629:
606:
40:
1217:
1094:, in particular, may lead to severe or even fatal liver-damage or hepatotoxicity. Seventy-five percent of all cases of severe liver damage reported until early 2001 were seen under combined drug therapy leflunomide plus methotrexate. However, some studies have shown that the combination of methotrexate and leflunomide in patients with rheumatoid arthritis gave better results than either drug alone.
31:
1256:"Regardless of the substance administered (leflunomide or teriflunomide), it is the same molecule (teriflunomide)—the one exerting the pharmacological, immunological or metabolic action in view of restoring, correcting or modifying physiological functions, and does not present, in clinical use, a new chemical entity to patients." Because of this, the
4280:
804:
827:
and approved by the U.S. Food and Drug
Administration in 1998. Clinical studies regarding the following diseases have been conducted: There has been reports on potential re-purposing of leflunomide for treatment of solid tumors with tumor suppressor, PTEN, loss. In PTEN negative tumors, leflunomide
1142:. Teriflunomide prevents the expansion of activated and autoimmune lymphocytes by interfering with their cell cycle progression while nonlymphoid cells are able to use another pathway to make their ribonucleotides by use of salvage pyrimidine pathway, which makes them less dependent on
942:
The dose-limiting side effects are liver damage, lung disease and immunosuppression. The most common side effects (occurring in >1% of those treated with it) are, in approximately descending order of frequency: diarrhea, respiratory tract infections, hair loss,
2053:
Holtmann MH, Gerts AL, Weinman A, Galle PR, Neurath MF (April 2008). "Treatment of Crohn's disease with leflunomide as second-line immunosuppression : a phase 1 open-label trial on efficacy, tolerability and safety".
2559:
226:
2451:
2298:
1205:
metabolite of leflunomide. Upon administration of leflunomide, 70% of the drug administered converts into teriflunomide. The only difference between the molecules is the opening of the
2010:
Prajapati DN, Knox JF, Emmons J, Saeian K, Csuka ME, Binion DG (August 2003). "Leflunomide treatment of Crohn's disease patients intolerant to standard immunomodulator therapy".
181:
2366:
Lee SS, Park YW, Park JJ, Kang YM, Nam EJ, Kim SI, et al. (2009). "Combination treatment with leflunomide and methotrexate for patients with active rheumatoid arthritis".
4087:
1352:
4330:
1347:[Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese).
1780:
Dai L, Wei XN, Zheng DH, Mo YQ, Pessler F, Zhang BY (June 2011). "Effective treatment of Kimura's disease with leflunomide in combination with glucocorticoids".
239:
62:
2566:
2645:
718:
2412:
Fox RI, Herrmann ML, Frangou CG, Wahl GM, Morris RE, Strand V, et al. (December 1999). "Mechanism of action for leflunomide in rheumatoid arthritis".
1071:
Other immunomodulatory treatments should be avoided due to the potential for additive immunosuppressant effects, or in the case of immunostimulants like
113:
3561:
2462:
2309:
1123:(rUMP), which is required for the synthesis of DNA and RNA. Hence, leflunomide inhibits the reproduction of rapidly dividing cells, especially
4234:
1604:
4097:
4080:
704:
4315:
1340:
3504:
2874:
738:
1311:
955:, urinary tract infection, dizziness, infection, joint disorder, itchiness, weight loss, loss of appetite, cough, gastroenteritis,
2638:
862:
2173:
Pirildar T (May 2003). "Treatment of adult-onset Still's disease with leflunomide and chloroquine combination in two patients".
4073:
3913:
379:
211:
94:
1229:
of leflunomide, responsible for its therapeutic actions. It results from the reaction of isoxazole ring opening, which occurs
1406:
793:
1555:
1087:
vaccines) should be avoided due to the potential for severe infection due to the immunosuppressive nature of the treatment.
887:
1134:, the active metabolite of leflunomide, occurs at levels (approximately 600 nM) that are achieved during treatment of
1348:
1344:
1080:
4270:
1044:
2710:
1112:
847:
797:
505:
130:
4305:
4251:
2819:
2631:
585:
1741:"Current recommendations for diagnosis and management of polyoma BK virus nephropathy in renal transplant recipients"
2531:"Clinical Pharmacology/Biopharmaceutics Review. Product: ARAVA (leflunomide tablets). Application Number: NDA 20905"
2452:"Assessment report. AUBAGIO (international non-proprietary name: teriflunomide). Procedure No. EMEA/H/C/002514/0000"
2221:
for "Mitoxantrone and
Prednisone With or Without Leflunomide in Treating Patients With Stage IV Prostate Cancer" at
2689:
1040:
1869:
Sanders S, Harisdangkul V (April 2002). "Leflunomide for the treatment of rheumatoid arthritis and autoimmunity".
1692:"PTEN Regulates Glutamine Flux to Pyrimidine Synthesis and Sensitivity to Dihydroorotate Dehydrogenase Inhibition"
3367:
2530:
1257:
1052:
108:
3747:
454:
1823:
Wu GC, Xu XD, Huang Q, Wu H (February 2013). "Leflunomide: friend or foe for systemic lupus erythematosus?".
1507:"When a DMARD fails, should patients switch to sulfasalazine or add sulfasalazine to continuing leflunomide?"
1345:"RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial"
828:
causes synthetic lethality potentially due to increased demand on pyrimidines in these faster growing cells.
4239:
3742:
991:. Whereas uncommon side effects (occurring in 0.1–1% of those treated with the drug) include: constipation,
167:
624:
4141:
3547:
2973:
2654:
1179:
1151:
867:
316:
1278:"FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)"
4136:
3734:
1187:
1120:
445:
1479:
574:
2623:
1277:
1135:
837:
816:
785:
348:
1690:
Mathur D, Stratikopoulos E, Ozturk S, Steinbach N, Pegno S, Schoenfeld S, et al. (April 2017).
39:
4310:
2955:
1048:
948:
852:
842:
820:
789:
601:
400:
174:
4065:
2450:
Melchiorri D, van
Zwieten-Boot B, Maciulaitis R, Vilceanu M, Bruins Slot K, Hudson I, et al.
2263:
Teschner S, Burst V (September 2010). "Leflunomide: a drug with a potential beyond rheumatology".
