310:
287:
184:
209:
561:
568:
316:
215:
3402:
29:
1320:
In the ER, PPIB interacts with proteins such as P3H1, CRTAP, BiP, GRP94, PDI, and calreticulin to form foldase and chaperone complexes and facilitate protein folding, especially for type I collagen. This protein is the major PPIase for type I collagen, since the collagen contains an abundance of
1324:
In addition, it is associated with the secretory pathway and released in biological fluids. This protein can bind to cells derived from T- and B-lymphocytes, and may regulate cyclosporine A-mediated immunosuppression. In one experiment, the addition of PPIB into cell cultures in vitro induced
55:
2558:"Nascent lipidated apolipoprotein B is transported to the Golgi as an incompletely folded intermediate as probed by its association with network of endoplasmic reticulum molecular chaperones, GRP94, ERp72, BiP, calreticulin, and cyclophilin B"
1383:. Thus, therapeutic targeting of PPIB with selective inhibitors may prove effective in combating viral infections and inflammation. Currently, PPIB is employed as a biomarker for various types of cancer. Moreover, there are two
1321:
prolines that require cis-trans isomerization for proper folding. Thus, PPIB is essential for collagen biosynthesis and post-translational modification and affects fibril assembly, matrix cross-linking, and bone mineralization.
2445:
Göthel, S. F.; Scholz, C.; Schmid, F. X.; Marahiel, M. A. (1998-09-22). "Cyclophilin and trigger factor from
Bacillus subtilis catalyze in vitro protein folding and are necessary for viability under starvation conditions".
2601:
Rasmussen HH, van Damme J, Puype M, Gesser B, Celis JE, Vandekerckhove J (1993). "Microsequences of 145 proteins recorded in the two-dimensional gel protein database of normal human epidermal keratinocytes".
2219:"An additional function of the rough endoplasmic reticulum protein complex prolyl 3-hydroxylase 1·cartilage-associated protein·cyclophilin B: the CXXXC motif reveals disulfide isomerase activity in vitro"
2489:
Pandey, Saurabh; Sharma, Ashish; Tripathi, Deeksha; Kumar, Ashutosh; Khubaib, Mohd; Bhuwan, Manish; Chaudhuri, Tapan Kumar; Hasnain, Seyed
Ehtesham; Ehtesham, Nasreen Zafar (2016-03-16).
2161:
Cabral WA, Perdivara I, Weis M, Terajima M, Blissett AR, Chang W, Perosky JE, Makareeva EN, Mertz EL, Leikin S, Tomer KB, Kozloff KM, Eyre DR, Yamauchi M, Marini JC (Jun 2014).
1185:
at the top and bottom. In addition, the β-turns and loops in the strands contribute to the flexibility of the barrel. In particular, PPIB is a 21 kDa protein which contains a
323:
222:
3086:"Native recombinant cyclophilins A, B, and C degrade DNA independently of peptidylprolyl cis-trans-isomerase activity. Potential roles of cyclophilins in apoptosis"
1966:
Wang T, Yun CH, Gu SY, Chang WR, Liang DC (Aug 2005). "1.88 A crystal structure of the C domain of hCyP33: a novel domain of peptidyl-prolyl cis-trans isomerase".
1764:
Wang T, Yun CH, Gu SY, Chang WR, Liang DC (Aug 2005). "1.88 A crystal structure of the C domain of hCyP33: a novel domain of peptidyl-prolyl cis-trans isomerase".
1285:. It is also associated with viral infections. In eukaryotes, cyclophilins localize ubiquitously to many cell and tissue types. In addition to PPIase and protein
2282:
Skagia, Aggeliki; Vezyri, Eleni; Sigala, Markezina; Kokkinou, Areti; Karpusas, Michael; Venieraki, Anastasia; Katinakis, Panagiotis; Dimou, Maria (January 2017).
2331:
Wiemels, Richard E.; Cech, Stephanie M.; Meyer, Nikki M.; Burke, Caleb A.; Weiss, Andy; Parks, Anastacia R.; Shaw, Lindsey N.; Carroll, Ronan K. (2017-01-01).
