1223:
782:
224:
1465:
1101:
1474:
1564:
29:
493:
3956:
Aptroot, André; Feuerstein, Shirley Cunha; Cunha-Dias, Iane Paula Rego; de Lucena Nunes, Álvaro
Rogerio; Honorato, Maykon Evangelista; da Silva Cáceres, Marcela Eugenia (2017). "New lichen species and lichen reports from Amazon forest remnants and Cerrado vegetation in the Tocantina Region, northern
4085:
Wijayawardene, N.N.; Phillips, A.J.L.; Tibpromma, S.; Dai, D.Q.; Selbmann, L.; Monteiro, J.S.; Aptroot, A.; Flakus, A.; Rajeshkumar, K.C.; Coleine, C.; Pereira, D.S.; Fan, X.; Zhang, L.; Maharachchikumbura, S.S.N.; Souza, M.F.; Kukwa, M.; Suwannarach, N.; Rodriguez-Flakus, P.; Ashtekar, N.; Dauner,
1594:
have been reported, some isolated from a variety of lichen species, and some produced synthetically. These derivatives are variously mono-, bi-, or trichlorinated with the chlorines at positions 2, 4, 5, and 7. As of 2016, 62 molecules with the lichexanthone scaffold had been described, and another
1554:
chemical in the cortex may allow them to survive in otherwise inhospitable habitats, like on exposed trees in tropical areas or high mountains. It has been pointed out, however, that lichexanthone is also found in lichens living in less stressed environments, and from species that are in families
4176:
Aptroot, André; Fernanda de Souza, Maria; Alves dos Santos, Lidiane; Oliveira Junior, Isaias; Cardoso
Barbosa, Bruno Micael; Cáceres da Silva, Marcela Eugenia (2022). "New species of lichenized fungi from Brazil, with a record report of 492 species in a small area of the Amazon Forest".
1279:
relies heavily on thallus chemistry to distinguish and classify species, some of which differ only in the presence or absence of a single secondary chemical. Lichexanthone, norlichexanthone, and their chlorinated derivatives are common in this genus.
3043:
Arriaga, Ângela M.C.; Feitosa, Edinilza M.A.; Lemos, Telma L.G.; Santiago, Gilvandete M.P.; Lima, Jefferson Q.; De
Oliveira, Maria C.F.; Vasconcelos, Jackson N. e; Rodrigues, Francisco E.A.; Gomes, Tathilene B.M.; Braz-Filho, Raimundo (2008).
3292:
Calderón, Angela I.; Terreaux, Christian; Schenk, Kurt; Pattison, Phil; Burdette, Joanna E.; Pezzuto, John M.; Gupta, Mahabir P.; Hostettmann, K. (2002). "Isolation and structure elucidation of an isoflavone and a sesterterpenoic acid from
3540:
Nissanka, Ajith P.K.; Karunaratne, Veranja; Bandara, B.M.Ratnayake; Kumar, Vijaya; Nakanishi, Tsutomu; Nishi, Masatoshi; Inada, Akira; Tillekeratne, L.M.V; Wijesundara, D.S.A.; Gunatilaka, A.A. Leslie (2001). "Antimicrobial alkaloids from
2608:
Wang, Quan-Xin; Bao, Li; Yang, Xiao-Li; Guo, Hui; Yang, Rui-Nan; Ren, Biao; Zhang, Li-Xin; Dai, Huan-Qin; Guo, Liang-Dong; Liu, Hong-Wei (2012). "Polyketides with antimicrobial activity from the solid culture of an endolichenic fungus
2508:
Micheletti, Ana; Honda, Neli; Pavan, Fernando; Leite, Clarice; Matos, Maria; Perdomo, Renata; Bogo, Danielle; Alcantara, Glaucia; Beatriz, Adilson (2013). "Increment of antimycobacterial activity on lichexanthone derivatives".
3005:
Costa, Emmanoel V.; Marques, Francisco de Assis; Pinheiro, Maria Lúcia B.; Braga, Raquel M.; Delarmelina, Camila; Duarte, Marta
Cristina T.; Ruiz, Ana Lúcia T.G.; Carvalho, João Ernesto de; Maia, Beatriz H.L.N.S. (2011).
1555:
where cortical substances are rare. In some instances, similar or related species exist that lack cortical substances entirely, suggesting that the actual ecological function of lichexanthone is not fully understood.
2942:
Yamthe, Lauve; Fokou, Patrick; Mbouna, Cedric; Keumoe, Rodrigue; Ndjakou, Bruno; Djouonzo, Paul; Mfopa, Alvine; Legac, Jennifer; Tsabang, Nole; Gut, Jiri; Rosenthal, Philip; Boyom, Fabrice (2015).
1534:
were exposed to long-wavelength UV light (365 nm) for three to four hours every week over a time span of three to four months. In the natural lichen, the compound is present in both the outer
1265:; of about 70 species in the genus, 20 contain lichexanthone. This represents the largest group of foliose lichens with the compound, as it is generally restricted to some groups of tropical
2470:
Ingólfsdóttir, Kristı́n; Chung, Gavin A.C.; Skúlason, Vilhjálmur G.; Gissurarson, Stefán R.; Vilhelmsdóttir, Margrét (1998). "Antimycobacterial activity of lichen metabolites in vitro".
1104:
Part of a proposed biosynthetic pathway for lichexanthone-type lichen xanthones, depicting an aldol cyclization step followed by a cyclodehydration, which would lead to norlichexanthone.
2651:
Gachet, M. Salomé; Kunert, Olaf; Kaiser, Marcel; Brun, Reto; Zehl, Martin; Keller, Walter; Muñoz, Ricardo A.; Bauer, Rudolf; Schuehly, Wolfgang (2011). "Antiparasitic compounds from
2698:
Brandão, Luiz Fabrício
Gardini; Alcantara, Glaucia Braz; Matos, Maria de Fátima Cepa; Bogo, Danielle; Freitas, Deisy dos Santos; Oyama, Nathália Mitsuko; Honda, Neli Kika (2013).
506:
3402:
Lim, Pei Cee; Ramli, Hanizah; Kassim, Nur
Kartinee; Ali, Zulfiqar; Khan, Ikhlas A.; Shaari, Khozirah; Ismail, Amin (2019). "Chemical constituents from the stem bark of
754:(2-hydroxy-4-methoxy-6-methylbenzoic acid) and phloroglucinol, was proposed in 1956. These early syntheses also helped to confirm the structure of lichexanthone before
1206:. In this way, lichexanthone is detected by monitoring its retention time, and verifying the presence of three peaks representing wavelengths of maximum absorption (λ
2347:
Carvalho, Adriana E.; Alcantara, Glaucia B.; Oliveira, Sebastião M.; Micheletti, Ana C.; Honda, Neli K.; Maia, Gilberto (2009). "Electroreduction of lichexanthone".
3363:
Pettit, George R.; Meng, Yanhui; Herald, Delbert L.; Graham, Keith A.N.; Pettit, Robin K.; Doubek, Dennis L. (2003). "Isolation and structure of ruprechstyril from
3140:
Suárez, Alírica I.; Blanco, Zuleyma; Compagnone, Reinaldo S.; Salazar-Bookaman, María M.; Zapata, Varlin; Alvarado, Claudia (2006). "Anti-inflammatory activity of
2824:
Feige, G.B.; Lumbsch, H.T.; Huneck, S.; Elix, J.A. (1993). "Identification of lichen substances by a standardized high-performance liquid chromatographic method".
3253:
Tsamo, Armelle Tontsa; Melong, Raduis; Mkounga, Pierre; Nkengfack, Augustin Ephrem (2018). "Rubescins I and J, further limonoid derivatives from the stem bark of
1198:, this technique can be used to identify complex mixtures of structurally similar derivatives. The technique was later refined to couple the HPLC output with a
2429:
Honda, N.K.; Pavan, F.R.; Coelho, R.G.; de
Andrade Leite, S.R.; Micheletti, A.C.; Lopes, T.I.B.; Misutsu, M.Y.; Beatriz, A.; Brum, R.L.; Leite, C.Q.F. (2010).
263:
1028:
105:
3433:
Sriyatep, Teerayut; Chakthong, Suda; Leejae, Sukanlaya; Voravuthikunchai, Supayang P. (2014). "Two lignans, one alkaloid, and flavanone from the twigs of
1651:
and ω-aminoalkoxylxanthones; lichexanthone and several derivatives were found to have weak antimycobacterial activity. According to the authors, this
3790:
Mostafavi, Najmeh; Ebrahimi, Ali (2018). "The role of chlorine substituents in lichexanthones properties: the ionic and halogen bond interactions".
1190:(HPLC) assay has been described to identify many lichen-derived substances, including lichexanthone and many other xanthones; because many xanthone
1283:
Although normally considered a secondary metabolite of lichens, lichexanthone has also been isolated from several plants, listed here organized by
2404:
Ranković, Branislav; Kosanić, Marijana (2019). "Lichens as a potential source of bioactive secondary metabolites". In
Ranković, Branislav (ed.).
3886:
Menezes, Aline Anjos; Xavier-Leite, Amanda
Barreto; de Jesus, Katia Almeida; Aptroot, André; Cáceres, Marcela Eugenia da Silva (2013). "Two new
1505:, although in that case it was suspected to have originated from a lichen growing on the bark. Additionally, two non-lichenised fungus species,
1187:
3743:
2926:
2808:
2413:
2266:
2190:
1203:
3925:
Lima, Edvaneide
Leandro de; Mendonça, Cléverton de Oliveira; Aptroot, André; Cáceres, Marcela Eugenia da Silva (2013). "Two new species of
954:
758:
of analysis were widely available. In 1977, Harris and Hay proposed a biogenetically modelled synthesis of lichexanthone starting from the
1247:, it is found in about a dozen species; when present, it usually completely replaces other cortical substances common in that genus, like
2225:
Harris, Thomas M.; Hay, James V. (1977). "Biogenetically modeled syntheses of heptaacetate metabolites. Alternariol and lichexanthone".