4185:
3904:
3005:
2512:
2391:
2240:
2222:
2198:
2079:
2035:
1943:
1894:
1848:
1805:
1076:
857:
141:
3944:
3802:
872:
3954:
3640:
3081:
4320:
4300:
4128:
3909:
3899:
2700:
2658:
2504:
2429:
2383:
2280:
2190:
2155:
2120:
2071:
2027:
1992:
1935:
1886:
1840:
1797:
1762:
1721:
1669:
1600:
1570:
1536:
1226:
263:
251:
52:
2239:
for "Leflunomide
Associated With Topical Corticosteroids for Bullous Pemphigoid (ARABUL)" at
746:
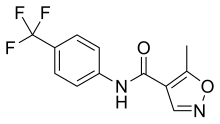
InChI=1S/C12H9F3N2O2/c1-7-10(6-16-19-7)11(18)17-9-4-2-8(3-5-9)12(13,14)15/h2-6H,1H3,(H,17,18)
554:
3705:
3608:
3271:
3167:
2609:
2496:
2421:
2375:
2334:
2272:
2182:
2147:
2110:
2063:
2019:
1982:
1974:
1925:
1878:
1832:
1789:
1752:
1711:
1703:
1659:
1651:
1526:
1518:
1139:
1000:
992:
641:
298:
1216:
514:
409:
4325:
4284:
4151:
4004:
3889:
1620:
1432:
1175:
1147:
1108:
1020:
892:
494:
326:
306:
823:
are the only indications that have received regulatory approval. Arava was developed by
628:
605:
4245:
3894:
3762:
3410:
3244:
3223:
3219:
3215:
2843:
2680:
1987:
1962:
1716:
1691:
1664:
1639:
1531:
1506:
1146:
synthesis. Teriflunomide also has antiviral effects against numerous viruses including
984:
824:
4294:
4195:
4172:
4118:
4044:
3974:
3884:
3791:
3577:
3277:
3199:
3151:
3131:
2989:
2905:
2756:
2722:
2500:
2395:
2151:
2023:
1882:
1377:
1221:
1198:
1131:
1060:
964:
617:
341:
276:
86:
2516:
2202:
2083:
2039:
1947:
1898:
4219:
4207:
4101:
4054:
4014:
3999:
3994:
3710:
3660:
3655:
3635:
3515:
3510:
3373:
3358:
3306:
3282:
3229:
3106:
3096:
2915:
2832:
2827:
2785:
2738:
2695:
2662:
1852:
1809:
1236:
1159:
1091:
1084:
1032:
1024:
968:
944:
194:
189:
72:
2235:
2217:
1480:"Arava (leflunomide) dosing, indications, interactions, adverse effects, and more"
2614:
2597:
1930:
1913:
1707:
1178:
of 80%, protein binding of >99%, metabolism sites of the GI mucosa and liver,
434:
4181:
4176:
4156:
4049:
3989:
3979:
3969:
3869:
3859:
3849:
3844:
3809:
3720:
3715:
3690:
3680:
3670:
3645:
3630:
3625:
3583:
3553:
3539:
3534:
3520:
3463:
3421:
3416:
3402:
3397:
3383:
3363:
3316:
3292:
3250:
3234:
3205:
3189:
3157:
3141:
3136:
3111:
3061:
3051:
3046:
3041:
3030:
3020:
2925:
2837:
2795:
2780:
2774:
2770:
1124:
1012:
1008:
956:
924:
920:
877:
80:
1505:
Dougados M, Emery P, Lemmel EM, Zerbini CA, Brin S, van Riel P (January 2005).
1260:(EMA) initially had not considered teriflunomide to be a new active substance.
4214:
4202:
4039:
4034:
4019:
3959:
3949:
3934:
3929:
3924:
3874:
3864:
3854:
3819:
3797:
3783:
3695:
3685:
3675:
3665:
3650:
3593:
3588:
3567:
3482:
3477:
3435:
3429:
3330:
3287:
3183:
3173:
3126:
3116:
3091:
3086:
3076:
3071:
3066:
3056:
3015:
3000:
2995:
2979:
2920:
2900:
2849:
2809:
2790:
2732:
2379:
2186:
2138:
Roy M (August 2007). "Early clinical experience with leflunomide in uveitis".
2067:
1836:
1793:
1655:
1036:
1028:
996:
960:
897:
680:
485:
336:
1963:"Six months open label trial of leflunomide in active ankylosing spondylitis"
4146:
4110:
4029:
4024:
4009:
3984:
3939:
3919:
3879:
3814:
3778:
3700:
3496:
3449:
3378:
3344:
3311:
3121:
3101:
3010:
2910:
2750:
1978:
1640:"Leflunomide triggers synthetic lethality in PTEN-deficient prostate cancer"
1522:
1206:
1072:
1056:
1004:
980:
972:
952:
780:
among others, is an immunosuppressive disease-modifying antirheumatic drug (
361:
66:
22:
2508:
2433:
2425:
2387:
2284:
2194:
2159:
2124:
2075:
2031:
1996:
1939:
1890:
1844:
1801:
1766:
1725:
1673:
1574:
1540:
1407:"Arava (leflunomide) tablet, film coated [sanofi-aventis U.S. LLC]"
1213:, the isoxazole ring of leflunomide is opened and teriflunomide is formed.
2115:
2098:
3964:
3839:
3528:
3036:
2946:
2882:
2804:
1757:
1740:
1155:
988:
976:
834:
465:
125:
1158:, which it achieves by inhibiting viral replication by interfering with
474:
30:
3457:
1231:
1016:
967:, vomiting, weakness, allergic reaction, chest pain, dry skin, eczema,
882:
420:
2276:
1638:
Ozturk S, Mathur D, Zhou RW, Mulholland D, Parsons R (December 2020).
1111:
drug that achieves its effects by inhibiting the mitochondrial enzyme
916:
Pregnancy, women of childbearing potential (unless contraception used)
803:
3772:
3603:
2560:"Summary of Opinion (Initial Authorisation): Aubagio (teriflunomide)"
1163:
565:
1190:
of 14–18 days and excretion routes of faeces (48%) and urine (43%).
2308:. Sanofi-Aventis Deutschland GmbH. 21 November 2013. Archived from
534:
3471:
3324:
1215:
802:
781:
703:
694:
545:
3443:
3391:
3352:
3338:
2892:
2766:
1252:-enol being the most stable and therefore most predominant form.
1245:
525:
285:
257:
112:
103:
4069:
2627:
947:, rash, nausea, bronchitis, headache, abdominal pain, abnormal
270:
120:
3300:
1285:
2487:
Rozman B (2002). "Clinical pharmacokinetics of leflunomide".
1079:, reduced therapeutic effects. Likewise live vaccines (like
590:
220:
152:
1599:. Adelaide: The Australian Medicines Handbook Unit Trust.
1007:. Rarely (in 0.1% of those treated with it) it can cause:
2596:
Shankaranarayana S, Barrett C, Kubler P (February 2013).
1961:
Haibel H, Rudwaleit M, Braun J, Sieper J (January 2005).
1378:"Arava 10mg Tablets - Summary of Product Characteristic"
246:
233:
763:
4268:
1914:"New treatment strategies in large-vessel vasculitis"
1685:
1683:
4165:
4127:
4109:
3832:
3760:
3733:
3618:
3261:
2963:
2954:
2945:
2938:
2891:
2873:
2864:
2818:
2749:
2679:
2670:
1248:forms (and the corresponding keto-amide), with the
692:
679:
640:
635:
616:
584:
564:
544:
524:
504:
484:
464:
453:
444:
419:
399:
370:
360:
347:
335:
325:
315:
305:
297:
210:
205:
180:
166:
140:
93:
79:
61:
51:
46:
2344:. sanofi-aventis new zealand limited. 29 June 2012
1235:. Teriflunomide then can interconvert between the
1401:
1399:
1321:. sanofi-aventis australia pty ltd. 7 August 2012
433:
1864:
1862:
408:
2099:"Acute pulmonary exacerbations of sarcoidosis"
1912:Unizony S, Stone JH, Stone JR (January 2013).
1209:ring. Upon oral administration of leflunomide
4081:
2639:
2445:
2443:
8:
1871:The American Journal of the Medical Sciences
1739:Blanckaert K, De Vriese AS (December 2006).
1372:
1370:
129:
21:
2565:. European Medicines Agency. Archived from
4088:
4074:
4066:
2960:
2951:
2942:
2870:
2676:
2646:
2632:
2624:
1138:(RA). Teriflunomide also inhibits several
627:
604:
493:
38:
29:
2613:
2540:. Center for Drug Evaluation and Research
2407:
2405:
2299:"Arava : EPAR - Product Information"
2258:
2256:
2254:
2252:
2250:
2248:
2114:
1986:
1929:
1756:
1715:
1663:
1530:
513:
2097:Panselinas E, Judson MA (October 2012).