3199:"Maturation-induced conformational changes of HIV-1 capsid protein and identification of two high affinity sites for cyclophilins in the C-terminal domain"
2639:
Peddada LB, McPherson JD, Law R, Wasmuth JJ, Youderian P, Deans RJ (1992). "Somatic cell mapping of the human cyclophilin B gene (PPIB) to chromosome 15".
2333:"An Intracellular Peptidyl-Prolyl cis/trans Isomerase Is Required for Folding and Activity of the Staphylococcus aureus Secreted Virulence Factor Nuclease"
861:
842:
145:
2834:
Allain F, Boutillon C, Mariller C, Spik G (1995). "Selective assay for CyPA and CyPB in human blood using highly specific anti-peptide antibodies".
2163:"Abnormal type I collagen post-translational modification and crosslinking in a cyclophilin B KO mouse model of recessive osteogenesis imperfecta"
1229:, and is often the rate-limiting step in protein refolding. The PPIase family is further divided into three structurally distinct subfamilies:
2052:"Comparing human pancreatic cell secretomes by in vitro aptamer selection identifies cyclophilin B as a candidate pancreatic cancer biomarker"
1241:(Pvn). While each family demonstrates PPIase activity, the families have no sequence of structural similarities. As a cyclophilin, PPIB binds
3430:
3381:
3004:
Luban J, Bossolt KL, Franke EK, Kalpana GV, Goff SP (1993). "Human immunodeficiency virus type 1 Gag protein binds to cyclophilins A and B".
2670:"s-cyclophilin is retained intracellularly via a unique COOH-terminal sequence and colocalizes with the calcium storage protein calreticulin"
1590:
1572:
2284:"Structural and functional analysis of cyclophilin PpiB mutants supports an in vivo function not limited to prolyl isomerization activity"
2918:"X-ray structure of a cyclophilin B/cyclosporin complex: comparison with cyclophilin A and delineation of its calcineurin-binding domain"
3164:"The V3 loop of human immunodeficiency virus type-1 envelope protein is a high-affinity ligand for immunophilins present in human blood"
309:
1061:
1068:
286:
3401:
3302:
Yurchenko V, O'Connor M, Dai WW, Guo H, Toole B, Sherry B, Bukrinsky M (2001). "CD147 is a signaling receptor for cyclophilin B".
2268:
1559:
1538:
1492:
208:
183:
1555:
52:
1534:
125:
3043:"The hydrophobic pocket of cyclophilin is the binding site for the human immunodeficiency virus type 1 Gag polyprotein"
1814:
Yao Q, Li M, Yang H, Chai H, Fisher W, Chen C (Mar 2005). "Roles of cyclophilins in cancers and other organ systems".
1504:
1467:
1274:
2390:"Intracellularly Induced Cyclophilins Play an Important Role in Stress Adaptation and Virulence of Brucella abortus"
322:
221:
1448:
1349:
As a cyclophilin, PPIB binds the immunosuppressive drug CsA to form a CsA-cyclophilin complex, which then targets
3374:
2754:
Spik G, Haendler B, Delmas O, Mariller C, Chamoux M, Maes P, Tartar A, Montreuil J, Stedman K, Kocher HP (1991).
2491:"Mycobacterium tuberculosis Peptidyl-Prolyl Isomerases Also Exhibit Chaperone like Activity In-Vitro and In-Vivo"
315:
214:
1198:
906:
133:
2789:
Bram RJ, Crabtree GR (1994). "Calcium signalling in T cells stimulated by a cyclophilin B-binding protein".