1595:
eight additional lichexanthone derivatives were considered "putative"–thought to exist in nature, but not yet discovered in lichens.
1580:(1,3,6-trihydroxy-8-methylxanthone) differs from lichexanthone in having hydroxy rather than methoxy groups at positions 3 and 6. In
3464:
Jiménez, Carlos; Marcos, Manuel; Villaverde, Mary Carmen; Riguera, Ricardo; Castedo, Luis; Stermitz, Frank (1989). "A chromone from
2110:
Masters, Kye-Simeon; Bräse, Stefan (2012). "Xanthones from fungi, lichens, and bacteria: the natural products and their synthesis".
950:
238:
3989:
da Silva Cáceres, Marcela Eugenia; Aptroot, André (2017). "Lichens from the Brazilian Amazon, with special reference to the genus
1586:(1,6-dihydroxy-3-methoxy-8-methylxanthen-9-one), the methoxy at position 6 of lichexanthone is replaced with a hydroxy. Dozens of
576:; this feature is used to help identify some species. Lichexanthone is also found in several plants (many are from the families
1842:
1798:
1788:
2320:
Letcher, R.M. (1968). "Chemistry of lichen constituents—VI: Mass spectra of usnic acid, lichexanthone and their derivatives".
1768:
957:(C NMR) spectral assignments for lichexanthone were reported in 2010, as well as its crystal structure determined using
1222:
3332:
El-Seedi, Hesham R.; Hazell, Alan C.; Torssell, Kurt B.G. (1994). "Triterpenes, lichexanthone and an acetylenic acid from
2853:"Analysis of secondary metabolites from lichen by high-performance liquid chromatography with a photodiode array detector"
1778:
4057:
513:
1828:
1748:
1718:
934:
4029:
Aptroot, André; da Silva Cáceres, Marcela Eugenia (2018). "New lichen species from Chapada Diamantina, Bahia, Brazil".
2739:
Kathirgamanathar, Selvaluxmy; Ratnasooriya, W.D.; Baekstrom, Peter; Andersen, Raymond J.; Karunaratne, Veranja (2006).
1933:
Buitrago Díaz, Alexis; Rojas Vera, Janne; Cote, Valentina; Bruno-Colmenárez, Julia; Díaz de Delgado, Graciela (2010).
1862:
1808:
1738:
1688:
1546:. Lichexanthone may function as a light filter to protect the UV-sensitive algal layer in lichens from high-intensity
202:
1976:"Untersuchungen über Flechtenstoffe, XCVIII. Mitteil.: Über Lichexanthon, ein neues Stoffwechselprodukt der Flechte"
4252:
3734:
Galloway, D.J. (1993). "Global environmental change: lichens and chemistry". In Feige, G.B.; Lumbsch, H.T. (eds.).
2792:
1872:
1818:
1708:
1643:
995:
3081:
Wairata, Johanis; Sukandar, Edwin Risky; Fadlan, Arif; Purnomo, Adi Setyo; Taher, Muhammad; Ersam, Taslim (2021).
3007:
1998:"Da dasselbe ein in der Flechte zum ersten mal entdeckte Xanthone-Derivat ist, so nennen wir es 'Lichexanthone'."
868:
3861:
1852:
718:. This method, one of six standard ways of synthesising xanthone derivatives, enables the creation of partially
4257:
1758:
1680:
1378:
1341:
1441:
1302:
1641:. A series of lichexanthone derivatives were synthesized and assessed for antimycobacterial activity against
1423:
1728:
1241:
species, lichexanthone has since been found in a wide variety of lichens. For example, in the foliose genus
1010:
985:
930:
564:. More than a dozen lichen species have a variation of the word lichexanthone incorporated as part of their
1435:
2784:
1698:
1591:
1526:
1507:
1400:
1308:
946:
770:
691:
669:
640:
4151:
607:
into a double-ring structure. Although it has been suggested that lichexanthone functions in nature as a
145:
3495:
Walker, Tameka M.; Vogler, Bernhard; Moriarity, Debra M.; Haber, William A.; Setzer, William N. (2011).
1483:
1429:
1389:
1176:
1141:
1117:
1085:
1040:
735:
41:
4123:
species with strepsilin and other new lichens from the Serra de Maracaju, Mato Grosso do Sul, Brazil".
3736:
Phytochemistry and Chemotaxonomy of Lichenized Ascomycetes: A Festschrift in Honour of Siegfried Huneck
3694:: anthraquinone production in selected cultured mycobionts as a response to stress and nutrient supply"
3083:"Evaluation of the antioxidant, antidiabetic, and antiplasmodial activities of xanthones isolated from
2851:
Yoshimura, Isao; Kinoshita, Yasuhiro; Yamamoto, Yoshikazu; Huneck, Siegfried; Yamada, Yasuyuki (1994).
1671:
of their published lichen species, thereby acknowledging the presence of this compound as an important
3689:
1577:
781:
773:
between positions 1 and 6 leads to the formation of a group of compounds that includes lichexanthone.
4267:
4088:"Looking for the undiscovered asexual taxa: case studies from lesser studied life modes and habitats"
3619:, a new griseofulvin, chrysogine and roquefortine C producing species from Qinghai province, China".
2053:
1627:
1607:
1513:
1330:
1165:
989:
is largely attributed to the presence of lichexanthone. Chemically unmodified lichexanthone has weak
918:
876:
795:
2206:
Grover, P.K.; Shah, G.D.; Shah, R.C. (1956). "Xanthones: part V. A new synthesis of lichexanthone".
1501:
751:
219:
1367:
1125:
1001:
970:
695:
624:
71:
2882:
Aptroot, André; Jungbluth, Patrícia; Cáceres, Marcela E.S. (2014). "A world key to the species of
1520:
Xanthones are known to have strong UV-absorbing properties. In experiments using laboratory-grown
4247:
3865:
2035:
1672:
1227:
1046:
926:
891:
703:
679:
644:
596:
545:
3818:
2376:"Antimicrobial activity of extracts and various fractions of chloroform extract from the lichen
906:. The presence of the compound in lichens causes them to fluoresce yellow under long-wavelength
673:), a lichen that is widespread in Asia. Another early publication described its isolation from
247:
InChI=1S/C16H14O5/c1-8-4-9(19-2)6-12-14(8)16(18)15-11(17)5-10(20-3)7-13(15)21-12/h4-7,17H,1-3H3
3739:
3636:
3566:
3522:
3384:
3314:
3274:
3235:
3214:
Anyanwu, Gabriel O.; Onyeneke, Chukwu E.; Rauf, Khalid (2015). "Medicinal plants of the genus
3161:
3122:
2987:
2922:
2804:
2721:
2680:
2630:
2590:
2526:
2487:
2452:
2409:
2272:
2262:
2186:
2178:
2127:
1603:
1489:
1417:
1411:
1319:
1237:
1113:
1109:
1023:
990:
958:
938:
864:
832:
723:
687:
to be reported from lichens, and it was given its name by Asahina and Nogami for this reason.
371:
921:) of 286, and weaker-intensity rearrangement peaks at 257, 243, and 200. A 2009 study on the
165:
4262:
4222:
4186:
4132:
4099:
4038:
4002:
3966:
3938:
3907:
3857:
3799:
3776:
3772:
3716:
3708:
3628:
3597:
3558:
3512:
3477:
3446:
3415:
3376:
3345:
3306:
3266:
3227:
3196:
3153:
3112:
3102:
3061:
3023:
2977:
2967:
2895:
2864:
2833:
2796:
2760:
2711:
2672:
2622:
2580:
2570:
2518:
2479:
2442:
2356:
2329:
2302:
2234:
2159:
2119:
2027:
1987:
1623:
1582:
1352:
1284:
1256:
1006:
656:
529:
434:
405:
286:
4136:
4119:
Aptroot, André; Souza, Maria Fernanda; Spielmann, Adriano Afonso (2021). "Two new crustose
3584:
Ferrari, F.; Monache, G.Delle; de Lima, R.Alves (1985). "Two naphthopyran derivatives from
1464:
81:
3655:
1836:
1551:
1547:
1535:
1452:
1373:
1296:
1266:
1169:
1153:
1137:
922:
766:
746:, leads to a xanthone with three methoxy groups. Afterwards, one of the methoxy groups is
707:
620:
608:
604:
223:
3632:
536:. Lichexanthone was first isolated and identified by Japanese chemists from a species of
1563:
1100:
125:
3117:
3082:
2982:
2943:
2780:
2585:
2558:
2061:
1668:
1195:
1063:
852:
848:
763:
711:
660:
636:
537:
484:
3601:
3562:
3481:
3349:
3200:
2483:
1602:
on some structural and electronic properties of lichexanthones have been studied with
4241:
4104:
4087:
2852:
2837:
2375:
1935:"NMR elucidation and crystal structure analysis of 1-hydroxy-3,6-dimethoxy-8-methyl-9
1325:
1080:
1035:
1018:
980:
914:
903:
860:
820:
812:
628:
565:
355:
345:
3028:
2360:
190:
3894:
from Chapada do Araripe, Ceará, NE Brazil (Ascomycota: Arthoniales), with a key to
3844:
Guderley, Roland; Lumbsch, H. Thorsten; Elix, John A. (2000). "Four new species of
2082:
Aghoramurthy, K.; Seshadri, T.R. (1953). "An improved synthesis of lichexanthone".