1413:. sanofi-aventis U.S. LLC. November 2012
902:Prevention of organ transplant rejection
807:Bottle of Leflunomide (Arava) and tablet
4275:
1269:
1115:(DHODH), which plays a key role in the
743:
723:
600:
473:
384:
85:
2361:
2359:
1644:Prostate Cancer and Prostatic Diseases
1590:
1588:
1586:
1584:
1474:
618:
20:
4331:Disease-modifying antirheumatic drugs
1745:Nephrology, Dialysis, Transplantation
1472:
1470:
1468:
1466:
1464:
1462:
1460:
1458:
1456:
1454:
1306:
1304:
1302:
784:), used in active moderate-to-severe
573:
553:
71:
7:
2368:Scandinavian Journal of Rheumatology
2012:Journal of Clinical Gastroenterology
1627:. U.S. National Library of Medicine.
193:
533:
424:
1556:"Leflunomide in clinical practice"
1355:from the original on 3 August 2023
726:O=C(Nc1ccc(cc1)C(F)(F)F)c2c(onc2)C
14:
2538:U.S. Food and Drug Administration
2140:Canadian Journal of Ophthalmology
1130:The inhibition of human DHODH by
4278:
2501:10.2165/00003088-200241060-00003
2152:10.3129/can.j.ophthalmol.i07-085
2024:10.1097/00004836-200308000-00006
1967:Annals of the Rheumatic Diseases
1883:10.1097/00000441-200204000-00004
1511:Annals of the Rheumatic Diseases
863:Granulomatosis with polyangiitis
664:
658:
652:
57:Arava, Lefumide, Arabloc, others
2056:Digestive Diseases and Sciences
1918:Current Opinion in Rheumatology
1382:electronic Medicines Compendium
751:Key:VHOGYURTWQBHIL-UHFFFAOYSA-N
794:pyrimidine synthesis inhibitor
670:
646:
1:
2461:. p. 119. Archived from
1597:Australian Medicines Handbook
1563:Acta Reumatologica Portuguesa
1081:haemophilus influenzae type b
242:(Other controlled substances)
2711:dihydroorotate dehydrogenase
2615:10.18773/austprescr.2013.010
1931:10.1097/BOR.0b013e32835b133a
1708:10.1158/2159-8290.CD-16-0612
1554:Pinto P, Dougados M (2006).
1113:dihydroorotate dehydrogenase
848:Systemic lupus erythematosus
798:dihydroorotate dehydrogenase
776:, sold under the brand name
2690:purine synthesis inhibitors
2598:"The safety of leflunomide"
1312:"Arava Product Information"
912:Contraindications include:
4347:
1825:Rheumatology International
1384:. Sanofi. 21 February 2014
1351:(published 4 April 2023).
1041:toxic epidermal necrolysis
636:Chemical and physical data
4316:Trifluoromethyl compounds
4229:
2875:IL-1 receptor antagonists
2489:Clinical Pharmacokinetics
2459:European Medicines Agency
2380:10.1080/03009740802360632
2306:European Medicines Agency
2187:10.1007/s10067-002-0667-0
2068:10.1007/s10620-007-9953-7
1837:10.1007/s00296-012-2508-z
1794:10.1007/s10067-011-1689-2
1656:10.1038/s41391-020-0251-1
1437:European Medicines Agency
1258:European Medicines Agency
1053:interstitial lung disease
930:Active serious infections
796:that works by inhibiting
759:
734:
714:
391:--isoxazole-4-carboxamide
375:
366:Faeces (48%), urine (43%)
37:
28:
3748:Anti-lymphocyte globulin
1162:tegumentation and hence
1045:Stevens–Johnson syndrome
3743:Anti-thymocyte globulin
2655:Immunosuppressive drugs
1979:10.1136/ard.2003.019174
1523:10.1136/ard.2003.016709
1349:Diário Oficial da União
1090:The concomitant use of
4142:Sodium aurothiosulfate
3548:Interleukin-6 receptor
2974:Complement component 5
2426:10.1006/clim.1999.4777
2233:Clinical trial number
2215:Clinical trial number
1319:TGA eBusiness Services
1253:
1194:Leflunomide metabolism
1180:volume of distribution
868:Ankylosing spondylitis
808:
4137:Sodium aurothiomalate
2602:Australian Prescriber
2175:Clinical Rheumatology
2116:10.1378/chest.12-1060
1782:Clinical Rheumatology
1595:Rossi S, ed. (2013).
1219:
1188:elimination half-life
1186:) of 0.13 L/kg,
1121:uridine monophosphate
999:, taste disturbance,
806:
1621:"Leflunomide Search"
1136:rheumatoid arthritis
1051:, severe infection,
949:liver function tests
817:Rheumatoid arthritis
786:rheumatoid arthritis
4100:products / DMARDs (
2414:Clinical Immunology
1201:is the main active
1103:Mechanism of action
1049:lupus erythematosus
989:shortness of breath
945:high blood pressure
821:psoriatic arthritis
790:psoriatic arthritis
331:GI mucosa and liver
266:(Prescription only)
229:(Prescription only)
157: X (High risk)
25:
4306:Immunosuppressants
4256:Never to phase III
4186:Hydroxychloroquine
3905:Diroximel fumarate
3578:IL-2 receptor/CD25
3006:Certolizumab pegol
2659:Immunosuppressants
2335:"Data Sheet Arava"
2241:ClinicalTrials.gov
2223:ClinicalTrials.gov
1758:10.1093/ndt/gfl404
1625:ClinicalTrials.gov
1484:Medscape Reference
1254:
1107:Leflunomide is an
858:Takayasu arteritis
809:
4266:
4265:
4129:Gold preparations
4063:
4062:
3910:Efgartigimod alfa
3900:Dimethyl fumarate
3828:
3827:
3756:
3755:
3729:
3728:
2934:
2933:
2860:
2859:
2701:Mycophenolic acid
2277:10.2217/imt.10.52
1606:978-0-9805790-9-3
1343:(31 March 2023).