887:
1610:"Human cyclophilin B: a second cyclophilin gene encodes a peptidyl-prolyl isomerase with a signal sequence"
1415:
1411:
1391:
1434:
1310:
1254:
197:
1217:
and regulate protein folding and maturation. Proline is the only amino acid known to exist in both the
1134:. As a member of the peptidyl-prolyl cis-trans isomerase (PPIase) family, this protein catalyzes the
3367:
2929:
2876:
2798:
2502:
2101:"Depletion of cyclophilins B and C leads to dysregulation of endoplasmic reticulum redox homeostasis"
1621:
1439:
1334:
1253:
PPIB is the second of 18 cyclophilins to be identified in humans, after CypA. PPIB localizes to the
112:
1429:
1286:
3121:"Distribution of cyclophilin B-binding sites in the subsets of human peripheral blood lymphocytes"
3029:
2822:
2627:
2029:
1904:
1839:
1741:
1361:
157:
2865:"Cyclophilin B trafficking through the secretory pathway is altered by binding of cyclosporin A"
2388:
Roset, Mara S.; García Fernández, Lucía; DelVecchio, Vito G.; Briones, Gabriel (February 2013).
1927:"Selective cerebral perfusion during operation for aneurysms of the aortic arch: a reassessment"
1672:"Selective cerebral perfusion during operation for aneurysms of the aortic arch: a reassessment"
1044:
1023:
997:
976:
3339:
3319:
3290:
3255:
3220:
3185:
3150:
3107:
3072:
3021:
2992:
2957:
2904:
2851:
2814:
2777:
2742:
2699:
2656:
2619:
2579:
2538:
2520:
2471:
2463:
2427:
2409:
2370:
2352:
2313:
2305:
2250:
2194:
2132:
2081:
2021:
2004:
Hoffmann H, Schiene-Fischer C (Jul 2014). "Functional aspects of extracellular cyclophilins".
1983:
1948:
1896:
1878:
1831:
1781:
1733:
1716:
Hoffmann H, Schiene-Fischer C (Jul 2014). "Functional aspects of extracellular cyclophilins".
1693:
1649:
1454:
1357:
105:
45:
1356:
In cardiac myogenic cells, cyclophilins have been observed to be activated by heat shock and
3311:
3280:
3245:
3210:
3175:
3140:
3132:
3097:
3062:
3054:
3013:
2982:
2947:
2937:
2894:
2884:
2843:
2806:
2767:
2732:
2724:
2689:
2681:
2648:
2611:
2569:
2528:
2510:
2455:
2417:
2401:
2360:
2344:
2295:
2240:
2230:
2184:
2174:
2122:
2112:
2071:
2063:
2013:
1975:
1938:
1886:
1870:
1823:
1773:
1725:
1683:
1639:
1629:
1474:
1420:
1210:
560:
402:
333:
277:
232:
3232:
Bristow R, Byrne J, Squirell J, Trencher H, Carter T, Rodgers B, Saman E, Duncan J (1999).
3234:"Human cyclophilin has a significantly higher affinity for HIV-1 recombinant p55 than p24"
2971:"Characterization of surface binding sites for cyclophilin B on a human tumor T-cell line"
1480:
1438:, PPIB has been shown to have PPIase activity, and to directly assist in the refolding of
1364:. Thus, cyclophilins may function in cardioprotection during ischemia-reperfusion injury.
1338:
1146:
567:
377:
153:
2933:
2880:
2802:
2506:
1891:
1858:
1625:
3269:"Role of cyclophilin B in prolactin signal transduction and nuclear retrotranslocation"
3180:
3163:
3145:
3120:
2694:
2669:
2533:
2490:
2422:
2389:
2365:
2332:
2245:
2218:
2189:
2162:
2127:
2100:
2076:
2051:
1158:
3067:
3042:
2987:
2970:
2772:
2755:
2737:
2712:
1367:
PPIB contributes to the replication and infection of viruses causing diseases such as
776:
771:
766:
761:
756:
751:
746:
741:
736:
731:
715:
710:
705:
700:
695:
690:
685:
680:
675:
670:
665:
649:
644:
639:
634:
629:
624:
619:
614:
609:
28:
3424:
3348:
3250:
3233:
3136:
3017:
2952:
2917:
2899:
2864:
2847:
1943:
1926:
1859:"Peptidyl-prolyl cis-trans isomerases, a superfamily of ubiquitous folding catalysts"
1688:
1671:
1644:
1609:
1242:
1138:
596:
3058:
3033:
2631:
2033:
1908:
1843:
1745:
1317:
homeostasis. Depletion of these two cyclophilins leads to hyperoxidation of the ER.