1652:
1619:
1395:
1347:
1336:
1270:
1243:
1173:
1172:). The exact mechanism is not known, but this ring closure might proceed through a
1089:
1014:
899:
816:
759:
755:
747:
592:
451:
4152:"New lichen species and records from Santa Catarina and Rio Grande do Sul, Brazil"
3270:
1934:
1070:; there are only a few compounds known to have this effect. The chemical also has
4227:
4210:
3654:
Frisvad, Jens C.; Smedsgaard, Jørn; Larsen, Thomas O.; Samson, Robert A. (2004).
2800:
2626:
2447:
2430:
4190:
4042:
4006:
3970:
3107:
2783:; Stocker-Wörgötter, Elfie (2008). "Biochemistry and secondary metabolites". In
2700:"Cytotoxic evaluation of phenolic compounds from lichens against melanoma cells"
2522:
1611:
1599:
1473:
1447:
1133:
1067:
917:
of lichexanthone was reported in 1968. It features a strong parent peak at m/z (
895:
872:
727:
719:
472:
385:
3517:
3496:
3066:
3045:
2406:
Lichen Secondary Metabolites. Bioactive Properties and Pharmaceutical Potential
3942:
3911:
3803:
3738:. Bibliotheca Lichenologica. Vol. 53. Berlin: J. Cramer. pp. 87–95.
3712:
3450:
3419:
3231:
3157:
2899:
2765:
2740:
2575:
1890:
1884:
1615:
1314:
1291:
1275:
1252:
1199:
1121:
1108:
In lichens, biosynthesis of lichexanthone occurs through the acetate-malonate
1071:
1009:
derivative of lichexanthone had antimycobacterial activity similar to that of
824:
722:
polyhydroxyxanthones. In the reaction, the two substrates, in the presence of
647:; like lichexanthone, some of these derivatives are also biologically active.
632:
616:
600:
577:
554:
377:
314:
156:
2972:
2276:
1902:
1631:
1521:
1384:
1362:
1248:
1211:
1056:
1052:
790:
743:
569:
3640:
3570:
3526:
3388:
3318:
3278:
3239:
3165:
3126:
2991:
2868:
2725:
2684:
2634:
2594:
2530:
2456:
2374:
Manojlovic, Nedeljko T.; Vasiljevic, Perica J.; Marković, Zoran S. (2010).
2333:
2306:
2131:
28:
2716:
2699:
2491:
1896:
1587:
1543:
1406:
1149:
975:
907:
804:
739:
731:
715:
684:
581:
573:
533:
416:
3848:
sensu stricto (Lecanorales, Ascomycotina) from tropical South America".
3615:
Wang, Long; Zhou, Han-Bai; C. Frisvad, Jens; A. Samson, Robert (2004). "
2921:. Bibliotheca Lichenologica. Vol. 69. Berlin/Stuttgart: J. Cramer.
2238:
2163:
1992:
1975:
1950:
Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas
762:
compound 3,5,7,9,11,13-hexaoxotetradecanoic acid. In this synthesis, an
3869:
2039:
1659:
antimycobacterial activity among the group of ω-aminoalkoxylxanthones.
1655:
approach was useful to correlate structural and chemical features with
1648:
1635:
1539:
1531:
1145:
945:
mechanism of lichexanthone, and to better understand the nature of its
887:
856:
786:
699:
627:
of lichexanthone that have been demonstrated in the laboratory include
335:
177:
4024:
4022:
4020:
4018:
4016:
3761:"New crustose lichens from a tropical coastal area in Paraná (Brazil)"
3720:
3380:
3310:
3012:(Annonaceae) and their antiproliferative and antimicrobial activities"
2676:
2123:
910:, a property that is used as a tool in lichen species identification.
799:; the yellowish colour results from the fluorescence of lichexanthone.
603:
and sequentially add successive units, forming a longer chain that is
1907:
1676:
1261:
1191:
1180:
1157:
1129:
1075:
560:
549:
541:
136:
3760:
2261:. Berlin, Heidelberg: Springer Berlin Heidelberg. pp. 209–212.
2031:
1132:
control a number of enzymatic reactions through several coordinated
803:
Lichexanthone is a member of the class of chemical compounds called
483:
Except where otherwise noted, data are given for materials in their
3690:"Intraspecific chemical variation within the crustose lichen genus
2177:
Diderot, Noungoue Tchamo; Silvere, Ngouela; Etienne, Tsamo (2006).
3218:—A review of their ethnobotany, phytochemistry and pharmacology".
1562:
1231:
is one of the first lichens from which lichexanthone was isolated.
1221:
1161:
1099:
979:
experiments, have been recorded in the scientific literature. The
942:
780:
643:
are known, some produced naturally in lichens, and others created
585:
116:
104:
94:
2185:. Advances in Phytomedicine. Elsevier Science. pp. 284–285.
1259:
used in classifying species of the predominantly tropical genus
1062:
In laboratory tests, the presence of lichexanthone enhances the
690:
Asahina and Nogami used a chemical method called potash fusion (
623:—its complete ecological function is not fully understood. Some
612:
4211:"Lichens from the Roosevelt River Area in the Brazilian Amazon"
3656:"Mycotoxins, drugs and other extrolites produced by species in
3179:
Okorie, Dominic A. (1976). "A new phthalide and xanthones from
1683:
and year of publication. All of these species occur in Brazil:
3688:
Stocker-Wörgötter, Elfie; Hager, Armin; Elix, John A. (2009).
2552:
2550:
2548:
2546:
2544:
2542:
2540:
2183:
Lead Molecules from Natural Products: Discovery and New Trends
2179:"Xanthones as therapeutic agents: chemistry and pharmacology"
1499:
Lichexanthone has also been reported to occur in the bark of
4080:
4078:
207:
3862:
10.1639/0007-2745(2000)103[0139:FNSOLS]2.0.CO;2
1969:
1967:
1965:
1963:
1928:
1926:
1924:
1922:
750:
to yield lichexanthone. A simpler synthesis, starting from
2408:(2 ed.). Springer Nature Switzerland AG. p. 13.
2150:
Roberts, John C. (1961). "Naturally occurring xanthones".
2105:
2103:
2101:
2099:
2097:
568:. The presence of lichexanthone in lichens causes them to
2503:
2501:
1140:. The structure of lichen xanthones is derived by linear
1667:
Some authors have explicitly named lichexanthone in the
1530:, the synthesis of lichexanthone was induced when young
1124:
are created by the sequential reactions of a variety of
3984:
3982:
3980:
2293:
Hale, Mason E. (1975). "A Revision of the Lichen Genus
898:
solution containing lichexanthone will emit a greenish
501:
4086:
L.; Tang, L.Z.; Jin, X.C.; Karunarathna, S.C. (2021).
3881:
3879:
655:
Lichexanthone was first reported by Japanese chemists
4150:
Aptroot, A.; Spielmann, A.A.; Gumboski, E.L. (2021).
3819:"Two new species and new reports in the Parmeliaceae
2886:
with lichexanthone, with a new species from Brazil".
2646:
2644:
2301:(25). Washington: Smithsonian Institution Press: 10.
2208:
Journal of Scientific and Industrial Research (India)
2084:
Journal of Scientific and Industrial Research (India)
540:
in the 1940s. The compound is known to occur in many
3046:"Chemical constituents and insecticidal activity of
742:
derivative, which, after subsequent methylation and
1906:, the listed species are the only members of those
1493:
are two tropical plants that contain lichexanthone.
2741:"Chemistry and bioactivity of Physciaceae lichens
925:reduction of the compound used techniques such as
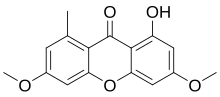
350:189–190 °C (372–374 °F; 462–463 K)
3008:"Chemical constituents isolated from the bark of
2952:L. (Annonaceae) potently and selectively inhibit
2431:"Antimycobacterial activity of lichen substances"
1156:-type cyclisation. The two rings are joined by a
4058:"New Graphidaceae from South and Central Brazil"
2399:
2397:
1255:. The presence or absence of lichexanthone is a
1202:to screen for xanthones based on their specific
189:
4204:
4202:
4200:
1164:-oxygen arising from cyclodehydration (i.e., a
572:a greenish-yellow colour under long-wavelength
80:
3759:Aptroot, André; Souza, Maria Fernanda (2021).
2252:
2250:
2248:
2145:
2143:
2141:
532:in the structural class of chemicals known as
1939:-xanthen-9-one (lichexanthone) isolated from
1235:Although first isolated from foliose (leafy)
859:form, it exists as long yellow prisms with a
659:and Hisasi Nogami in 1942. They isolated the
59:1-hydroxy-3,6-dimethoxy-8-methylxanthen-9-one
8:
4056:Aptroot, André; Feuerstein, Shirley (2020).
3817:Eliasaro, Sionara; Adler, Monica T. (1997).
2288:
2286:
2559:"Xanthones of lichen source: a 2016 update"
2472:European Journal of Pharmaceutical Sciences
2384:Journal of Biological Research-Thessaloniki
1713:A.A.Menezes, M.Cáceres & Aptroot (2013)
2058:(Willd.) Vain., Term. Füz. 22: 279 (1899)"
2018:(Parmeliaceae: lichenized Ascomycotina)".
1974:Asahina, Yasuhiko; Nogami, Hisasi (1942).
