:
2482:
2478:
2471:
2446:
2440:
2434:
2409:
2405:
2399:
2356:
2321:
2275:
2229:
2225:
2216:
2197:
2191:
2172:
2166:
2141:
2137:
2131:
2106:
2102:
2095:
2085:
2080:
2045:
2041:
2031:
2004:
1998:
1988:
1953:
1949:
1939:
1920:
1914:
1900:
1895:
1862:
1858:
1848:
1827:
1819:
1717:
1715:
1671:
1596:
1435:
1319:
1179:
1155:
1151:
1087:Iodine–xenon
1075:
1066:
1039:
1016:
979:
975:neutron flux
964:
930:nuclear bomb
915:
908:
881:
868:Ale's Stones
831:protactinium
820:
816:
789:strontium-87
782:
748:
726:
711:
709:techniques.
698:
669:
658:
623:Faraday cups
600:
584:
579:
575:
571:
564:
558:
550:
546:
542:
540:
519:
513:
509:
505:
494:
490:
479:
470:
458:
452:
448:
444:
437:
433:
428:
387:
372:
364:
332:
312:Pb–Pb system
308:
289:zirconium-89
285:strontium-85
261:
257:samarium-147
234:
167:
135:Fundamentals
130:
122:evolutionary
91:
83:age of Earth
71:absolute age
38:
34:
30:
29:
4112:Radiocarbon
3787:Dual dating
3646:Regnal year
3624:Short Count
3564:Bostran era
3545:Anno Domini
3478:Big History
3458:Archaeology
2901:Radiocarbon
2226:Radiocarbon
1081:Argon–argon
892:cosmic rays
785:rubidium-87
767:hornblendes
729:alpha decay
683:baddeleyite
649:Pfunze Belt
281:beryllium-7
265:temperature
249:decay chain
206:alpha decay
150:decay chain
4274:Categories
3707:Vietnamese
3619:Long Count
3554:Anno Mundi
3549:Common Era
3451:Key topics
3444:Chronology
3249:: 105348.
2701:(1): 562.
2257:10289/3690
1812:References
1496:decays to
1476:chondrules
1174:See also:
1159:meteorites
934:solar wind
336:alteration
303:See also:
220:emission,
214:beta decay
55:impurities
4244:Year zero
4224:Chronicle
4167:Seriation
4102:Lead–lead
3976:Standards
3957:Deep time
3917:Ephemeris
3803:Lunisolar
3767:Gregorian
3760:Gregorian
3721:Calendars
3684:Era names
3654:Anka year
3533:Human Era
3463:Astronomy
3314:100441969
3273:248907159
2806:161729853
2786:Antiquity
2741:210670668
2641:129408440
1887:131688682
1800:Radiohalo
1516:half-life
1141:Beryllium
1099:Lead–lead
922:biosphere
918:volcanoes
835:sediments
763:feldspars
733:half-life
695:zirconium
535:half-life
391:diffusion
241:half-life
156:) via an
4239:Timeline
4077:Ice core
3950:Concepts
3697:Japanese
3629:Tzolk'in
3594:Egyptian
3045:17615355
2733:31953465
2348:27937350
2302:37783103
1980:28947247
1956:: 6–12.
1807:(SHRIMP)
1730:See also
998:titanite
986:tektites
755:positron
687:monazite
665:Mesozoic
653:Zimbabwe
603:invented
340:isochron
273:magnetic
269:pressure
222:positron
218:electron
194:neutrons
190:isotopes
4249:Floruit
3997:Methods
3858:Iranian
3826:Islamic
3692:Chinese
3503:Periods
3473:History
3468:Geology
3327:Bibcode
3251:Bibcode
3187:Bibcode
3146:Bibcode
3053:7423309
3036:2694912
3015:Bibcode
3007:Science
2939:Bibcode
2877:6 April
2840:Bibcode
2765:9 March
2724:6969261
2703:Bibcode
2664:Bibcode
2621:Science
2594:Bibcode
2559:Bibcode
2523:Bibcode
2487:Bibcode
2451:Bibcode
2414:Bibcode
2330:Longman
2234:Bibcode
2146:Bibcode
2111:Bibcode
2072:4252035
2050:Bibcode
2009:Bibcode
1958:Bibcode
1867:Bibcode
1514:with a
1241:) into
1143:(Be–Be)
1137:(Kr–Kr)
1125:(Re–Os)
1107:(Lu–Hf)
1101:(Pb–Pb)
1095:(La–Ba)
1083:(Ar–Ar)
1002:epidote
994:apatite
959:Apatite
827:thorium
823:uranium
793:igneous
699:In situ
672:mineral
667:rocks.
529:is the
253:tritium
198:nuclide
184:in the
182:protons
162:β decay
158:α decay
118:fossils
81:or the
4045:dating
3841:Others
3807:Hebrew
3702:Korean
3513:Epochs
3402:
3383:
3364:
3345:
3312:
3271:
3224:
3185:: 14.
3085:
3051:
3043:
3033:
2984:
2804:
2739:
2731:
2721:
2639:
2346:
2336:
2326:Harlow
2300:
2290:
2204:
2179:
2070:
2042:Nature
1978:
1927:
1885:
1119:(K–Ca)
1113:(Hf-W)
1089:(I–Xe)
1050:quartz
1006:garnet
990:zircon
876:Sweden
843:ionium
821:While
765:, and
703:ICP-MS
689:(see:
677:(ZrSiO
675:zircon
561:closed
459:where
395:closed
350:, the
287:, and
212:) and
170:matter
51:carbon
3878:Aztec
3822:Lunar
3817:Solar
3811:Hindu
3674:Limmu
3634:Haab'
3589:Hijri
3310:S2CID
3294:(PDF)
3269:S2CID
3103:(PDF)
3049:S2CID
2897:(PDF)
2828:(PDF)
2802:S2CID
2737:S2CID
2637:S2CID
2366:(PDF)
2068:S2CID
1883:S2CID
1861:. 4.