1227:active metabolite
908:Contraindications
771:
770:
705:Interactive image
586:CompTox Dashboard
289:
274:
261:
249:
237:
224:
156:
123:
106:
16:Chemical compound
4338:
4283:
4282:
4281:
4274:
4090:
4083:
4076:
4067:
3706:Telimomab aritox
3609:Zolimomab aritox
3430:CD62L/L-selectin
3168:Immunoglobulin E
2961:
2952:
2943:
2871:
2677:
2648:
2641:
2634:
2625:
2619:
2617:
2582:
2581:
2579:
2577:
2572:on 13 March 2016
2571:
2564:
2556:
2550:
2549:
2547:
2545:
2535:
2527:
2521:
2520:
2484:
2478:
2477:
2475:
2473:
2467:
2456:
2447:
2438:
2437:
2409:
2400:
2399:
2363:
2354:
2353:
2351:
2349:
2339:
2331:
2325:
2324:
2322:
2320:
2315:on 11 March 2014
2314:
2303:
2295:
2289:
2288:
2260:
2243:
2231:
2225:
2213:
2207:
2206:
2170:
2164:
2163:
2135:
2129:
2128:
2118:
2094:
2088:
2087:
2050:
2044:
2043:
2007:
2001:
2000:
1990:
1958:
1952:
1951:
1933:
1909:
1903:
1902:
1866:
1857:
1856:
1820:
1814:
1813:
1777:
1771:
1770:
1760:
1736:
1730:
1729:
1719:
1696:Cancer Discovery
1687:
1678:
1677:
1667:
1635:
1629:
1628:
1617:
1611:
1610:
1592:
1579:
1578:
1560:
1551:
1545:
1544:
1534:
1502:
1496:
1495:
1493:
1491:
1476:
1449:
1448:
1446:
1444:
1439:. 25 August 2023
1429:
1423:
1422:
1420:
1418:
1403:
1394:
1393:
1391:
1389:
1374:
1365:
1364:
1362:
1360:
1337:
1331:
1330:
1328:
1326:
1316:
1308:
1297:
1296:
1294:
1292:
1282:nctr-crs.fda.gov
1274:
1170:Pharmacokinetics
1140:tyrosine kinases
1109:immunomodulatory
1001:thrombocytopenia
933:Hypersensitivity
853:Felty's syndrome
843:Kimura's disease
767:
766:
707:
687:
672:
666:
660:
654:
648:
631:
620:
609:
608:
594:
592:
577:
557:
537:
517:
497:
477:
457:
437:
427:
426:
412:
352:
287:
284:
279:
272:
269:
259:
256:
248:
245:
235:
232:
222:
219:
197:
154:
151:
133:
122:
119:
116:
105:
102:
89:
75:
42:
33:
26:
24:
4346:
4345:
4341:
4340:
4339:
4337:
4336:
4335:
4291:
4290:
4289:
4279:
4277:
4269:
4267:
4262:
4261:
4246:Clinical trials
4225:
4161:
4152:Aurothioglucose
4123:
4105:
4094:
4064:
4059:
4005:Rozanolixizumab
3890:Deucravacitinib
3824:
3752:
3725:
3614:
3263:
3257:
2965:
2930:
2887:
2866:
2856:
2814:
2754:
2745:
2681:Antimetabolites
2672:
2666:
2652:
2622:
2595:
2591:
2589:Further reading
2586:
2585:
2575:
2573:
2569:
2562:
2558:
2557:
2553:
2543:
2541:
2533:
2529:
2528:
2524:
2486:
2485:
2481:
2471:
2469:
2468:on 17 July 2015
2465:
2454:
2449:
2448:
2441:
2411:
2410:
2403:
2365:
2364:
2357:
2347:
2345:
2337:
2333:
2332:
2328:
2318:
2316:
2312:
2301:
2297:
2296:
2292:
2262:
2261:
2246:
2232:
2228:
2214:
2210:
2172:
2171:
2167:
2137:
2136:
2132:
2096:
2095:
2091:
2052:
2051:
2047:
2009:
2008:
2004:
1960:
1959:
1955:
1911:
1910:
1906:
1868:
1867:
1860:
1822:
1821:
1817:
1779:
1778:
1774:
1738:
1737:
1733:
1689:
1688:
1681:
1637:
1636:
1632:
1619:
1618:
1614:
1607:
1594:
1593:
1582:
1558:
1553:
1552:
1548:
1504:
1503:
1499:
1489:
1487:
1478:
1477:
1452:
1442:
1440:
1431:
1430:
1426:
1416:
1414:
1405:
1404:
1397:
1387:
1385:
1376:
1375:
1368:
1358:
1356:
1339:
1338:
1334:
1324:
1322:
1314:
1310:
1309:
1300:
1290:
1288:
1276:
1275:
1271:
1266:
1196:
1185:
1176:bioavailability
1174:It has an oral
1172:
1105:
1100:
1069:
1021:agranulocytosis
940:
938:Adverse effects
919:Liver disease,
910:
905:
893:Prostate cancer
888:Still's disease
873:Crohn's disease
814:
762:
760:
755:
752:
747:
742:
741:
730:
727:
722:
721:
710:
685:
675:
669:
663:
657:
651:
612:
588:
580:
560:
540:
520:
500:
480:
460:
440:
423:
415:
395:
392:
383:
382:
350:
317:Protein binding
307:Bioavailability
299:Pharmacokinetic
293:
277:
201:
169:
162:
143:
136:
17:
12:
11:
5:
4344:
4342:
4334:
4333:
4328:
4323:
4318:
4313:
4308:
4303:
4293:
4292:
4288:
4287:
4264:
4263:
4260:
4259:
4258:
4257:
4254:
4243:
4237:
4231:
4230:
4227:
4226:
4224:
4223:
4211:
4199:
4193:
4188:
4179:
4169:
4167:
4163:
4162:
4160:
4159:
4154:
4149:
4144:
4139:
4133:
4131:
4125:
4124:
4122:
4121:
4115:
4113:
4107:
4106:
4095:
4093:
4092:
4085:
4078:
4070:
4061:
4060:
4058:
4057:
4052:
4047:
4042:
4037:
4032:
4027:
4022:
4017:
4012:
4007:
4002:
3997:
3992:
3987:
3982:
3977:
3972:
3967:
3962:
3957:
3952:
3947:
3942:
3937:
3932:
3927:
3922:
3917:
3914:+hyaluronidase
3907:
3902:
3897:
3895:Deuruxolitinib
3892:
3887:
3882:
3877:
3872:
3867:
3862:
3857:
3852:
3847:
3842:
3836:
3834:
3830:
3829:
3826:
3825:
3823:
3822:
3817:
3812:
3807:
3806:
3805:
3800:
3788:
3787:
3786:
3781:
3768:
3766:
3758:
3757:
3754:
3753:
3751:
3750:
3745:
3739:
3737:
3731:
3730:
3727:
3726:
3724:
3723:
3718:
3713:
3708:
3703:
3698:
3693:
3688:
3683:
3678:
3673:
3668:
3663:
3658:
3653:
3648:
3643:
3638:
3633:
3628:
3622:
3620:
3616:
3615:
3613:
3612:
3599:
3598:
3597:
3596:
3591:
3586:
3573:
3572:
3571:
3570:
3558:
3557:
3556:
3544:
3543:
3542:
3537:
3525:
3524:
3523:
3518:
3513:
3501:
3500:
3499:
3488:
3487:
3486:
3485:
3480:
3468:
3467:
3466:
3454:
3453:
3452:
3440:
3439:
3438:
3426:
3425:
3424:
3419:
3407:
3406:
3405:
3400:
3388:
3387:
3386:
3381:
3376:
3371:
3368:+hyaluronidase
3361:
3349:
3348:
3347:
3335:
3334:
3333:
3321:
3320:
3319:
3314:
3309:
3297:
3296:
3295:
3290:
3285:
3280:
3267:
3265:
3259:
3258:
3256:
3255:
3254:
3253:
3240:
3239:
3238:
3237:
3232:
3211:
3210:
3209:
3208:
3195:
3194:
3193:
3192:
3179:
3178:
3177:
3176:
3163:
3162:
3161:
3160:
3147:
3146:
3145:
3144:
3139:
3134:
3129:
3124:
3119:
3114:
3109:
3104:
3099:
3094:
3089:
3084:
3079:
3074:
3069:
3064:
3059:
3054:
3049:
3044:
3039:
3026:
3025:
3024:
3023:
3018:
3013:
3008:
3003:
2998:
2985:
2984:
2983:
2982:
2969:
2967:
2958:
2949:
2940:
2936:
2935:
2932:
2931:
2929:
2928:
2923:
2918:
2913:
2908:
2903:
2897:
2895:
2889:
2888:
2886:
2885:
2879:
2877:
2868:
2862:
2861:
2858:
2857:
2855:
2854:
2853:
2852:
2844:PDE4 inhibitor
2840:
2835:
2830:
2824:
2822:
2816:
2815:
2813:
2812:
2807:
2801:
2800:
2799:
2798:
2793:
2788:
2783:
2762:
2760:
2747:
2746:
2744:
2743:
2742:
2741:
2728:
2727:
2726:
2725:
2720:
2706:
2705:
2704:
2703:
2698:
2685:
2683:
2674:
2668:
2667:
2653:
2651:
2650:
2643:
2636:
2628:
2621:
2620:
2592:
2590:
2587:
2584:
2583:
2551:
2522:
2479:
2439:
2420:(3): 198–208.