1179:that could dehydrate to yield the central
863:of 189–190 °C (372–374 °F). Its
639:-enhancing activities. Many lichexanthone
271:CC1=CC(=CC2=C1C(=O)C3=C(C=C(C=C3O2)OC)O)OC
222:
164:
20:
4226:
4103:
3516:
3116:
3106:
3065:
3027:
3016:Journal of the Brazilian Chemical Society
2981:
2971:
2764:
2715:
2584:
2574:
2446:
1991:
1980:Bulletin of the Chemical Society of Japan
1614:of the three rings, interactions between
769:between positions 8 and 13 followed by a
595:of lichexanthone occurs through a set of
2557:Le Pogam, Pierre; Boustie, Joël (2016).
2227:Journal of the American Chemical Society
1723:E.L.Lima, Aptroot & M.Cáceres (2013)
3777:10.5252/cryptogamie-mycologie2021v42a12
1918:
694:with a hot solution of the strong base
268:
243:
218:
18:Chemical compound found in some lichens
4137:10.5252/cryptogamie-mycologie2021v42a8
3823:(lichenized Ascomycotina) from Brazil"
1606:, to better understand things such as
1188:high-performance liquid chromatography
890:solution of lichexanthone reacts with
2297:(Parmeliaceae) in Tropical America".
2181:. In Khan, M.T.H.; Ather, A. (eds.).
1055:activity against a variety of cancer
1038:activity was detected against either
983:activity of the bark-dwelling lichen
827:is 1-hydroxy-3,6-dimethoxy-8-methyl-9
250:Key: QDLAGTHXVHQKRE-UHFFFAOYSA-N
144:
124:
7:
2704:Chemical and Pharmaceutical Bulletin
955:carbon-13 nuclear magnetic resonance
902:after adding a drop of concentrated
3408:Biochemical Systematics and Ecology
2299:Smithsonian Contributions to Botany
2259:Identification of Lichen Substances
1835:– named for both lichexanthone and
1679:are listed here, followed by their
1647:. These derivatives consisted of ω-
1567:Numbering scheme for lichexanthone;
180:
3633:10.1023/b:anto.0000036140.86059.51
3497:"A phytochemical investigation of
1027:, and also inhibits the growth of
45:1-Hydroxy-3,6-dimethoxy-8-methyl-9
14:
951:proton nuclear magnetic resonance
939:controlled-potential electrolysis
619:) in lichens from high-intensity
2010:Park, Y.S.; Hale, M.E. (1989). "
1863:Ocellularia fuscolichexanthonica
1542:and in the exciple (rim) of the
1517:, can synthesize lichexanthone.
1472:
1463:
973:of lichexanthone, studied using
831:-xanthen-9-one. Lichexanthone's
811:-xanthen-9-one substituted by a
734:derivative that is subsequently
491:
298:
27:
3029:10.1590/s0103-50532011000600016
2361:10.1016/j.electacta.2008.10.035
1843:Aggregatorygma lichexanthonicum
1799:Diorygma isidiolichexanthonicum
1789:Pertusaria lichexanthoverrucosa
1074:activity against second-instar
894:to produce a purple colour; an
683:). Lichexanthone was the first
487:(at 25 °C , 100 kPa).
330:long yellow prismatic crystals
3792:Theoretical Chemistry Accounts
3505:Natural Product Communications
3054:Natural Product Communications
1823:Aptroot & M.F.Souza (2021)
1813:Aptroot & M.F.Souza (2021)
1769:Pertusaria lichexanthofarinosa
1753:M.Cáceres & Aptroot (2017)
1743:M.Cáceres & Aptroot (2017)
1733:Aptroot & M.Cáceres (2017)
1630:formed between lichexanthone,
1168:leading to the formation of a
698:) on lichexanthone to produce
477:186.9 °C (368.4 °F)
304:
292:
1:
3602:10.1016/s0031-9422(00)80719-0
3563:10.1016/s0031-9422(00)00402-7
3482:10.1016/s0031-9422(00)97907-x
3350:10.1016/s0031-9422(00)94841-6
3271:10.1080/14786419.2018.1443087
3201:10.1016/s0031-9422(00)97499-5
2661:Trypanosoma bruceirhodesiense
2484:10.1016/s0928-0987(97)00078-x
1873:Ocellularia lichexanthocavata
1779:Pertusaria lichexanthoimmersa
1017:. Lichexanthone has a strong
714:as starting reactants in the
599:that start with the molecule
544:, and it is important in the
4228:10.3390/microbiolres14020054
3220:Journal of Ethnopharmacology
3146:Journal of Ethnopharmacology
2838:10.1016/0021-9673(93)83356-w
2801:10.1017/CBO9780511790478.008
2627:10.1016/j.fitote.2011.10.013
2448:10.1016/j.phymed.2009.07.018
1910:that contain lichexanthone.
1829:Lepra lichexanthonorstictica
1793:Aptroot & Cáceres (2018)
1783:Aptroot & Cáceres (2018)
1773:Aptroot & Cáceres (2018)
1763:Aptroot & Cáceres (2018)
1749:Enterographa lichexanthonica
1719:Cryptothecia lichexanthonica
1210:) at 208, 242, and 310
423: = 7.5444 Å,
4191:10.1639/0007-2745-125.3.433
4043:10.1639/0007-2745-121.1.067
4007:10.1639/0007-2745-120.2.166
3971:10.1639/0007-2745-120.3.320
3369:Journal of Natural Products
3299:Journal of Natural Products
3108:10.3390/biomedicines9101380
2826:Journal of Chromatography A
2665:Journal of Natural Products
2523:10.2174/1573406411309070003
1809:Caprettia lichexanthotricha
1739:Chiodecton lichexanthonicum
1693:Eliasaro & Adler (1997)
1689:Parmotrema lichexanthonicum
1204:ultraviolet–visible spectra
427: = 15.2341 Å
4284:
4105:10.5943/mycosphere/12/1/17
3518:10.1177/1934578x1100601204
3067:10.1177/1934578x0800301021
2793:Cambridge University Press
2791:(2nd ed.). New York:
2257:Huneck, Siegfried (1996).
1853:Allographa lichexanthonica
1819:Lecanora lichexanthoxylina
1709:Crypthonia lichexanthonica
1644:Mycobacterium tuberculosis
1269:, chiefly pyrenocarps and
996:Mycobacterium tuberculosis
823:at positions 3 and 6. Its
588:that do not form lichens.
415: = 11.6405
360:494 °C (921 °F)
3943:10.1017/s0024282912000862
3912:10.1017/s0024282913000406
3804:10.1007/s00214-018-2294-0
3713:10.1007/s11101-009-9149-1
3451:10.1016/j.tet.2014.01.023
3420:10.1016/j.bse.2018.12.010
3295:Henriettella fascicularis
3232:10.1016/j.jep.2015.09.032
3158:10.1016/j.jep.2005.10.006
2900:10.1017/s0024282914000231
2766:10.1080/13880200600686624
2576:10.3390/molecules21030294
2322:Organic Mass Spectrometry
1604:quantum mechanical theory
1379:Henriettella fascicularis
1200:photodiode array detector
869:monoclinic crystal system
807:. Specifically, it is a 9
481:
466:
364:
279:
259:
234:
64:
54:
40:
35:
26:
3543:Zanthoxylum tetraspermum
3259:Natural Product Research
3181:Anthocleista djalonensis
3010:Guatteria blepharophylla
2973:10.3390/medicines2020055
2655:with activities against
1759:Cladonia lichexanthonica
1598:The effects of chlorine
1342:Anthocleista djalonensis
1303:Guatteria blepharophylla
935:rotating ring electrodes
4209:Aptroot, André (2023).
4159:Archive for Lichenology
4065:Archive for Lichenology
3621:Antonie van Leeuwenhoek
2747:Heterodermia leucomelos
2014:, the correct name for
1729:Buellia lichexanthonica
1424:Zanthoxylum microcarpum
1183:core of lichexanthone.
1136:on a large multienzyme
1013:commonly used to treat
986:Marcelaria benguelensis
793:of the crustose lichen
584:), and some species of
4125:Cryptogamie, Mycologie
3765:Cryptogamie, Mycologie
3701:Phytochemistry Reviews
3617:Penicillium persicinum
2869:10.1002/PCA.2800050405
2857:Phytochemical Analysis
2753:Pharmaceutical Biology
2334:10.1002/oms.1210010409
2307:10.5479/si.0081024X.25
2016:Hypotrachyna formosana
2012:Hypotrachyna osseoalba
1699:Lecanora lichexanthona
1675:characteristic. These
1574:
1550:. The presence of the
1527:Haematomma fluorescens
1508:Penicillium persicinum
1232:
1105:
1029:methicillin-resistant
800:
706:of lichexanthone used
670:Hypotrachyna osseoalba
548:of species in several
4215:Microbiology Research
3499:Zanthoxylum setulosum
3334:Minquartia guianensis
2954:Plasmodium falciparum
2913:Archer, Alan (1997).
2717:10.1248/cpb.c12-00739
2657:Plasmodium falciparum
1566:
1484:Minquartia guianensis
1390:Minquartia guianensis
1225:
1103:
1041:Plasmodium falciparum
1031:Staphylococcus aureus
971:biological activities
965:Biological activities
784:
625:biological activities
611:—protecting resident
3365:Ruprechtia tangarana
3185:Anthocleista vogelli
3048:Rollinia leptopetala
2795:. pp. 118–119.
2378:Laurera benguelensis
1514:Penicillium vulpinum
1442:Z. tetraspermum
1401:Ruprechtia tangarana
1309:Rollinia leptopetala
1166:dehydration reaction
1126:polyketide synthases
1051:nor did it have any
941:to characterise the
919:mass-to-charge ratio
796:Ochrolechia africana
3671:Studies in Mycology
3255:Trichilia rubescens
2785:Nash III, Thomas H.