1824:IUPAC
1217:16.14
1131:(U–U)
982:micas
872:Ystad
759:micas
523:, and
423:Lu-Hf
346:. In
293:clock
160:or a
63:decay
47:rocks
3873:Maya
3608:Yuga
3508:Eras
3490:Time
3400:ISBN
3381:ISBN
3362:ISBN
3343:ISBN
3222:ISBN
3083:ISBN
3041:PMID
2982:ISBN
2879:2016
2767:2009
2729:PMID
2344:OCLC
2334:ISBN
2298:OCLC
2288:ISBN
2202:ISBN
2177:ISBN
1976:PMID
1925:ISBN
1672:The
1052:and
1004:and
829:and
795:and
707:SIMS
685:and
512:) =
455:− 1)
436:* =
399:open
112:and
43:date
3335:doi
3302:doi
3259:doi
3247:142
3183:650
3154:doi
3115:doi
3067:pdf
3031:PMC
3023:doi
3011:317
2974:doi
2947:doi
2909:doi
2848:doi
2794:doi
2719:PMC
2711:doi
2672:doi
2660:164
2629:doi
2625:286
2602:doi
2590:104
2567:doi
2555:175
2531:doi
2495:doi
2483:113
2459:doi
2422:doi
2252:hdl
2242:doi
2154:doi
2142:143
2119:doi
2058:doi
2046:264
2017:doi
1966:doi
1954:134
1875:doi
1838:doi
1836:".
1522:of
1338:to
906:).
902:(CO
787:to
753:or
705:or
605:by
582:).
451:) (
275:or
259:).
49:or
37:or
4276::
3809:,
3341:.
3333:.
3308:.
3296:.
3267:.
3257:.
3245:.
3241:.
3181:.
3177:.
3152:.
3142:41
3140:.
3136:.
3111:45
3109:.
3105:.
3047:.
3039:.
3029:.
3021:.
3009:.
3005:.
2980:.
2945:.
2935:33
2933:.
2905:43
2903:.
2899:.
2870:.
2846:.
2836:20
2834:.
2830:.
2800:.
2790:49
2788:.
2784:.
2735:.
2727:.
2717:.
2709:.
2699:10
2697:.
2693:.
2670:.
2658:.
2635:.
2623:.
2600:.
2588:.
2565:.
2553:.
2529:.
2519:40
2517:.
2493:.
2481:.
2457:.
2447:32
2445:.
2420:.
2410:26
2408:.
2389:.
2378:^
2368:.
2342:.
2332:.
2328::
2324:.
2310:^
2296:.
2286:.
2282::
2266:^
2250:.
2240:.
2230:46
2228:.
2224:.
2152:.
2140:.
2117:.
2107:65
2105:.
2066:.
2056:.
2044:.
2040:.
2015:.
2005:22
2003:.
1997:.
1974:.
1964:.
1948:.
1906:,
1881:.
1873:.
1863:23
1857:.
1826:,
1726:.
1699:Mg
1690:–
1681:Al
1660:Mg
1642:Mg
1624:Mg
1606:Mg
1594:.
1585:Mg
1567:Al
1549:Al
1531:Mg
1505:Mg
1487:Al
1478:.
1465:Mg
1456:–
1446:Al
1383:Xe
1365:Xe
1347:Xe
1308:Xe
1290:Xe
1250:Xe
1206:Xe
1019:Cl
1000:,
996:,
992:,
984:,
977:.
874:,
849:.
761:,
651:,
556:.
443:+
370:.
360:Ga
314:.
283:,
267:,
108:,
89:.
33:,
3828:)
3824:(
3813:)
3805:(
3610:)
3606:(
3436:e
3429:t
3422:v
3408:.
3389:.
3370:.
3351:.
3337::
3329::
3316:.
3304::
3275:.
3261::
3253::
3207:.
3193:.
3189::
3162:.
3156::
3148::
3121:.
3117::
3055:.
3025::
3017::
2990:.
2976::
2953:.
2949::
2941::
2917:.
2911::
2881:.
2856:.
2850::
2842::
2823:C
2808:.
2796::
2769:.
2743:.
2713::
2705::
2678:.
2674::
2666::
2643:.
2631::
2608:.
2604::
2596::
2573:.
2569::
2561::
2537:.
2533::
2525::
2501:.
2497::
2489::
2465:.
2461::
2453::
2428:.
2424::
2416::
2372:.
2350:.
2304:.
2260:.
2254::
2244::
2236::
2210:.
2185:.
2160:.
2156::
2148::
2125:.
2121::
2113::
2074:.
2060::
2052::
2025:.
2019::
2011::
1982:.
1968::
1960::
1933:.
1889:.
1877::
1869::
1840::
1651:/
1576:/
1419:I
1410:/
1401:I
1374:/
1329:I
1299:/
1268:I
1232:I
1219:±
1188:I
904:2
679:4
580:t
578:(
576:N
572:λ
568:0
565:D
553:o
551:N
547:t
545:(
543:N
527:λ
520:e
517:0
514:N
510:t
508:(
506:N
501:t
497:)
495:t
493:(
491:N
483:0
480:D
473:*
471:D
464:t
453:e
449:t
447:(
445:N
441:0
438:D
434:D
216:(
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.