2401:
2355:
2326:
2290:
2244:
2226:
2208:
2165:
2130:
2109:(4): 827–836.
2089:
2062:(4): 1025–32.
2045:
2002:
1953:
1904:
1858:
1815:
1772:
1751:(12): 3364–7.
1731:
1702:(4): 380–390.
1679:
1650:(4): 718–723.
1630:
1612:
1605:
1580:
1546:
1497:
1450:
1424:
1395:
1366:
1332:
1298:
1268:
1267:
1265:
1262:
1195:
1192:
1183:
1171:
1168:
1104:
1101:
1099:
1096:
1068:
1065:
985:cholelithiasis
939:
936:
935:
934:
931:
928:
917:
909:
906:
904:
903:
900:
895:
890:
885:
880:
875:
870:
865:
860:
855:
850:
845:
840:
830:
825:Sanofi Aventis
813:
810:
769:
768:
757:
756:
754:
753:
750:
748:
745:
737:
736:
735:
732:
731:
729:
728:
725:
717:
716:
715:
712:
711:
709:
708:
700:
698:
690:
689:
683:
677:
676:
673:
667:
661:
655:
649:
644:
638:
637:
633:
632:
622:
614:
613:
611:
610:
597:
595:
582:
581:
579:
578:
570:
568:
562:
561:
559:
558:
550:
548:
542:
541:
539:
538:
530:
528:
522:
521:
519:
518:
510:
508:
502:
501:
499:
498:
490:
488:
482:
481:
479:
478:
470:
468:
462:
461:
459:
458:
450:
448:
442:
441:
439:
438:
430:
428:
417:
416:
414:
413:
405:
403:
397:
396:
394:
393:
386:
378:
377:
376:
373:
372:
368:
367:
364:
358:
357:
354:
345:
344:
339:
333:
332:
329:
323:
322:
319:
313:
312:
309:
303:
302:
295:
294:
292:
291:
282:
267:
254:
243:
230:
216:
214:
208:
207:
203:
202:
200:
199:
186:
184:
178:
177:
172:
170:administration
164:
163:
161:
160:
158:
148:
146:
138:
137:
135:
134:
117:
99:
97:
91:
90:
83:
77:
76:
69:
59:
58:
55:
49:
48:
44:
43:
35:
34:
15:
13:
10:
9:
6:
4:
3:
2:
4343:
4332:
4329:
4327:
4324:
4322:
4319:
4317:
4314:
4312:
4309:
4307:
4304:
4302:
4299:
4298:
4296:
4286:
4276:
4272:
4255:
4253:
4250:
4249:
4247:
4244:
4241:
4238:
4236:
4233:
4232:
4228:
4221:
4217:
4216:
4212:
4209:
4205:
4204:
4200:
4197:
4196:Sulfasalazine
4194:
4192:
4189:
4187:
4183:
4180:
4178:
4174:
4173:Penicillamine
4171:
4170:
4168:
4164:
4158:
4155:
4153:
4150:
4148:
4145:
4143:
4140:
4138:
4135:
4134:
4132:
4130:
4126:
4120:
4119:Oxycinchophen
4117:
4116:
4114:
4112:
4108:
4103:
4099:
4098:antirheumatic
4091:
4086:
4084:
4079:
4077:
4072:
4071:
4068:
4056:
4053:
4051:
4048:
4046:
4045:Tildrakizumab
4043:
4041:
4038:
4036:
4033:
4031:
4028:
4026:
4023:
4021:
4018:
4016:
4013:
4011:
4008:
4006:
4003:
4001:
3998:
3996:
3993:
3991:
3988:
3986:
3983:
3981:
3978:
3976:
3975:Pegcetacoplan
3973:
3971:
3968:
3966:
3963:
3961:
3958:
3956:
3953:
3951:
3948:
3946:
3943:
3941:
3938:
3936:
3933:
3931:
3928:
3926:
3923:
3921:
3918:
3915:
3911:
3908:
3906:
3903:
3901:
3898:
3896:
3893:
3891:
3888:
3886:
3885:Darvadstrocel
3883:
3881:
3878:
3876:
3873:
3871:
3868:
3866:
3863:
3861:
3858:
3856:
3853:
3851:
3848:
3846:
3843:
3841:
3838:
3837:
3835:
3831:
3821:
3818:
3816:
3813:
3811:
3808:
3804:
3801:
3799:
3796:
3795:
3794:
3793:
3792:TNF inhibitor
3789:
3785:
3782:
3780:
3777:
3776:
3775:
3774:
3770:
3769:
3767:
3764:
3759:
3749:
3746:
3744:
3741:
3740:
3738:
3736:
3732:
3722:
3719:
3717:
3714:
3712:
3709:
3707:
3704:
3702:
3699:
3697:
3694:
3692:
3689:
3687:
3684:
3682:
3679:
3677:
3674:
3672:
3669:
3667:
3664:
3662:
3659:
3657:
3654:
3652:
3649:
3647:
3644:
3642:
3639:
3637:
3634:
3632:
3629:
3627:
3624:
3623:
3621:
3617:
3610:
3606:
3605:
3601:
3600:
3595:
3592:
3590:
3587:
3585:
3582:
3581:
3580:
3579:
3575:
3574:
3569:
3566:
3565:
3564:
3563:
3559:
3555:
3552:
3551:
3550:
3549:
3545:
3541:
3538:
3536:
3533:
3532:
3531:
3530:
3526:
3522:
3519:
3517:
3514:
3512:
3509:
3508:
3507:
3506:
3502:
3498:
3495:
3494:
3493:
3490:
3489:
3484:
3481:
3479:
3476:
3475:
3474:
3473:
3469:
3465:
3462:
3461:
3460:
3459:
3458:CD147/Basigin
3455:
3451:
3448:
3447:
3446:
3445:
3441:
3437:
3434:
3433:
3432:
3431:
3427:
3423:
3420:
3418:
3415:
3414:
3413:
3412:
3408:
3404:
3401:
3399:
3396:
3395:
3394:
3393:
3389:
3385:
3382:
3380:
3377:
3375:
3372:
3369:
3365:
3362:
3360:
3357:
3356:
3355:
3354:
3350:
3346:
3343:
3342:
3341:
3340:
3336:
3332:
3329:
3328:
3327:
3326:
3322:
3318:
3315:
3313:
3310:
3308:
3305:
3304:
3303:
3302:
3298:
3294:
3291:
3289:
3286:
3284:
3281:
3279:
3278:Muromonab-CD3
3276:
3275:
3274:
3273:
3269:
3268:
3266:
3260:
3252:
3249:
3248:
3247:
3246:
3242:
3241:
3236:
3233:
3231:
3228:
3227:
3226:
3225:
3221:
3217:
3213:
3212:
3207:
3204:
3203:
3202:
3201:
3197:
3196:
3191:
3188:
3187:
3186:
3185:
3181:
3180:
3175:
3172:
3171:
3170:
3169:
3165:
3164:
3159:
3156:
3155:
3154:
3153:
3152:Interleukin 5
3149:
3148:
3143:
3140:
3138:
3135:
3133:
3132:Tildrakizumab
3130:
3128:
3125:
3123:
3120:
3118:
3115:
3113:
3110:
3108:
3105:
3103:
3100:
3098:
3095:
3093:
3090:
3088:
3085:
3083:
3080:
3078:
3075:
3073:
3070:
3068:
3065:
3063:
3060:
3058:
3055:
3053:
3050:
3048:
3045:
3043:
3040:
3038:
3035:
3034:
3033:
3032:
3028:
3027:
3022:
3019:
3017:
3014:
3012:
3009:
3007:
3004:
3002:
2999:
2997:
2994:
2993:
2992:
2991:
2987:
2986:
2981:
2978:
2977:
2976:
2975:
2971:
2970:
2968:
2966:(noncellular)
2962:
2959:
2957:
2953:
2950:
2948:
2944:
2941:
2939:Extracellular
2937:
2927:
2924:
2922:
2919:
2917:
2914:
2912:
2909:
2907:
2906:Ridaforolimus
2904:
2902:
2899:
2898:
2896:
2894:
2890:
2884:
2881:
2880:
2878:
2876:
2872:
2869:
2865:Intracellular
2863:
2851:
2848:
2847:
2846:
2845:
2841:
2839:
2836:
2834:
2831:
2829:
2826:
2825:
2823:
2821:
2817:
2811:
2808:
2806:
2803:
2802:
2797:
2794:
2792:
2789:
2787:
2784:
2782:
2779:
2778:
2777:
2776:
2772:
2768:
2764:
2763:
2761:
2758:
2752:
2748:
2740:
2737:
2736:
2735:
2734:
2730:
2729:
2724:
2723:Teriflunomide
2721:
2719:
2716:
2715:
2713:
2712:
2708:
2707:
2702:
2699:
2697:
2694:
2693:
2692:
2691:
2687:
2686:
2684:
2682:
2678:
2675:
2671:Intracellular
2669:
2664:
2660:
2656:
2649:
2644:
2642:
2637:
2635:
2630:
2629:
2626:
2616:
2611:
2607:
2603:
2599:
2594:
2593:
2588:
2568:
2561:
2555:
2552:
2539:
2532:
2526:
2523:
2518:
2514:
2510:
2506:
2502:
2498:
2495:(6): 421–30.