2511:Medicinal Chemistry
2349:Electrochimica Acta
2239:10.1021/ja00447a058
2164:10.1021/cr60214a003
1993:10.1246/bcsj.17.202
1368:Trichilia rubescens
1120:. In this pathway,
947:chemical reactivity
771:Claisen cyclization
696:potassium hydroxide
597:enzymatic reactions
322: g·mol
23:
3890:species and a new
3144:aqueous extract".
1575:
1273:. The large genus
1233:
1228:Parmelina quercina
1106:
1047:Trypanosoma brucei
1034:. In contrast, no
927:cyclic voltammetry
892:iron(III) chloride
855:. In its purified
819:at position 8 and
801:
680:Parmelina quercina
665:Parmelia formosana
514:Infobox references
21:
4253:Methoxy compounds
3931:The Lichenologist
3929:from NE Brazil".
3900:The Lichenologist
3745:978-3-443-58032-2
3596:(11): 2753–2755.
3511:(12): 1807–1808.
3435:Feroniella lucida
3404:Clausena excavata
3381:10.1021/np0300986
3311:10.1021/np0201164
3305:(12): 1749–1753.
3195:(11): 1799–1800.
3085:Garcinia forbesii
3060:(10): 1687–1688.
2950:Annona reticulata
2928:978-3-443-58048-3
2915:The Lichen Genus
2888:The Lichenologist
2810:978-0-521-69216-8
2743:Pyxine consocians
2677:10.1021/np100415m
2415:978-3-030-16813-1
2268:978-3-642-85245-9
2192:978-0-08-045933-2
2124:10.1021/cr100446h
2056:Parmelia quercina
2054:"Record Details:
1669:specific epithets
1559:Related compounds
1490:Feroniella lucida
1436:Z. setulosum
1418:Feroniella lucida
1412:Clausena excavata
1320:Garcinia forbesii
1160:carbon and by an
1114:acetyl coenzyme A
1110:metabolic pathway
1024:Bacillus subtilis
993:activity against
991:antimycobacterial
959:X-ray diffraction
953:(H NMR) and
865:crystal structure
833:molecular formula
815:at position 1, a
724:hydrochloric acid
675:Parmelia quercina
522:Chemical compound
520:
519:
372:Crystal structure
203:CompTox Dashboard
106:Interactive image
4275:
4233:
4232:
4230:
4206:
4195:
4194:
4173:
4167:
4166:
4156:
4147:
4141:
4140:
4116:
4110:
4109:
4107:
4098:(1): 1290–1333.
4082:
4073:
4072:
4062:
4053:
4047:
4046:
4026:
4011:
4010:
3986:
3975:
3974:
3953:
3947:
3946:
3922:
3916:
3915:
3883:
3874:
3873:
3841:
3835:
3834:
3814:
3808:
3807:
3787:
3781:
3780:
3756:
3750:
3749:
3731:
3725:
3724:
3698:
3685:
3679:
3678:
3668:
3651:
3645:
3644:
3612:
3606:
3605:
3581:
3575:
3574:
3537:
3531:
3530:
3520:
3492:
3486:
3485:
3476:(7): 1992–1993.
3461:
3455:
3454:
3445:(9): 1773–1779.
3430:
3424:
3423:
3399:
3393:
3392:
3375:(8): 1065–1069.
3360:
3354:
3353:
3344:(5): 1297–1299.
3329:
3323:
3322:
3289:
3283:
3282:
3250:
3244:
3243:
3211:
3205:
3204:
3176:
3170:
3169:
3137:
3131:
3130:
3120:
3110:
3078:
3072:
3071:
3069:
3040:
3034:
3033:
3031:
3022:(6): 1111–1117.
3002:
2996:
2995:
2985:
2975:
2939:
2933:
2932:
2910:
2904:
2903:
2879:
2873:
2872:
2848:
2842:
2841:
2821:
2815:
2814:
2777:
2771:
2770:
2768:
2736:
2730:
2729:
2719:
2695:
2689:
2688:
2648:
2639:
2638:
2605:
2599:
2598:
2588:
2578:
2554:
2535:
2534:
2505:
2496:
2495:
2467:
2461:
2460:
2450:
2426:
2420:
2419:
2401:
2392:
2391:
2371:
2365:
2364:
2355:(8): 2290–2297.
2344:
2338:
2337:
2317:
2311:
2310:
2290:
2281:
2280:
2254:
2243:
2242:
2233:(5): 1631–1637.
2222:
2216:
2215:
2203:
2197:
2196:
2174:
2168:
2167:
2152:Chemical Reviews
2147:
2136:
2135:
2118:(7): 3717–3776.
2112:Chemical Reviews
2107:
2092:
2091:
2079:
2073:
2072:
2070:
2068:
2050:
2044:
2043:
2007:
2001:
2000:
1995:
1971:
1958:
1957:
1947:
1941:Vismia baccifera
1930:
1878:
1868:
1858:
1848:
1834:
1824:
1814:
1804:
1794:
1784:
1774:
1764:
1754:
1744:
1734:
1724:
1714:
1704:
1694:
1624:binding energies
1585:
1583:griseoxanthone C
1578:Norlichexanthone
1569:Me = methyl (–CH
1524:from the lichen
1476:
1467:
1353:Vismia baccifera
1267:crustose lichens
1078:of the mosquito
756:spectral methods
667:(known today as
657:Yasuhiko Asahina
591:In lichens, the
530:organic compound
504:
498:
495:
494:
406:Lattice constant
321:
306:
300:
294:
287:Chemical formula
227:
226:
211:
209:
193:
182:
168:
148:
128:
108:
84:
31:
24:
4283:
4282:
4278:
4277:
4276:
4274:
4273:
4272:
4258:Lichen products
4238:
4237:
4236:
4208:
4207:
4198:
4175:
4174:
4170:
4154:
4149:
4148:
4144:
4118:
4117:
4113:
4084:
4083:
4076:
4060:
4055:
4054:
4050:
4028:
4027:
4014:
3988:
3987:
3978:
3955:
3954:
3950:
3924:
3923:
3919:
3885:
3884:
3877:
3843:
3842:
3838:
3816:
3815:
3811:
3789:
3788:
3784:
3771:(12): 191–197.
3758:
3757:
3753:
3746:
3733:
3732:
3728:
3696:
3687:
3686:
3682:
3666:
3653:
3652:
3648:
3614:
3613:
3609:
3583:
3582:
3578:
3539:
3538:
3534:
3494:
3493:
3489:
3463:
3462:
3458:
3432:
3431:
3427:
3401:
3400:
3396:
3362:
3361:
3357:
3331:
3330:
3326:
3291:
3290:
3286:
3252:
3251:
3247:
3213:
3212:
3208:
3178:
3177:
3173:
3152:(1–2): 99–101.
3142:Croton cuneatus
3139:
3138:
3134:
3080:
3079:
3075:
3042:
3041:
3037:
3004:
3003:
2999:
2946:Annona muricata
2944:"Extracts from
2941:
2940:
2936:
2929:
2912:
2911:
2907:
2881:
2880:
2876:
2850:
2849:
2845:
2823:
2822:
2818:
2811:
2779:
2778:
2774:
2738:
2737:
2733:
2697:
2696:
2692:
2653:Cupania cinerea
2650:
2649:
2642:
2607:
2606:
2602:
2556:
2555:
2538:
2507:
2506:
2499:
2469:
2468:
2464:
2428:
2427:
2423:
2416:
2403:
2402:
2395:
2373:
2372:
2368:
2346:
2345:
2341:
2319:
2318:
2314:
2292:
2291:
2284:
2269:
2256:
2255:
2246:
2224:
2223:
2219:
2205:
2204:
2200:
2193:
2176:
2175:
2171:
2149:
2148:
2139:
2109:
2108:
2095:
2081:
2080:
2076:
2066:
2064:
2052:
2051:
2047:
2032:10.2307/1220900
2009:
2008:
2004:
1973:
1972:
1961:
1945:
1932:
1931:
1920:
1916:
1882:In the case of
1876:
1866:
1856:
1846:
1837:norstictic acid
1832:
1822:
1812:
1802:
1792:
1782:
1772:
1762:
1752:
1742:
1732:
1722:
1712:
1703:Guderley (2000)
1702:
1692:
1681:author citation
1665:
1639:
1581:
1572:
1568:
1561:
1552:photoprotective
1548:solar radiation
1497:
1496:
1495:
1494:
1479:
1478:
1477:
1469:
1468:
1453:Cupania cinerea
1374:Melastomataceae
1331:Croton cuneatus
1297:Annona muricata
1220:
1209:
1196:retention times
1194:have different
1186:A standardized
1170:cyclic compound
1154:orsellinic acid
1152:units with one
1138:protein complex
1098:
1021:effect towards
967:
949:. The complete
923:electrochemical
880:
867:is part of the
851:of 286.27
846:
842:
838:
785:UV-illuminated
779:
702:. The earliest
653:
621:solar radiation
609:photoprotectant
523:
516:
511:
510:
509: ?)
500:
496:
492:
488:
459:
445:1307.26 Å
442:
428:
408:
394:
388:
374:
319:
309:
303:
297:
289:
275:
272:
267:
266:
255:
252:
251:
248:
242:
241:
230:
212:
205:
196:
183:
171:
151:
131:
111:
98:
87:
74:
60:
58:
57:Lichenxanthone,
50:
19:
12:
11:
5:
4281:
4279:
4271:
4270:
4265:
4260:
4255:
4250:
4240:
4239:
4235:
4234:
4221:(2): 755–786.
4196:
4185:(3): 435–467.
4179:The Bryologist
4168:
4142:
4131:(8): 137–148.
