520:
256:
153:
441:
436:
446:
431:
515:
24:
593:
672:. Samples of lead styphnate vary in color from yellow to gold, orange, reddish-brown, to brown. Lead styphnate is known in various polymorphs, hydrates, and basic salts. Normal lead styphnate monohydrate, monobasic lead styphnate, tribasic lead styphnate dihydrate, and pentabasic lead styphnate dehydrate as well as α, β
794:
Normal lead styphnate exists as α and β polymorphs, both being monoclinic crystals. The lead centres are seven-coordinate and are bridged via oxygen bridges. The water molecule is coordinated to the metal and is also hydrogen-bonded to the anion. Many of the Pb-O distances are short, indicating some
803:
Lead styphnate's heat of formation is −835 kJ mol. The loss of water leads to the formation of a sensitive anhydrous material with a density of 2.9 g cm. The variation of colors remains unexplained. Lead styphnate has a detonation velocity of 5.2 km/s and an explosion temperature of
543:
518:
110:
454:
411:
722:
In 1919, Austrian chemist Edmund von Herz first established a preparation of anhydrous normal lead styphnate by the reaction of magnesium styphnate with lead acetate in the presence of
519:
606:
521:
1085:
Hyman Henkin; Russell McGill (1952). "Rates of
Explosive Decomposition of Explosives. Experimental and Theoretical Kinetic Study as a Function of Temperature".
305:
841:
1017:
Pierce-Butler, M.A. (1982). "Structures of the barium salt of 2,4,6-trinitro-1,3-benzenediol monohydrate and the isomorphous lead salt (beta-polymorph)".
687:. Long thin crystals are particularly sensitive. Lead styphnate does not react with other metals and is less sensitive to shock and friction than
711:
Lead styphnate (or, as it was then called, trinitro-orcinate) was discovered along with many other thrinitroresorcinate salts by
British chemist
1061:
930:
1163:
1132:
904:
1188:
270:
673:
990:
Pierce-Butler, M.A. (1984). "The structure of the lead salt of 2,4,6-trinitro-1,3-benzenediol monohydrate (alpha-polymorph)".
842:"Support document for identification of lead styphnate as a substance of very high concern because of its CMR properties"
613:
1183:
203:
445:
440:
1178:
234:
435:
490:
160:
848:
820:
used in firearms primers, which will ignite upon a simple impact. It is similarly used in blank cartridges for
556:
486:
529:
430:
700:
148:
688:
468:
423:
824:
nail guns. Lead styphnate is also used as primer in microthrusters for small satellite stationkeeping.
683:
and small rectangular crystals. Lead styphnate is particularly sensitive to fire and the discharge of
386:
36:
494:
251:
1193:
661:
396:
76:
887:
Boileau, Jacques; Fauquignon, Claude; Hueber, Bernard; Meyer, Hans H. (2009-04-15), "Explosives",
1067:
795:
degree of covalency. The styphnate ions lie in approximately parallel planes linked by Pb atoms.
684:
1057:
926:
920:
900:
817:
569:
376:
1140:
1113:
1094:
1049:
1026:
999:
972:
892:
653:
328:
212:
130:
86:
821:
949:
Contributions to the
History of Orcin.--No. I. Nitro-Substitution Compounds of the Orcins
255:
152:
712:
692:
584:
279:
InChI=1S/C6H3N3O8.Pb/c10-5-2(7(12)13)1-3(8(14)15)6(11)4(5)9(16)17;/h1,10-11H;/q;+2/p-2
1172:
1071:
976:
645:
289:
InChI=1/C6H3N3O8.Pb/c10-5-2(7(12)13)1-3(8(14)15)6(11)4(5)9(16)17;/h1,10-11H;/q;+2/p-2
141:
716:
575:
896:
192:
723:
680:
947:
1053:
1030:
1003:
813:
629:
351:
121:
657:
649:
223:
1133:"MEMS Mega-pixel Micro-thruster Arrays for Small Satellite Stationkeeping"
872:
23:
695:. It is stable in storage, even at elevated temperatures. As with other
669:
542:
535:
528:
501:
1098:
715:
in 1871, the synthesis route involving action of trinitroresorcinol on
361:
179:
161:
583:
Except where otherwise noted, data are given for materials in their
478:
1137:
Honeywell
Technology 14th Annual/USU Conference on Small Satellites
665:
482:
474:
109:
99:
1164:
National
Pollutant Inventory - Lead and Lead Compounds Fact Sheet
536:
696:
239:
891:, Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA,
816:
for military and commercial applications. It serves as a
513:
699:-containing compounds, lead styphnate is toxic owing to
963:
J.R. Payne (1994). "Thermochmistry of lead styphnate".