2494:
2490:
2483:
2480:
2464:
2460:
2453:
2446:
2444:
2440:
2435:
2431:
2427:
2423:
2419:
2415:
2408:
2406:
2402:
2397:
2393:
2389:
2385:
2381:
2377:
2373:
2369:
2362:
2360:
2356:
2343:
2336:
2330:
2327:
2311:
2307:
2300:
2294:
2291:
2286:
2282:
2278:
2274:
2271:(5): 637–50.
2270:
2266:
2265:Immunotherapy
2259:
2257:
2255:
2253:
2251:
2249:
2245:
2242:
2238:
2237:
2230:
2227:
2224:
2220:
2219:
2212:
2209:
2204:
2200:
2196:
2192:
2188:
2184:
2180:
2176:
2169:
2166:
2161:
2157:
2153:
2149:
2145:
2141:
2134:
2131:
2126:
2122:
2117:
2112:
2108:
2104:
2100:
2093:
2090:
2085:
2081:
2077:
2073:
2069:
2065:
2061:
2057:
2049:
2046:
2041:
2037:
2033:
2029:
2025:
2021:
2017:
2013:
2006:
2003:
1998:
1994:
1989:
1984:
1980:
1976:
1972:
1968:
1964:
1957:
1954:
1949:
1945:
1941:
1937:
1932:
1927:
1923:
1919:
1915:
1908:
1905:
1900:
1896:
1892:
1888:
1884:
1880:
1876:
1872:
1865:
1863:
1859:
1854:
1850:
1846:
1842:
1838:
1834:
1830:
1826:
1819:
1816:
1811:
1807:
1803:
1799:
1795:
1791:
1788:(6): 859–65.
1787:
1783:
1776:
1773:
1768:
1764:
1759:
1754:
1750:
1746:
1742:
1735:
1732:
1727:
1723:
1718:
1713:
1709:
1705:
1701:
1697:
1693:
1686:
1684:
1680:
1675:
1671:
1666:
1661:
1657:
1653:
1649:
1645:
1641:
1634:
1631:
1626:
1622:
1616:
1613:
1608:
1602:
1598:
1591:
1589:
1587:
1585:
1581:
1576:
1572:
1569:(3): 215–24.
1568:
1564:
1557:
1550:
1547:
1542:
1538:
1533:
1528:
1524:
1520:
1516:
1512:
1508:
1501:
1498:
1485:
1481:
1475:
1473:
1471:
1469:
1467:
1465:
1463:
1461:
1459:
1457:
1455:
1451:
1438:
1434:
1428:
1425:
1412:
1408:
1402:
1400:
1396:
1383:
1379:
1373:
1371:
1367:
1354:
1350:
1346:
1342:
1336:
1333:
1320:
1313:
1307:
1305:
1303:
1299:
1287:
1283:
1279:
1273:
1270:
1263:
1261:
1259:
1251:
1247:
1244:
1243:
1239:
1234:
1233:
1228:
1224:
1223:
1222:Teriflunomide
1218:
1214:
1212:
1208:
1204:
1200:
1199:Teriflunomide
1193:
1191:
1189:
1181:
1177:
1169:
1167:
1165:
1161:
1157:
1153:
1149:
1145:
1141:
1137:
1133:
1132:teriflunomide
1128:
1126:
1122:
1119:synthesis of
1118:
1114:
1110:
1102:
1097:
1095:
1093:
1088:
1086:
1082:
1078:
1074:
1066:
1064:
1062:
1061:liver failure
1058:
1054:
1050:
1046:
1042:
1038:
1034:
1030:
1026:
1022:
1018:
1014:
1010:
1006:
1002:
998:
994:
990:
986:
982:
978:
974:
970:
966:
965:tenosynovitis
962:
958:
954:
951:, back pain,
950:
946:
937:
932:
929:
926:
922:
918:
915:
914:
913:
907:
901:
899:
896:
894:
891:
889:
886:
884:
881:
879:
876:
874:
871:
869:
866:
864:
861:
859:
856:
854:
851:
849:
846:
844:
841:
839:
836:
832:
831:
829:
826:
822:
818:
811:
805:
801:
799:
795:
791:
787:
783:
779:
775:
765:
758:
749:
744:
740:
733:
724:
720:
713:
706:
702:
701:
699:
696:
691:
684:
682:
678:
645:
643:
639:
634:
630:
626:
623:
621:
619:ECHA InfoCard
615:
607:
603:
602:DTXSID9023201
599:
598:
596:
587:
583:
576:
572:
571:
569:
567:
563:
556:
552:
551:
549:
547:
543:
536:
532:
531:
529:
527:
523:
516:
512:
511:
509:
507:
503:
496:
492:
491:
489:
487:
483:
476:
472:
471:
469:
467:
463:
456:
452:
451:
449:
447:
443:
436:
432:
431:
429:
422:
418:
411:
407:
406:
404:
402:
398:
390:
385:
381:
374:
369:
365:
363:
359:
355:
353:
346:
343:
342:Teriflunomide
340:
338:
334:
330:
328:
324:
320:
318:
314:
310:
308:
304:
300:
296:
290: Rx-only
283:
280:
268:
265:
255:
253:
244:
241:
231:
228:
218:
217:
215:
213:
209:
204:
196:
191:
188:
187:
185:
183:
179:
176:
173:
171:
165:
159:
150:
149:
147:
145:
139:
132:
127:
118:
115:
110:
101:
100:
98:
96:
92:
88:
84:
82:
78:
74:
70:
68:
64:
60:
56:
54:
50:
47:Clinical data
45:
41:
36:
32:
27:
19:
4220:Azathioprine
4213:
4208:Methotrexate
4201:
4190:
4055:Upadacitinib
4015:Satralizumab
4000:Ritlecitinib
3995:Risankizumab
3790:
3771:
3711:Teprotumumab
3661:Inebilizumab
3656:Fontolizumab
3636:Atorolimumab
3604:T-lymphocyte
3602:
3576:
3560:
3546:
3527:
3516:Lerdelimumab
3511:Bertilimumab
3503:
3491:
3470:
3456:
3442:
3428:
3409:
3390:
3374:Pascolizumab
3359:Obinutuzumab
3351:
3337:
3323:
3307:Clenoliximab
3299:
3283:Otelixizumab
3270:
3243:
3230:Lebrikizumab
3214:
3198:
3182:
3166:
3150:
3107:Satralizumab
3097:Risankizumab
3029:
2988:
2972:
2964:Serum target
2916:Temsirolimus
2842:
2833:Pomalidomide
2828:Lenalidomide
2786:Pimecrolimus
2765:
2739:Methotrexate
2731:
2717:
2709:
2696:Azathioprine
2688:
2673:(initiation)
2608:(1): 28–32.