4111:
4074:
4048:
4031:The Bryologist
4012:
4001:(2): 166–182.
3995:The Bryologist
3976:
3965:(3): 320–328.
3959:The Bryologist
3948:
3937:(3): 361–365.
3917:
3906:(5): 657–664.
3875:
3856:(1): 139–144.
3850:The Bryologist
3836:
3809:
3782:
3751:
3744:
3726:
3707:(3): 561–569.
3680:
3646:
3627:(2): 173–179.
3607:
3590:Phytochemistry
3586:Faramea cyanea
3576:
3557:(8): 857–861.
3551:Phytochemistry
3532:
3487:
3470:Phytochemistry
3456:
3425:
3394:
3355:
3338:Phytochemistry
3324:
3284:
3265:(2): 196–203.
3257:(Meliaceae)".
3245:
3206:
3189:Phytochemistry
3171:
3132:
3073:
3035:
2997:
2934:
2927:
2905:
2894:(5): 669–672.
2874:
2863:(4): 197–205.
2843:
2832:(2): 417–427.
2816:
2809:
2789:Lichen Biology
2772:
2759:(3): 217–220.
2731:
2710:(2): 176–183.
2690:
2671:(4): 559–566.
2640:
2621:(1): 209–214.
2600:
2536:
2517:(7): 904–910.
2497:
2478:(2): 141–144.
2462:
2441:(5): 328–332.
2421:
2414:
2393:
2366:
2339:
2328:(4): 551–561.
2312:
2282:
2267:
2244:
2217:
2198:
2191:
2169:
2158:(6): 591–605.
2137:
2093:
2074:
2062:Index Fungorum
2045:
2002:
1986:(4): 202–207.
1959:
1917:
1915:
1912:
1880:
1879:
1877:Aptroot (2023)
1869:
1867:Aptroot (2023)
1859:
1857:Aptroot (2023)
1849:
1847:Aptroot (2022)
1839:
1833:Aptroot (2021)
1825:
1815:
1805:
1803:Aptroot (2020)
1795:
1785:
1775:
1765:
1755:
1745:
1735:
1725:
1715:
1705:
1695:
1664:
1661:
1637:
1610:interactions,
1608:intramolecular
1590:lichexanthone
1570:
1560:
1557:
1502:Faramea cyanea
1481:
1480:
1471:
1470:
1462:
1461:
1460:
1459:
1458:
1457:
1456:
1445:
1430:Z. valens
1404:
1393:
1382:
1371:
1360:
1345:
1334:
1323:
1312:
1219:
1216:
1207:
1097:
1094:
966:
963:
878:
853:grams per mole
849:molecular mass
844:
840:
836:
821:methoxy groups
778:
775:
752:everninic acid
712:phloroglucinol
661:lichen product
652:
649:
637:sperm motility
521:
518:
517:
512:
490:
489:
485:standard state
482:
479:
478:
475:
469:
468:
464:
463:
460:
450:
447:
446:
443:
435:Lattice volume
433:
430:
429:
411:
409:
404:
401:
400:
392:
389:
384:
381:
380:
375:
370:
367:
366:
362:
361:
358:
352:
351:
348:
342:
341:
338:
332:
331:
328:
324:
323:
317:
311:
310:
307:
301:
295:
290:
285:
282:
281:
277:
276:
274:
273:
270:
262:
261:
260:
257:
256:
254:
253:
249:
246:
245:
237:
236:
235:
232:
231:
229:
228:
220:DTXSID80164977
215:
213:
201:
198:
197:
195:
194:
186:
184:
176:
173:
172:
170:
169:
161:
159:
153:
152:
150:
149:
141:
139:
133:
132:
130:
129:
121:
119:
113:
112:
110:
109:
101:
99:
92:
89:
88:
86:
85:
77:
75:
70:
67:
66:
62:
61:
56:
52:
51:
49:-xanthen-9-one
44:
38:
37:
33:
32:
22:Lichexanthone
17:
13:
10:
9:
6:
4:
3:
2:
4280:
4269:
4266:
4264:
4261:
4259:
4256:
4254:
4251:
4249:
4246:
4245:
4243:
4229:
4224:
4220:
4216:
4212:
4205:
4203:
4201:
4197:
4192:
4188:
4184:
4180:
4172:
4169:
4164:
4160:
4153:
4146:
4143:
4138:
4134:
4130:
4126:
4122:
4115:
4112:
4106:
4101:
4097:
4093:
4089:
4081:
4079:
4075:
4070:
4066:
4059:
4052:
4049:
4044:
4040:
4036:
4032:
4025:
4023:
4021:
4019:
4017:
4013:
4008:
4004:
4000:
3996:
3992:
3985:
3983:
3981:
3977:
3972:
3968:
3964:
3960:
3952:
3949:
3944:
3940:
3936:
3932:
3928:
3921:
3918:
3913:
3909:
3905:
3901:
3897:
3893:
3889:
3882:
3880:
3876:
3871:
3867:
3863:
3859:
3855:
3851:
3847:
3840:
3837:
3832:
3828:
3824:
3822:
3821:sensu stricto
3813:
3810:
3805:
3801:
3797:
3793:
3786:
3783:
3778:
3774:
3770:
3766:
3762:
3755:
3752:
3747:
3741:
3737:
3730:
3727:
3722:
3718:
3714:
3710:
3706:
3702:
3695:
3693:
3684:
3681:
3676:
3672:
3665:
3663:
3659:
3650:
3647:
3642:
3638:
3634:
3630:
3626:
3622:
3618:
3611:
3608:
3603:
3599:
3595:
3591:
3587:
3580:
3577:
3572:
3568:
3564:
3560:
3556:
3552:
3548:
3544:
3536:
3533:
3528:
3524:
3519:
3514:
3510:
3506:
3502:
3500:
3491:
3488:
3483:
3479:
3475:
3471:
3467:
3460:
3457:
3452:
3448:
3444:
3440:
3436:
3429:
3426:
3421:
3417:
3413:
3409:
3405:
3398:
3395:
3390:
3386:
3382:
3378:
3374:
3370:
3366:
3359:
3356:
3351:
3347:
3343:
3339:
3335:
3328:
3325:
3320:
3316:
3312:
3308:
3304:
3300:
3296:
3288:
3285:
3280:
3276:
3272:
3268:
3264:
3260:
3256:
3249:
3246:
3241:
3237:
3233:
3229:
3225:
3221:
3217:
3210:
3207:
3202:
3198:
3194:
3190:
3186:
3182:
3175:
3172:
3167:
3163:
3159:
3155:
3151:
3147:
3143:
3136:
3133:
3128:
3124:
3119:
3114:
3109:
3104:
3100:
3096:
3092:
3090:
3086:
3077:
3074:
3068:
3063:
3059:
3055:
3051:
3050:(Annonaceae)"
3049:
3039:
3036:
3030:
3025:
3021:
3017:
3013:
3011:
3001:
2998:
2993:
2989:
2984:
2979:
2974:
2969:
2965:
2961:
2957:
2955:
2951:
2947:
2938:
2935:
2930:
2924:
2920:
2916:
2909:
2906:
2901:
2897:
2893:
2889:
2885:
2878:
2875:
2870:
2866:
2862:
2858:
2854:
2847:
2844:
2839:
2835:
2831:
2827:
2820:
2817:
2812:
2806:
2802:
2798:
2794:
2790:
2786:
2782:
2781:Elix, John A.
2776:
2773:
2767:
2762:
2758:
2754:
2750:
2748:
2744:
2735:
2732:
2727:
2723:
2718:
2713:
2709:
2705:
2701:
2694:
2691:
2686:
2682:
2678:
2674:
2670:
2666:
2662:
2658:
2654:
2647:
2645:
2641:
2636:
2632:
2628:
2624:
2620:
2616:
2612:
2604:
2601:
2596:
2592:
2587:
2582:
2577:
2572:
2568:
2564:
2560:
2553:
2551:
2549:
2547:
2545:
2543:
2541:
2537:
2532:
2528:
2524:
2520:
2516:
2512:
2504:
2502:
2498:
2493:
2489:
2485:
2481:
2477:
2473:
2466:
2463:
2458:
2454:
2449:
2444:
2440:
2436:
2435:Phytomedicine
2432:
2425:
2422:
2417:
2411:
2407:
2400:
2398:
2394:
2389:
2385:
2381:
2379:
2370:
2367:
2362:
2358:
2354:
2350:
2343:
2340:
2335:
2331:
2327:
2323:
2316:
2313:
2308:
2304:
2300:
2296:
2289:
2287:
2283:
2278:
2274:
2270:
2264:
2260:
2253:
2251:
2249:
2245:
2240:
2236:
2232:
2228:
2221:
2218:
2213:
2209:
2202:
2199:
2194:
2188:
2184:
2180:
2173:
2170:
2165:
2161:
2157:
2153:
2146:
2144:
2142:
2138:
2133:
2129:
2125:
2121:
2117:
2113:
2106:
2104:
2102:
2100:
2098:
2094:
2089:
2085:
2078:
2075:
2063:
2059:
2057:
2049:
2046:
2041:
2037:
2033:
2029:
2025:
2021:
2017:
2013:
2006:
2003:
1999:
1994:
1989:
1985:
1982:(in German).
1981:
1977:
1970:
1968:
1966:
1964:
1960:
1956:(6): 470–474.