601:
58:
1,3-Benzenediol, 2,4,6-trinitro-, lead(2+) salt (1:1)
191:
679:Lead styphnate forms six-sided crystals of the
517:
85:
889:Ullmann's Encyclopedia of Industrial Chemistry
664:. Lead styphnate is only slightly soluble in
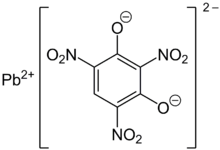
41:Lead(II) 2,4,6-trinitrobenzene-1,3-bis(olate)
8:
919:Matyáš, Robert; Pachman, Jiří (2013-03-12).
812:Lead styphnate is mainly used in small arms
254:
151:
129:
15:
1048:. Springer Science & Business Media.
925:. Springer Science & Business Media.
211:
1131:Daniel W. Youngner; et al. (2000).
832:
310:
275:
250:
564:330 °C (626 °F; 603 K)
142:
50:Lead 2,4,6-trinitrobenzene-1,3-diolate
282:Key: WETZJIOEDGMBMA-UHFFFAOYSA-L
7:
1044:Robert Matyáš; Ji í Pachman (2013).
882:
880:
804:265–280 °C after five seconds.
292:Key: WETZJIOEDGMBMA-NUQVWONBAY
182:
14:
644:Pb ), whose name is derived from
839:ECHA, European Chemicals Agency
591:
444:
439:
434:
429:
22:
587:(at 25 °C , 100 kPa).
1:
897:10.1002/14356007.a10_143.pub2
313:c1c(c(c(c(c1(=O)))(=O)))(=O).
977:10.1016/0040-6031(94)85003-8
946:Stenhouse, J. (March 1871).
660:mixtures for less sensitive
356:450.288 g/mol
1210:
952:. Royal Society of London.
1054:10.1007/978-3-642-28436-6
1031:10.1107/S0567740882010966
1004:10.1107/S0108270184003036
676:of lead styphnate exist.
581:
410:
405:
370:
321:
301:
266:
69:
47:
35:
30:
21:
1189:Nitrobenzene derivatives
62:Lead trinitroresorcinate
1112:Gray, Theodore (2009).
652:used as a component in
524:
523:
662:secondary explosives
506:(fire diamond)
387:Friction sensitivity
52:Lead 2,4,6-trinitro-
37:Preferred IUPAC name
1184:Explosive chemicals
1099:10.1021/ie50510a054
630:trinitroresorcinate
397:Detonation velocity
18:
1179:Lead(II) compounds
1046:Primary Explosives
965:Thermochimica Acta
922:Primary Explosives
685:static electricity
614:Infobox references
525:
56:-phenylene dioxide
16:
1063:978-3-642-28435-9
1025:(12): 3100–3104.
932:978-3-642-28436-6
818:primary explosive
689:mercury fulminate
622:Chemical compound
620:
619:
570:Safety data sheet
469:Hazard statements
377:Shock sensitivity
366:3.06 to 3.1 g cm
235:CompTox Dashboard
111:Interactive image
1201:
1152:
1151:
1149:
1148:
1139:. Archived from
1128:
1122:
1121:
1109:
1103:
1102:
1093:(6): 1391–1395.