2605:
2601:
2574:. Retrieved
2567:the original
2554:
2542:. Retrieved
2537:
2525:
2492:
2488:
2482:
2470:. Retrieved
2463:the original
2458:
2417:
2413:
2371:
2367:
2346:. Retrieved
2341:
2329:
2317:. Retrieved
2310:the original
2305:
2293:
2268:
2264:
2234:
2229:
2216:
2211:
2178:
2174:
2168:
2143:
2139:
2133:
2106:
2102:
2092:
2059:
2055:
2048:
2018:(2): 125–8.
2015:
2011:
2005:
1973:(1): 124–6.
1970:
1966:
1956:
1921:
1917:
1907:
1877:(4): 190–3.
1874:
1870:
1831:(2): 273–6.
1828:
1824:
1818:
1785:
1781:
1775:
1748:
1744:
1734:
1699:
1695:
1647:
1643:
1633:
1624:
1615:
1596:
1566:
1562:
1549:
1517:(1): 44–51.
1514:
1510:
1500:
1488:. Retrieved
1483:
1441:. Retrieved
1436:
1433:"Arava EPAR"
1427:
1415:. Retrieved
1410:
1386:. Retrieved
1381:
1357:. Retrieved
1335:
1323:. Retrieved
1318:
1289:. Retrieved
1281:
1272:
1255:
1249:
1241:
1237:
1230:
1220:
1210:
1202:
1197:
1173:
1160:nucleocapsid
1143:
1129:
1116:
1106:
1098:Pharmacology
1092:methotrexate
1089:
1085:yellow fever
1083:vaccine and
1070:
1067:Interactions
1047:, cutaneous
1033:pancytopenia
1025:eosinophilia
969:paraesthesia
941:
927:seropositive
911:
815:
777:
773:
772:
761:
388:
349:Elimination
212:Legal status
206:Legal status
95:License data
18:
4242:from market
4191:Leflunomide
4182:Chloroquine
4177:Bucillamine
4157:Aurotioprol
4050:Tofacitinib
3990:Ravulizumab
3980:Pirfenidone
3970:Peficitinib
3870:Canakinumab
3860:Briakinumab
3850:Bimekizumab
3845:Baricitinib
3810:Aflibercept
3721:Vepalimomab
3716:Vapaliximab
3691:Rovelizumab
3681:Pexelizumab
3671:Morolimumab
3646:Cedelizumab
3631:Anifrolumab
3626:Alemtuzumab
3584:Basiliximab
3554:Tocilizumab
3540:Vedolizumab
3535:Natalizumab
3521:Metelimumab
3464:Gavilimomab
3422:Toralizumab
3417:Teneliximab
3403:Lumiliximab
3398:Gomiliximab
3384:Ublituximab
3364:Ocrelizumab
3317:Zanolimumab
3293:Visilizumab
3251:Secukinumab
3235:Ustekinumab
3206:Elsilimomab
3190:Faralimomab
3158:Mepolizumab
3142:Ustekinumab
3137:Tocilizumab
3112:Secukinumab
3062:Canakinumab
3052:Briakinumab
3047:Bimekizumab
3042:Basiliximab
3031:Interleukin
3021:Nerelimomab
2926:Zotarolimus
2867:(reception)
2838:Thalidomide
2796:Voclosporin
2781:Ciclosporin
2775:Calcineurin
2771:Cyclophilin
2718:Leflunomide
2714:inhibitors
2374:(1): 11–4.
2236:NCT00802243
2218:NCT00004071
1125:lymphocytes
1013:angiooedema
1009:anaphylaxis
993:oral thrush
957:pharyngitis
953:indigestion
921:hepatitis B
878:Sarcoidosis
838:nephropathy
812:Medical use
774:Leflunomide
688: g·mol
625:100.123.883
371:Identifiers
337:Metabolites
131:Leflunomide
81:MedlinePlus
53:Trade names
23:Leflunomide
4311:Isoxazoles
4295:Categories
4215:thiopurine
4203:antifolate
4111:Quinolines
4040:Sutimlimab
4035:Spesolimab
4020:Siltuximab
3960:Olokizumab
3950:Ixekizumab
3945:Itacitinib
3935:Guselkumab
3930:Fingolimod
3925:Filgotinib
3875:Crovalimab
3865:Brodalumab
3855:Blisibimod
3820:Rilonacept
3803:Opinercept
3798:Etanercept
3784:Belatacept
3735:Polyclonal
3696:Siplizumab
3686:Reslizumab
3676:Ofatumumab
3666:Maslimomab
3651:Emapalumab
3594:Inolimomab
3589:Daclizumab
3568:Odulimomab
3483:Ruplizumab
3478:Frexalimab
3436:Aselizumab
3331:Efalizumab
3288:Teplizumab
3184:Interferon
3174:Omalizumab
3127:Spesolimab
3117:Siltuximab
3092:Rilonacept
3087:Olokizumab
3077:Ixekizumab
3072:Guselkumab
3067:Daclizumab
3057:Brodalumab
3016:Infliximab
3001:Afelimomab
2996:Adalimumab
2980:Eculizumab
2956:Monoclonal
2947:Antibodies
2921:Umirolimus
2901:Everolimus
2850:Apremilast
2810:Gusperimus
2791:Tacrolimus
2759:inhibitors
2751:Macrolides
2733:antifolate
2181:(2): 157.
2146:(4): 634.
1924:(1): 3–9.
1291:22 October
1264:References
1166:assembly.