1955:
1951:
1944:
1943:(Guttiferae)"
1942:
1938:
1929:
1927:
1925:
1923:
1919:
1913:
1911:
1909:
1905:
1904:
1899:
1898:
1893:
1892:
1887:
1886:
1875:
1874:
1870:
1865:
1864:
1860:
1855:
1854:
1850:
1845:
1844:
1840:
1838:
1831:
1830:
1826:
1821:
1820:
1816:
1811:
1810:
1806:
1801:
1800:
1796:
1791:
1790:
1786:
1781:
1780:
1776:
1771:
1770:
1766:
1761:
1760:
1756:
1751:
1750:
1746:
1741:
1740:
1736:
1731:
1730:
1726:
1721:
1720:
1716:
1711:
1710:
1706:
1701:
1700:
1696:
1691:
1690:
1686:
1685:
1684:
1682:
1678:
1674:
1670:
1662:
1660:
1658:
1654:
1650:
1646:
1645:
1640:
1634:ion (Mg) and
1633:
1629:
1625:
1621:
1620:halogen bonds
1617:
1613:
1609:
1605:
1601:
1596:
1593:
1589:
1584:
1579:
1565:
1558:
1556:
1553:
1549:
1545:
1541:
1538:layer of the
1537:
1533:
1529:
1528:
1523:
1518:
1516:
1515:
1510:
1509:
1504:
1503:
1492:
1491:
1486:
1485:
1475:
1466:
1455:
1454:
1449:
1446:
1444:
1443:
1438:
1437:
1433:
1431:
1426:
1425:
1420:
1419:
1414:
1413:
1408:
1405:
1403:
1402:
1397:
1394:
1392:
1391:
1386:
1383:
1381:
1380:
1375:
1372:
1370:
1369:
1364:
1361:
1359:
1355:
1354:
1349:
1346:
1344:
1343:
1338:
1335:
1333:
1332:
1327:
1326:Euphorbiaceae
1324:
1322:
1321:
1316:
1313:
1311:
1310:
1305:
1304:
1299:
1298:
1293:
1290:
1289:
1288:
1286:
1281:
1278:
1277:
1272:
1268:
1264:
1263:
1258:
1254:
1250:
1246:
1245:
1240:
1239:
1230:
1229:
1224:
1217:
1215:
1213:
1205:
1201:
1197:
1193:
1189:
1184:
1182:
1178:
1175:
1171:
1167:
1163:
1159:
1155:
1151:
1147:
1143:
1139:
1135:
1131:
1127:
1123:
1119:
1115:
1112:, which uses
1111:
1102:
1095:
1093:
1091:
1087:
1083:
1082:
1081:Aedes aegypti
1077:
1073:
1069:
1065:
1060:
1058:
1054:
1050:
1048:
1043:
1042:
1037:
1036:antiparasitic
1033:
1032:
1026:
1025:
1020:
1019:antibacterial
1016:
1012:
1008:
1007:dihydropyrane
1005:. However, a
1004:
1003:
1002:M. aurum
998:
997:
992:
988:
987:
982:
981:antimicrobial
978:
977:
972:
964:
962:
960:
956:
952:
948:
944:
940:
936:
932:
931:rotating disc
928:
924:
920:
916:
915:mass spectrum
911:
909:
905:
904:sulfuric acid
901:
897:
893:
889:
885:
884:
874:
870:
866:
862:
861:melting point
858:
854:
850:
834:
830:
826:
822:
818:
814:
813:hydroxy group
810:
806:
798:
797:
792:
788:
783:
776:
774:
772:
768:
765:
761:
757:
753:
749:
745:
741:
737:
733:
729:
725:
721:
717:
716:Tanase method
713:
710:aldehyde and
709:
705:
701:
697:
693:
692:decomposition
688:
686:
682:
681:
676:
672:
671:
666:
662:
658:
650:
648:
646:
645:synthetically
642:
638:
634:
630:
629:antibacterial
626:
622:
618:
615:populations (
614:
610:
606:
602:
598:
594:
589:
587:
583:
579:
575:
571:
567:
566:binomial name
563:
562:
557:
556:
551:
547:
543:
539:
535:
531:
527:
526:Lichexanthone
515:
508:
503:
486:
480:
476:
474:
471:
470:
465:
461:
457:
453:
452:Formula units
449:
448:
444:
440:
436:
432:
431:
426:
422:
418:
414:
410:
407:
403:
402:
398:
390:
387:
383:
382:
379:
376:
373:
369:
368:
363:
359:
357:
356:Boiling point
354:
353:
349:
347:
346:Melting point
344:
343:
339:
337:
334:
333:
329:
326:
325:
318:
316:
313:
312:
291:
288:
284:
283:
278:
269:
265:
258:
244:
240:
233:
225:
221:
217:
216:
214:
204:
200:
199:
192:
188:
187:
185:
179:
175:
174:
167:
163:
162:
160:
158:
155:
154:
147:
143:
142:
140:
138:
135:
134:
127:
123:
122:
120:
118:
115:
114:
107:
103:
102:
100:
96:
91:
90:
83:
79:
78:
76:
73:
69:
68:
63:
53:
48:
43:
39:
34:
30:
25:
16:
4218:
4214:
4182:
4178:
4171:
4162:
4158:
4145:
4128:
4124:
4120:
4114:
4095:
4091:
4068:
4064:
4051:
4037:(1): 67–79.
4034:
4030:
3998:
3994:
3991:Astrothelium
3990:
3962:
3958:
3951:
3934:
3930:
3927:Cryptothecia
3926:
3920:
3903:
3899:
3895:
3891:
3887:
3853:
3849:
3845:
3839:
3830:
3826:
3820:
3812:
3795:
3791:
3785:
3768:
3764:
3754:
3735:
3729:
3704:
3700:
3691:
3683:
3674:
3670:
3661:
3657:
3649:
3624:
3620:
3616:
3610:
3593:
3589:
3585:
3579:
3554:
3550:
3546:
3542:
3535:
3508:
3504:
3498:
3490:
3473:
3469:
3465:
3459:
3442:
3438:
3434:
3428:
3411:
3407:
3403:
3397:
3372:
3368:
3364:
3358:
3341:
3337:
3333:
3327:
3302:
3298:
3294:
3287:
3262:
3258:
3254:
3248:
3223:
3219:
3216:Anthocleista
3215:
3209:
3192:
3188:
3184:
3180:
3174:
3149:
3145:
3141:
3135:
3101:(10): 1380.
3098:
3095:Biomedicines
3094:
3088:
3084:
3076:
3057:
3053:
3047:
3038:
3019:
3015:
3009:
3000:
2966:(2): 55–66.
2963:
2959:
2953:
2949:
2945:
2937:
2919:in Australia
2918:
2914:
2908:
2891:
2887:
2883:
2877:
2860:
2856:
2846:
2829:
2825:
2819:
2788:
2775:
2756:
2752:
2746:
2742:
2734:
2707:
2703:
2693:
2668:
2664:
2660:
2656:
2652:
2618:
2614:
2610:
2603:
2566:
2562:
2514:
2510:
2475:
2471:
2465:
2438:
2434:
2424:
2405:
2387:
2383:
2377:
2369:
2352:
2348:
2342:
2325:
2321:
2315:
2298:
2295:Hypotrachyna
2294:
2258:
2230:
2226:
2220:
2211:
2207:
2201:
2182:
2172:
2155:
2151:
2115:
2111:
2087:
2083:
2077:
2065:. Retrieved
2055:
2048:
2023:
2019:
2015:
2011:
2005:
1997:
1983:
1979:
1953:
1949:
1940:
1936:
1901:
1895:
1889:
1883:
1881:
1871:
1861:
1851:
1841:
1827:
1817:
1807:
1797:
1787:
1777:
1767:
1757:
1747:
1737:
1727:
1717:
1707:
1697:
1687:
1666:
1656:
1653:chemometrics
1642:
1600:substituents
1597:
1576:
1525:
1519:
1512:
1506:
1500:
1498:
1488:
1482:
1451:
1440:
1434:
1428:
1422:
1416:
1410:
1399:
1396:Polygonaceae
1388:
1377:
1366:
1357:
1351:
1348:Hypericaceae
1340:
1337:Gentianaceae
1329:
1318:
1307:
1301:
1295:
1282:
1274:
1271:Graphidaceae
1260:
1244:Hypotrachyna
1242:
1236:
1234:
1226:
1185:
1177:intermediate
1174:benzophenone
1142:condensation
1134:active sites
1107:
1096:Biosynthesis
1090:Dengue virus
1079:
1061:
1045:
1039:
1030:
1022:
1015:tuberculosis
1000:
994:
984:
974:
968:
912:
900:fluorescence
882:
828:
817:methyl group
808:
802:
794:
760:polycarbonyl
748:demethylated
730:, produce a
689:
678:
674:
668:
664:
654:
593:biosynthesis
590:
559:
553:
538:leafy lichen
525:
524:
455:
438:
424:
420:
412:
396:
146:ChEMBL470650
65:Identifiers
55:Other names
46:
15:
4268:Xanthonoids
3662:Penicillium
3658:Penicillium
3466:Zanthoxylum
3439:Tetrahedron
3226:: 648–667.
2615:Fitoterapia
1612:aromaticity
1592:derivatives
1588:chlorinated
1487:(left) and
1448:Sapindaceae
1122:polyketides
1068:human sperm
896:acetic acid
873:space group
857:crystalline
847:; it has a
767:cyclization
728:acetic acid
641:derivatives
617:photobionts
473:Flash point
386:Space group
340:1.323 g/cm
327:Appearance
280:Properties
126:CHEBI:67821
4242:Categories
4092:Mycosphere
3896:Crypthonia
3888:Crypthonia
3721:1885/57129
3692:Haematomma
3677:: 201–241.
3468:species".
3406:Burm. f".
3087:and their
2917:Pertusaria
2611:Ulocladium
2569:(3): 294.
2214:: 629–630.