2826:
1510:
1298:
1290:
1270:
1142:
395:
174:
1182:
137:
2515:
2179:
161:
1595:
National Center for
Biotechnology Information, U.S. National Library of Medicine
1577:
National Center for
Biotechnology Information, U.S. National Library of Medicine
1399:
1380:
1372:
1350:
1302:
1230:
1178:
1174:
1170:
1979:
1777:
478:
1827:
1390:(CypB84-92 and CypB91-99) recognized by HLA-A24-restricted and tumor-specific
1326:
1258:
1194:
1186:
1154:
1150:
294:
191:
141:
2615:
2524:
2467:
2413:
2356:
2309:
1882:
3102:
3085:
2942:
2235:
2117:
1294:
1262:
1257:(ER) and participates in many biological processes, including mitochondrial
1190:
1135:
806:
538:
416:
361:
348:
260:
247:
149:
3323:
3315:
3294:
3285:
3268:
3259:
3224:
3215:
3198:
2889:
2583:
2574:
2557:
2542:
2431:
2374:
2317:
2254:
2198:
2136:
2085:
2025:
1987:
1900:
1835:
1785:
1737:
1634:
3189:
3154:
3111:
3076:
3025:
2996:
2961:
2908:
2855:
2818:
2781:
2746:
2728:
2703:
2685:
2660:
2623:
2475:
2017:
1952:
1874:
1729:
1697:
1653:
1108:
1103:
2405:
1608:
Price ER, Zydowsky LD, Jin MJ, Baker CH, McKeon FD, Walsh CT (Apr 1991).
1387:
1330:
1238:
1092:
951:
932:
2348:
3353:
3343:
1395:
1384:
1376:
1214:
918:
873:
2652:
2459:
2300:
2283:
2050:
Ray P, Rialon-Guevara KL, Veras E, Sullenger BA, White RR (May 2012).
82:
78:
74:
2810:
2067:
1425:
1282:
1124:
1076:
828:
116:, CYP-S1, CYPB, HEL-S-39, OI9, SCYLP, peptidylprolyl isomerase B, B
1498:
1486:
1314:
1266:
791:
787:
1368:
1306:
1278:
1245:(CsA) and can be found within the cell or secreted by the cell.
1234:
1131:
129:
3363:
576:
3359:
2288:
Genes to Cells: Devoted to
Molecular & Cellular Mechanisms
2269:"Entrez Gene: PPIB peptidylprolyl isomerase B (cyclophilin B)"
1209:
PPIB is a member of the peptidyl-prolyl cis-trans isomerase (
3338:
Overview of all the structural information available in the
2863:
Price ER, Jin M, Lim D, Pati S, Walsh CT, McKeon FD (1994).
1442:. Aside from these bacteria, PPIB has been identified in
1273:, as well as in related diseases and conditions, such as
3267:
Rycyzyn MA, Reilly SC, O'Malley K, Clevenger CV (2001).
2099:
Stocki P, Chapman DC, Beach LA, Williams DB (Aug 2014).
1353:
to inhibit the signaling pathway for T-cell activation.
385:
1215:
cis-trans isomerization of proline imidic peptide bonds
1177:
core. This β-barrel is composed of eight anti-parallel
747:
positive regulation by host of viral genome replication
1189:
ER retention motif that directs the protein to the ER
737:
regulation of post-translational protein modification
550:
2711:
Hasel KW, Glass JR, Godbout M, Sutcliffe JG (1991).
1341:
by recruiting T cells into infected tissue in vivo.
762:
positive regulation of multicellular organism growth
2756:"A novel secreted cyclophilin-like protein (SCYLP)"
1968:
1766:
1037:
1016:
990:
969:
1925:Kazui T, Inoue N, Yamada O, Komatsu S (Jan 1992).