1077:astragalus
1037:vasculitis
1029:leucopenia
997:stomatitis
961:stomatitis
898:Pemphigoid
792:. It is a
693:3D model (
681:Molar mass
555:CHEBI:6402
515:G162GK9U4W
486:ChemSpider
446:IUPHAR/BPS
410:75706-12-6
401:CAS Number
380:IUPAC name
356:14–18 days
327:Metabolism
4252:Phase III
4240:Withdrawn
4147:Auranofin
4096:Specific
4030:Sirukumab
4025:Siponimod
4010:Sarilumab
3985:Ponesimod
3955:Netakimab
3940:Iptacopan
3920:Etrasimod
3880:Danicopan
3815:Alefacept
3779:Abatacept
3701:Talizumab
3641:Begelomab
3497:Belimumab
3450:Galiximab
3379:Rituximab
3345:Erlizumab
3312:Keliximab
3122:Sirukumab
3102:Sarilumab
3082:Netakimab
3011:Golimumab
2911:Sirolimus
2396:205543918
1443:26 August
1359:16 August
1207:isoxazole
1073:echinacea
1057:cirrhosis
981:synovitis
973:pneumonia
575:ChEMBL960
387:5-methyl-
362:Excretion
351:half-life
168:Routes of
142:Pregnancy
73:Monograph
67:Drugs.com
4321:Prodrugs
4301:Anilides
4285:Medicine
3965:Ozanimod
3840:Avacopan
3833:Unsorted
3619:Unsorted
3529:Integrin
3262:Cellular
3037:Anakinra
2883:Anakinra
2805:Abetimus
2657: /
2576:15 April
2544:15 April
2517:33745823
2509:12074690
2434:10600330
2388:19191187
2348:11 March
2319:11 March
2285:20874647
2203:41656726
2195:12740686
2160:17641721
2125:23032450
2084:29918308
2076:17934840
2040:21212960
2032:12869881
1997:15608310
1948:21101525
1940:23114585
1899:28479334
1891:12003373
1845:22961090
1802:21286771
1767:16998219
1726:28255082
1674:32661432
1575:17094333
1541:15271770
1490:11 March
1417:11 March
1411:DailyMed
1388:11 March
1353:Archived
1325:11 March
1156:BK virus
1154:and the
977:rhinitis
835:BK virus
833:Polyoma
764:(verify)
466:DrugBank
240:Class C1
182:ATC code
175:By mouth
144:category
126:DailyMed
3761:-cept (
2342:Medsafe
1988:1755172
1853:7202069
1810:1914281
1717:5562025
1665:7666085
1532:1755199
1486:. WebMD
1232:in vivo
1225:is the
1211:in vivo
1203:in vivo
1144:de novo
1117:de novo
1017:anaemia
883:Uveitis
686:270.211
642:Formula
475:DB01097
421:PubChem
321:>99%
281:Rx-only
278:WARNING
250::
198:)
192: (
190:L04AK01
128::
111::
87:a600032
4326:Sanofi
4271:Portal
4235:WHO-EM
3773:CTLA-4
3763:Fusion
3264:target
3245:IL-17A
3222:, and
2755:other
2515:
2507:
2472:5 June
2432:
2394:
2386:
2283:
2201:
2193:
2158:
2123:
2082:
2074:
2038:
2030:
1995:
1985:
1946:
1938:
1897:
1889:
1851:
1843:
1808:
1800:
1765:
1724:
1714:
1672:
1662:
1603:
1573:
1539:
1529:
1341:Anvisa
1246:enolic
1164:virion
719:SMILES
566:ChEMBL
535:D00749
275:
262:
252:℞-only
238:
225:
124:
114:by INN
107:
4166:Other
3562:LFA-1
3472:CD154
3325:CD11a
3224:IL-23
3220:IL-13
3216:IL-12
2820:IMiDs
2570:(PDF)
2563:(PDF)
2534:(PDF)
2513:S2CID
2466:(PDF)
2455:(PDF)
2392:S2CID
2338:(PDF)
2313:(PDF)
2302:(PDF)
2199:S2CID
2103:Chest
2080:S2CID
2036:S2CID
1944:S2CID
1895:S2CID
1849:S2CID
1806:S2CID
1559:(PDF)
1315:(PDF)
1005:hives
782:DMARD
778:Arava
739:InChI
695:JSmol
546:ChEBI
4102:M01C
3492:BLyS
3444:CD80
3411:CD40
3392:CD23
3353:CD20
3339:CD18
3200:IL-6
2893:mTOR
2767:FKBP
2757:IL-2
2578:2016
2546:2016
2505:PMID
2474:2015
2430:PMID
2384:PMID
2350:2014
2321:2014
2281:PMID
2191:PMID
2156:PMID
2121:PMID
2072:PMID
2028:PMID
1993:PMID
1936:PMID
1887:PMID
1841:PMID
1798:PMID
1763:PMID
1722:PMID
1670:PMID
1601:ISBN
1571:PMID
1537:PMID
1492:2014
1445:2023
1419:2014
1390:2014
1361:2023
1327:2014
1293:2023
1240:and
1152:HSV1
1059:and
1003:and
987:and
819:and
788:and
526:KEGG
506:UNII
495:3762
455:6825
435:3899
301:data
63:AHFS
4222:) #
3505:CAT
3301:CD4
3272:CD3
2990:TNF
2663:L04
2610:doi
2497:doi
2422:doi
2376:doi
2273:doi
2183:doi
2148:doi
2111:doi
2107:142
2064:doi
2020:doi
1983:PMC
1975:doi
1926:doi
1879:doi
1875:323
1833:doi
1790:doi
1753:doi
1712:PMC
1704:doi
1660:PMC
1652:doi
1527:PMC
1519:doi
1286:FDA
1148:CMV
1075:or
591:EPA
425:CID
311:80%
264:POM
195:WHO
109:EMA
4297::
4248::
4210:#)
4184:#/
4175:#/
3218:,
2606:36
2604:.
2600:.
2536:.
2511:.
2503:.
2493:41
2491:.
2457:.
2442:^
2428:.
2418:93
2416:.
2404:^
2390:.
2382:.
2372:38
2370:.
2358:^
2340:.
2304:.
2279:.
2267:.
2247:^
2197:.
2189:.
2179:22
2177:.
2154:.
2144:42
2142:.
2119:.
2105:.
2101:.
2078:.
2070:.
2060:53
2058:.
2034:.
2026:.
2016:37
2014:.
1991:.
1981:.
1971:64
1969:.
1965:.
1942:.
1934:.
1922:25
1920:.
1916:.
1893:.
1885:.
1873:.
1861:^
1847:.
1839:.
1829:33
1827:.
1804:.
1796:.
1786:30
1784:.
1761:.
1749:21
1747:.
1743:.
1720:.
1710:.
1698:.
1694:.
1682:^
1668:.
1658:.
1648:23
1646:.
1642:.
1623:.
1583:^
1567:31
1565:.
1561:.
1535:.
1525:.
1515:64
1513:.
1509:.
1482:.
1453:^
1435:.
1409:.
1398:^
1380:.
1369:^
1317:.
1301:^
1284:.
1280:.
1182:(V
1150:,
1127:.
1063:.
1055:,
1043:,
1039:,
1035:,
1031:,
1027:,
1023:,
1019:,
1015:,
1011:,
995:,
983:,
979:,
975:,
971:,
963:,
959:,
800:.
650:12
286:EU
271:US
258:UK
247:CA
234:BR
227:S4
221:AU
153:AU
121:US
104:EU
4273::
4218:(
4206:(
4198:#
4104:)
4089:e
4082:t
4075:v
3916:)
3912:(
3765:)
3611:)
3607:(
3370:)
3366:(
2773:/
2769:/
2753:/
2665:)
2661:(
2647:e
2640:t
2633:v
2618:.
2612::
2580:.
2548:.
2519:.
2499::
2476:.
2436:.
2424::
2398:.
2378::
2352:.
2323:.
2287:.
2275::
2269:2
2205:.
2185::
2162:.
2150::
2127:.
2113::
2086:.
2066::
2042:.
2022::
1999:.
1977::
1950:.
1928::
1901:.
1881::
1855:.
1835::
1812:.
1792::
1769:.
1755::
1728:.
1706::
1700:7
1676:.
1654::
1609:.
1577:.
1543:.
1521::
1494:.
1447:.
1421:.
1392:.
1363:.
1329:.
1295:.
1250:Z
1242:Z
1238:E
1184:d
925:C
923:/
697:)
674:2
671:O
668:2
665:N
662:3
659:F
656:9
653:H
647:C
593:)
589:(
389:N
288::
273::
260::
236::
223::
155::
65:/
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.