2090:: 350–352.
2067:4 February
1914:References
1891:Chiodecton
1885:Crypthonia
1522:mycobionts
1315:Clusiaceae
1292:Annonaceae
1276:Pertusaria
1253:usnic acid
1218:Occurrence
1072:larvicidal
1057:cell lines
825:IUPAC name
777:Properties
738:to give a
720:methylated
708:orsellinic
633:larvicidal
601:acetyl-CoA
578:Annonaceae
555:Pertusaria
552:, such as
378:Monoclinic
365:Structure
315:Molar mass
157:ChemSpider
93:3D model (
82:15222-53-4
72:CAS Number
42:IUPAC name
4248:Xanthones
3957:Brazil".
3827:Mycotaxon
3660:subgenus
3414:: 52–55.
3089:in silico
2960:Medicines
2563:Molecules
2277:851387266
2026:(1): 88.
1903:Caprettia
1673:taxonomic
1632:magnesium
1628:complexes
1385:Olacaceae
1363:Meliaceae
1257:character
1249:atranorin
1144:of seven
1118:precursor
1053:cytotoxic
943:reduction
888:ethanolic
871:, in the
805:xanthones
791:apothecia
744:oxidation
704:syntheses
570:fluoresce
534:xanthones
399:(No. 14)
4121:Cladonia
3892:Syncesia
3846:Lecanora
3833:: 49–56.
3641:15280651
3571:11324918
3547:caudatum
3527:22312711
3389:12932125
3319:12502307
3279:29502449
3240:26432351
3166:16314057
3127:34680496
3091:studies"
2992:28930201
2726:23207680
2685:21438586
2635:22061662
2595:26950106
2531:23106287
2457:19683421
2390:: 27–34.
2132:22617028
1897:Cladonia
1657:in vitro
1544:ascomata
1536:cortical
1407:Rutaceae
1358:dealbata
1238:Parmelia
1150:malonate
1128:. These
1064:motility
976:in vitro
969:Various
908:UV light
740:xanthene
732:fluorone
685:xanthone
605:cyclized
582:Rutaceae
574:UV light
546:taxonomy
467:Hazards
4263:Ketones
4165:: 1–18.
4071:: 1–10.
3870:3244290
3118:8533219
2983:5533161
2948:L. and
2787:(ed.).
2586:6273661
2492:9795033
2040:1220900
1677:eponyms
1663:Eponyms
1540:thallus
1532:mycelia
1192:isomers
1158:ketonic
1146:acetate
1130:enzymes
1088:of the
875:called
787:thallus
736:reduced
700:orcinol
651:History
542:lichens
507:what is
505: (
336:Density
320:286.283
191:5358904
178:PubChem
166:4513972
3868:
3742:
3639:
3569:
3525:
3387:
3317:
3277:
3238:
3164:
3125:
3115:
2990:
2980:
2925:
2884:Pyxine
2807:
2724:
2683:
2633:
2593:
2583:
2529:
2490:
2455:
2412:
2275:
2265:
2189:
2130:
2038:
1908:genera
1900:, and
1622:, and
1285:family
1262:Pyxine
1181:pyrone
1086:vector
1076:larvae
937:, and
635:, and
561:Pyxine
550:genera
528:is an
502:verify
499:
264:SMILES
137:ChEMBL
36:Names
4155:(PDF)
4061:(PDF)
3866:JSTOR
3798:(8).
3697:(PDF)
3667:(PDF)
2613:sp".
2036:JSTOR
2020:Taxon
1946:(PDF)
1649:bromo
1616:ionic
1356:var.
1162:ether
1116:as a
1011:drugs
929:with
886:. An
764:aldol
677:(now
663:from
613:algal
586:fungi
239:InChI
117:ChEBI
95:JSmol
3740:ISBN
3637:PMID
3567:PMID
3545:and
3523:PMID
3385:PMID
3315:PMID
3275:PMID
3236:PMID
3183:and
3162:PMID
3123:PMID
2988:PMID
2923:ISBN
2805:ISBN
2745:and
2722:PMID
2681:PMID
2659:and
2631:PMID
2591:PMID
2527:PMID
2488:PMID
2453:PMID
2410:ISBN
2273:OCLC
2263:ISBN
2187:ISBN
2128:PMID
2069:2022
1618:and
1511:and
1251:and
1148:and
1084:, a
999:and
933:and
913:The
835:is C
789:and
726:and
580:and
558:and
4223:doi
4187:doi
4183:125
4133:doi
4100:doi
4039:doi
4035:121
4003:doi
3999:120
3993:".
3967:doi
3963:120
3939:doi
3908:doi
3898:".
3858:doi
3854:103
3800:doi
3796:137
3773:doi
3717:hdl
3709:doi
3629:doi
3598:doi
3588:".
3559:doi
3549:".
3513:doi
3478:doi
3447:doi
3437:".
3416:doi
3377:doi
3367:".
3346:doi
3336:".
3307:doi
3297:".
3267:doi
3228:doi
3224:175
3197:doi
3187:".
3154:doi
3150:105
3113:PMC
3103:doi
3062:doi
3024:doi
2978:PMC
2968:doi
2896:doi
2865:doi
2834:doi
2830:646
2797:doi
2761:doi
2712:doi
2673:doi
2663:".
2623:doi
2581:PMC
2571:doi
2519:doi
2480:doi
2443:doi
2357:doi
2330:doi
2303:doi
2235:doi
2212:15B
2160:doi
2120:doi
2116:112
2088:12B
2028:doi
1988:doi
1626:of
1208:max
1066:of
1044:or
208:EPA
181:CID
4244::
4219:14
4217:.
4213:.
4199:^
4181:.
4163:23
4161:.
4157:.
4129:42
4127:.
4096:12
4094:.
4090:.
4077:^
4069:16
4067:.
4063:.
4033:.
4015:^
3997:.
3979:^
3961:.
3935:45
3933:.
3904:45
3902:.
3878:^
3864:.
3852:.
3831:63
3829:.
3825:.
3794:.
3769:42
3767:.
3763:.
3715:.
3703:.
3699:.
3675:49
3673:.
3669:.
3635:.
3625:86
3623:.
3594:24
3592:.
3565:.
3555:56
3553:.
3521:.
3507:.
3503:.
3474:28
3472:.
3443:70
3441:.
3412:82
3410:.
3383:.
3373:66
3371:.
3342:35
3340:.
3313:.
3303:65
3301:.
3273:.
3263:33
3261:.
3234:.
3222:.
3193:15
3191:.
3160:.
3148:.
3121:.
3111:.
3097:.
3093:.
3056:.
3052:.
3020:22
3018:.
3014:.
2986:.
2976:.
2962:.
2958:.
2892:46
2890:.
2859:.
2855:.
2828:.
2803:.
2757:44
2755:.
2751:.
2720:.
2708:61
2706:.
2702:.
2679:.
2669:74
2667:.
2643:^
2629:.
2619:83
2617:.
2589:.
2579:.
2567:21
2565:.
2561:.
2539:^
2525:.
2513:.
2500:^
2486:.
2474:.
2451:.
2439:17
2437:.
2433:.
2396:^
2388:13
2386:.
2382:.
2353:54
2351:.
2324:.
2285:^
2271:.
2247:^
2231:99
2229:.
2210:.
2156:61
2154:.
2140:^
2126:.
2114:.
2096:^
2086:.
2060:.
2034:.
2024:38
2022:.
1996:.
1984:17
1978:.
1962:^
1952:.
1948:.
1921:^
1894:,
1888:,
1636:NH
1450::
1439:,
1427:,
1421:,
1415:,
1409::
1398::
1387::
1376::
1365::
1350::
1339::
1328::
1317::
1306:,
1300:,
1294::
1287::
1214:.
1212:nm
1092:.
1059:.
961:.
877:P2
841:14
837:16
631:,
462:4
419:,
391:P2
302:14
296:16
4231:.
4225::
4193:.
4189::
4139:.
4135::
4108:.
4102::
4045:.
4041::
4009:.
4005::
3973:.
3969::
3945:.
3941::
3914:.
3910::
3872:.
3860::
3806:.
3802::
3779:.
3775::
3748:.
3723:.
3719::
3711::
3705:8
3664:"
3643:.
3631::
3604:.
3600::
3573:.
3561::
3529:.
3515::
3509:6
3501:"
3484:.
3480::
3453:.
3449::
3422:.
3418::
3391:.
3379::
3352:.
3348::
3321:.
3309::
3281:.
3269::
3242:.
3230::
3203:.
3199::
3168:.
3156::
3129:.
3105::
3099:9
3070:.
3064::
3058:3
3032:.
3026::
2994:.
2970::
2964:2
2956:"
2931:.
2902:.
2898::
2871:.
2867::
2861:5
2840:.
2836::
2813:.
2799::
2769:.
2763::
2749:"
2728:.
2714::
2687:.
2675::
2637:.
2625::
2597:.
2573::
2533:.
2521::
2515:9
2494:.
2482::
2476:6
2459:.
2445::
2418:.
2380:"
2363:.
2359::
2336:.
2332::
2326:1
2309:.
2305::
2279:.
2241:.
2237::
2195:.
2166:.
2162::
2134:.
2122::
2071:.
2042:.
2030::
1990::
1954:9
1937:h
1638:3
1573:)
1571:3
1432:,
1049:,
883:c
881:/
879:1
845:5
843:O
839:H
829:H
809:H
497:Y
458:)
456:Z
454:(
441:)
439:V
437:(
425:c
421:b
417:Å
413:a
397:c
395:/
393:1
308:5
305:O
299:H
293:C
210:)
206:(
97:)
47:H
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.