1670:Kazui T, Inoue N, Yamada O, Komatsu S (Jan 1992).
1551:
1549:
1547:
1530:
1528:
1526:
1999:
1997:
1145:, which allows it to regulate protein folding of
332:
231:
2045:
2043:
1920:
1918:
3352:(Peptidyl-prolyl cis-trans isomerase B) at the
3238:J. Acquir. Immune Defic. Syndr. Hum. Retrovirol
2713:"An endoplasmic reticulum-specific cyclophilin"
2212:
2210:
2208:
2156:
2154:
2152:
2150:
2148:
2146:
1809:
1807:
1805:
1803:
1801:
1799:
1797:
1795:
16:Protein-coding gene in the species Homo sapiens
1759:
1757:
1755:
1711:
1709:
1707:
1556:GRCm38: Ensembl release 89: ENSMUSG00000032383
3375:
3119:Denys A, Allain F, Foxwell B, Spik G (1997).
3084:Montague JW, Hughes FM, Cidlowski JA (1997).
1857:Göthel, S. F.; Marahiel, M. A. (March 1999).
1665:
1663:
8:
1337:(ECM), suggesting that it might function in
752:positive regulation by host of viral process
645:peptidyl-prolyl cis-trans isomerase activity
3410:: CYCLOPHILIN B COMPLEXED WITH -CYCLOSPORIN
1535:GRCh38: Ensembl release 89: ENSG00000166794
3382:
3368:
3360:
802:
592:
373:
272:
169:
63:
3284:
3249:
3214:
3179:
3144:
3101:
3066:
2986:
2951:
2941:
2916:Mikol V, Kallen J, Walkinshaw MD (1994).
2898:
2888:
2771:
2736:
2693:
2573:
2532:
2514:
2421:
2364:
2299:
2244:
2234:
2188:
2178:
2126:
2116:
2075:
1942:
1890:
1687:
1643:
1633:
1173:, PPIB forms a β-barrel structure with a
3197:Endrich MM, Gehrig P, Gehring H (1999).
3397:
1522:
1360:-reoxygenation as well as complex with
696:endoplasmic reticulum chaperone complex
1149:. Generally, PPIases are found in all
18:
3041:Braaten D, Ansari H, Luban J (1997).
2668:Arber S, Krause KH, Caroni P (1992).
2217:Ishikawa Y, Bächinger HP (Nov 2013).
2056:The Journal of Clinical Investigation
1333:-mediated adhesion of T cells to the
1289:activities, cyclophilins function in
1121:Peptidyl-prolyl cis-trans isomerase B
742:protein peptidyl-prolyl isomerization
337:
298:
293:
236:
195:
190:
7:
1863:Cellular and Molecular Life Sciences
513:endothelial cell of lymphatic vessel
2223:The Journal of Biological Chemistry
2105:The Journal of Biological Chemistry
1424:, PPIB has been shown to have both
3181:10.1046/j.1432-1327.1998.2520441.x
2969:Allain F, Denys A, Spik G (1994).
2556:Zhang J, Herscovitz H (Feb 2003).
1398:, and in fact, were used to treat
1034:
1013:
987:
966:
942:
923:
897:
878:
852:
833:
772:chaperone-mediated protein folding
620:protein-containing complex binding
555:
473:
411:
390:
14:
1410:PPIB has been identified in both
1161:, and thus are highly conserved.
3400:
3251:10.1097/00042560-199904010-00002
3137:10.1046/j.1365-2567.1997.00296.x
1418:as an intracellular protein. In
566:
559:
321:
314:
308:
285:
220:
213:
207:
182:
27:
3059:10.1128/JVI.71.3.2107-2113.1997
1213:) family. PPIases catalyze the
706:perinuclear region of cytoplasm
3162:Endrich MM, Gehring H (1998).