1082:
1076:
1075:
1041:
1035:
1034:
1019:Acta Crystallogr
1014:
1008:
1007:
992:Acta Crystallogr
987:
981:
980:
960:
954:
953:
943:
937:
936:
916:
910:
909:
884:
875:
869:
863:
862:
860:
859:
853:
847:. Archived from
846:
837:
604:
598:
595:
594:
545:
538:
531:
516:
496:
492:
488:
484:
480:
476:
448:
443:
438:
433:
329:Chemical formula
259:
258:
243:
241:
215:
195:
184:
163:
155:
144:
133:
113:
89:
26:
19:
1209:
1208:
1204:
1203:
1202:
1200:
1199:
1198:
1169:
1168:
1160:
1155:
1146:
1144:
1130:
1129:
1125:
1118:Popular Science
1111:
1110:
1106:
1084:
1083:
1079:
1064:
1043:
1042:
1038:
1016:
1015:
1011:
989:
988:
984:
962:
961:
957:
945:
944:
940:
933:
918:
917:
913:
907:
886:
885:
878:
870:
866:
857:
855:
851:
844:
840:
838:
834:
830:
822:powder-actuated
810:
801:
792:
785:
781:
777:
773:
769:
765:
761:
757:
753:
749:
745:
741:
737:
733:
709:
643:
639:
635:
623:
616:
611:
610:
609: ?)
600:
596:
592:
588:
561:
558:
550:
549:
548:
547:
540:
533:
526:
522:
514:
471:
457:
426:
371:Explosive data
345:
341:
337:
331:
317:
314:
309:
308:
297:
294:
293:
290:
284:
283:
280:
274:
273:
262:
244:
237:
218:
198:
185:
173:
136:
116:
103:
92:
79:
65:
63:
61:
59:
57:
51:
43:
42:
17:Lead styphnate
12:
11:
5:
1207:
1205:
1197:
1196:
1191:
1186:
1181:
1171:
1170:
1167:
1166:
1159:
1158:External links
1156:
1154:
1153:
1123:
1104:
1087:Ind. Eng. Chem
1077:
1062:
1036:
1009:
982:
955:
938:
931:
911:
906:978-3527306732
905:
876:
864:
831:
829:
826:
809:
806:
800:
797:
791:
788:
787:
786:
783:
779:
775:
771:
767:
763:
759:
755:
751:
747:
743:
739:
735:
731:
713:John Stenhouse
708:
705:
641:
637:
633:
626:Lead styphnate
621:
618:
617:
612:
590:
589:
585:standard state
582:
579:
578:
573:
566:
565:
562:
555:
552:
551:
541:
534:
527:
512:
511:
510:
509:
507:
498:
497:
472:
467:
464:
463:
458:
453:
450:
449:
427:
422:
419:
418:
408:
407:
403:
402:
399:
393:
392:
389:
383:
382:
379:
373:
372:
368:
367:
364:
358:
357:
354:
348:
347:
343:
339:
335:
332:
327:
324:
323:
319:
318:
316:
315:
312:
304:
303:
302:
299:
298:
296:
295:
291:
288:
287:
285:
281:
278:
277:
269:
268:
267:
264:
263:
261:
260:
252:DTXSID10890757
247:
245:
233:
230:
229:
226:
220:
219:
217:
216:
208:
206:
200:
199:
197:
196:
188:
186:
178:
175:
174:
172:
171:
167:
165:
157:
156:
146:
138:
137:
135:
134:
126:
124:
118:
117:
115:
114:
106:
104:
97:
94:
93:
91:
90:
82:
80:
75:
72:
71:
67:
66:
60:Lead tricinate
49:
45:
44:
40:
39:
33:
32:
28:
27:
13:
10:
9:
6:
4:
3:
2:
1206:
1195:
1192:
1190:
1187:
1185:
1182:
1180:
1177:
1176:
1174:
1165:
1162:
1161:
1157:
1143:on 2021-03-10
1142:
1138:
1134:
1127:
1124:
1119:
1115:
1108:
1105:
1100:
1096:
1092:
1088:
1081:
1078:
1073:
1069:
1065:
1059:
1055:
1051:
1047:
1040:
1037:
1032:
1028:
1024:
1020:
1013:
1010:
1005:
1001:
997:
993:
986:
983:
978:
974:
970:
966:
959:
956:
951:
950:
942:
939:
934:
928:
924:
923:
915:
912:
908:
902:
898:
894:
890:
883:
881:
877:
874:
873:GESTIS 490561
868:
865:
854:on 2014-10-22
850:
843:
836:
833:
827:
825:
823:
819:
815:
807:
805:
798:
796:
789:
729:
728:
727:
725:
720:
718:
714:
706:
704:
702:
698:
694:
690:
686:
682:
677:
675:
671:
667:
663:
659:
655:
651:
647:
646:styphnic acid
631:
627:
615:
608:
603:
586:
580:
577:
574:
571:
568:
567:
563:
560:
554:
553:
546:
539:
532:
508:
505:
504:
500:
499:
473:
470:
466:
465:
462:
459:
456:
452:
451:
447:
442:
437:
432:
428:
425:
421:
420:
416:
414:
409:
404:
400:
398:
395:
394:
390:
388:
385:
384:
380:
378:
375:
374:
369:
365:
363:
360:
359:
355:
353:
350:
349:
333:
330:
326:
325:
320:
311:
307:
300:
286:
276:
272:
265:
257:
253:
249:
248:
246:
236:
232:
231:
227:
225:
222:
221:
214:
210:
209:
207:
205:
202:
201:
194:
190:
189:
187:
181:
177:
176:
169:
168:
166:
164:
159:
158:
154:
150:
147:
145:
143:ECHA InfoCard
140:
139:
132:
128:
127:
125:
123:
120:
119:
112:
108:
107:
105:
101:
96:
95:
88:
84:
83:
81:
78:
74:
73:
68:
55:
46:
38:
34:
29:
25:
20:
1145:. Retrieved
1141:the original
1136:
1126:
1117:
1114:"Flash Bang"
1107:
1090:
1086:
1080:
1045:
1039:
1022:
1018:
1012:
995:
991:
985:
968:
964:
958:
948:
941:
921:
914:
888:
867:
856:. Retrieved
849:the original
835:
811:
808:Applications
802:
793:
721:
717:lead acetate
710:
678:
628:(lead 2,4,6-
625:
624:
557:Autoignition
502:
460:
412:
70:Identifiers
53:
48:Other names
724:nitric acid
707:Preparation
703:poisoning.
701:heavy metal
681:monohydrate
576:Oxford MSDS
559:temperature
455:Signal word
322:Properties
149:100.035.703
1194:Phenolates
1173:Categories
1147:2016-10-18
858:2014-10-17
828:References
814:ammunition
799:Properties
774:O + Mg(CH
693:lead azide
674:polymorphs
424:Pictograms
352:Molar mass
213:0T8SE91KOP
122:ChemSpider
98:3D model (
87:15245-44-0
77:CAS Number
1072:199492549
998:: 63–65.
971:: 13–21.
790:Structure
746:O + Pb(CH
658:detonator
650:explosive
415:labelling
401:5200 m/s
346:Pb
224:UN number
170:239-290-0
162:EC Number
670:methanol
648:, is an
503:NFPA 704
406:Hazards
64:Tricinat
607:what is
605: (
362:Density
180:PubChem
1070:
1060:
929:
903:
758:→ {C
654:primer
602:verify
599:
572:(SDS)
487:H360Df
461:Danger
306:SMILES
31:Names
1068:S2CID
871:GHS:
852:(PDF)
845:(PDF)
666:water
391:High
381:High
271:InChI
228:0130
193:61789
131:55674
100:JSmol
1058:ISBN
927:ISBN
901:ISBN
770:}PbH
742:}MgH
697:lead
668:and
656:and
495:H410
491:H373
483:H332
479:H302
475:H200
204:UNII
1095:doi
1050:doi
1027:doi
1000:doi
973:doi
969:242
893:doi
691:or
632:, C
413:GHS
240:EPA
183:CID
1175::
1135:.
1116:.
1091:44
1089:.
1066:.
1056:.
1023:38
1021:.
996:40
994:.
967:.
899:,
879:^
778:CO
750:CO
730:{C
726:.
719:.
636:HN
493:,
489:,
485:,
481:,
477:,
417::
338:HN
1150:.
1120:.
1101:.
1097::
1074:.
1052::
1033:.
1029::
1006:.
1002::
979:.
975::
935:.
895::
861:.
784:2
782:)
780:2
776:3
772:2
768:8
766:O
764:3
762:N
760:6
756:2
754:)
752:2
748:3
744:2
740:8
738:O
736:3
734:N
732:6
642:8
640:O
638:3
634:6
597:N
544:3
537:0
530:4
344:8
342:O
340:3
336:6
334:C
242:)
238:(
102:)
54:m
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.