1931:The Annals of Thoracic Surgery
1676:The Annals of Thoracic Surgery
1394:which could be used as cancer
1305:and proliferation. Along with
1233:(CyP), FK506-binding protein (
577:More reference expression data
539:More reference expression data
1:
3304:Biochem. Biophys. Res. Commun
2988:10.1016/S0021-9258(19)89421-8
2773:10.1016/S0021-9258(18)99078-2
1197:extension attaches it to its
497:vestibular sensory epithelium
306:
205:
3431:Genes on human chromosome 15
3018:10.1016/0092-8674(93)90637-6
2922:Proc. Natl. Acad. Sci. U.S.A
2869:Proc. Natl. Acad. Sci. U.S.A
2848:10.1016/0022-1759(94)00249-V
2516:10.1371/journal.pone.0150288
2180:10.1371/journal.pgen.1004465
1944:10.1016/0003-4975(92)90767-x
1689:10.1016/0003-4975(92)90767-x
691:smooth endoplasmic reticulum
1275:ischemic reperfusion injury
671:endoplasmic reticulum lumen
439:right lobe of thyroid gland
427:stromal cell of endometrium
3447:
1980:10.1016/j.bbrc.2005.06.006
1778:10.1016/j.bbrc.2005.06.006
1449:Mycobacterium tuberculosis
1297:, immunological response,
666:protein-containing complex
451:left lobe of thyroid gland
3395:
1828:10.1007/s00268-004-7812-7
1591:"Mouse PubMed Reference:"
1573:"Human PubMed Reference:"
1313:(ER), where it maintains
1107:
1102:
1098:
1091:
1075:
1056:
1041:
1020:
1009:
994:
973:
962:
949:
945:
930:
926:
917:
904:
900:
885:
881:
872:
859:
855:
840:
836:
827:
812:
805:
801:
785:
595:
591:
574:
558:
549:
536:
485:
476:
423:
414:
384:
376:
372:
355:
342:
305:
284:
275:
271:
254:
241:
204:
181:
172:
168:
123:
120:
110:
103:
98:
71:
66:
49:
44:
39:
35:
26:
21:
2616:10.1002/elps.11501301199
1816:World Journal of Surgery
1614:Proc Natl Acad Sci U S A
1309:, PPIB localizes to the
1062:Chr 15: 64.16 – 64.16 Mb
615:unfolded protein binding
3103:10.1074/jbc.272.10.6677
2943:10.1073/pnas.91.11.5183
2337:Journal of Bacteriology
2236:10.1074/jbc.M113.498063
2118:10.1074/jbc.M114.570911
1466:PPIB has been shown to
1440:Staphylococcal nuclease
1392:cytotoxic T lymphocytes
1127:that is encoded by the
1069:Chr 9: 65.97 – 65.97 Mb
493:internal carotid artery
489:external carotid artery
3316:10.1006/bbrc.2001.5847
3286:10.1210/mend.14.8.0508
3216:10.1074/jbc.274.9.5326
2890:10.1073/pnas.91.9.3931
2575:10.1074/jbc.M207976200
2394:Infection and Immunity
1635:10.1073/pnas.88.5.1903
1416:Gram-positive bacteria
1412:Gram-negative bacteria
1157:, as well as in a few
635:RNA polymerase binding
2729:10.1128/mcb.11.7.3484
2686:10.1083/jcb.116.1.113
2641:Cytogenet. Cell Genet
2018:10.1515/hsz-2014-0125
1875:10.1007/s000180050299
1730:10.1515/hsz-2014-0125
1435:Staphylococcus aureus
1402:in a clinical trial.
1345:Clinical significance
1311:endoplasmic reticulum
1255:endoplasmic reticulum
732:protein stabilization
711:extracellular exosome
701:endoplasmic reticulum
650:cyclosporin A binding
198:Chromosome 15 (human)
2406:10.1128/IAI.01125-12
2006:Biological Chemistry
1718:Biological Chemistry
1458:and other bacteria.
1335:extracellular matrix
300:Chromosome 9 (mouse)
67:List of PDB id codes
40:Available structures
2934:1994PNAS...91.5183M
2881:1994PNAS...91.3931P
2836:J. Immunol. Methods
2803:1994Natur.371..355B
2507:2016PLoSO..1150288P
2454:(38): 13392–13399.
2349:10.1128/JB.00453-16
1626:1991PNAS...88.1903P
1430:Chaperone (protein)
1362:heat shock proteins
1225:isomerization rate
1181:and capped by two
1141:of proline imidic
907:ENSMUSG00000032383
725:Biological process
659:Cellular component
625:isomerase activity
603:Molecular function
459:tail of epididymis
443:anterior pituitary
3418:
3417:
2653:10.1159/000133343
2460:10.1021/bi981253w
2301:10.1111/gtc.12452
1455:Bacillus subtilis
1118:
1117:
1114:
1113:
1087:
1086:
1052:
1051:
1031:
1030:
1005:
1004:
984:
983:
958:
957:
939:
938:
913:
912:
894:
893:
868:
867:
849:
848:
797:
796:
777:protein refolding
587:
586:
583:
582:
545:
544:
532:
531:
470:
469:
431:corpus epididymis
368:
367:
267:
266:
94:
93:
90:
89:
50:Ortholog search:
3438:
3404:
3384:
3377:
3370:
3361:
3327:
3298:
3288:
3263:
3253:
3228:
3218:
3193:
3183:
3158:
3148:
3115:
3105:
3080:
3070:
3037:
3000:
2990:
2981:(24): 16537–40.
2965:
2955:
2945:
2912:
2902:
2892:
2859:
2830:
2811:10.1038/371355a0
2785:
2775:
2750:
2740:
2707:
2697:
2664:
2635:
2588:
2587:
2577:
2553:
2547:
2546:
2536:
2518:
2486:
2480:
2479:
2442:
2436:
2435:
2425:
2385:
2379:
2378:
2368:
2328:
2322:
2321:
2303:
2279:
2273:
2272:
2265:
2259:
2258:
2248:
2238:
2229:(44): 31437–46.
2214:
2203:
2202:
2192:
2182:
2158:
2141:
2140:
2130:
2120:
2111:(33): 23086–96.
2096:
2090:
2089:
2079:
2068:10.1172/JCI62385
2047:
2038:
2037:
2001:
1992:
1991:
1963:
1957:
1956:
1946:
1922:
1913:
1912:
1894:
1854:
1848:
1847:
1811:
1790:
1789:
1761:
1750:
1749:
1713:
1702:
1701:
1691:
1667:
1658:
1657:
1647:
1637:
1605:
1599:
1598:
1587:
1581:
1580:
1569:
1563:
1553:
1542:
1532:
1475:Apolipoprotein B
1444:Brucella abortus
1421:Escherichia coli
1100:
1099:
1071:
1064:
1047:
1035:
1026:
1014:
1010:RefSeq (protein)
1000:
988:
979:
967:
943:
924:
898:
879:
853:
834:
803:
767:bone development
610:collagen binding
593:
579:
570:
563:
556:
541:
525:efferent ductule
481:
479:Top expressed in
474:
447:seminal vesicula
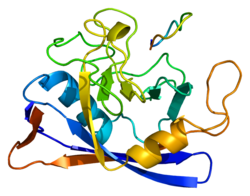
435:caput epididymis
419:
417:Top expressed in
412:
391:
374:
364:
351:
340:
325:
318:
312:
301:
289:
273:
263:
250:
239:
224:
217:
211:
200:
186:
170:
164:
162:PPIB - orthologs
115:
108:
85:
64:
58:
37:
36:
31:
19:
3446:
3445:
3441:
3440:
3439:
3437:
3436:
3435:
3421:
3420:
3419:
3414:
3411:
3405:
3391:
3388:
3335:
3330:
3301:
3273:Mol. Endocrinol
3266:
3231:
3196:
3168:Eur. J. Biochem
3161:
3118:
3096:(10): 6677–84.
3083:
3040:
3003:
2968:
2915:
2862:
2833:
2797:(6495): 355–8.
2788:
2766:(17): 10735–8.
2753:
2717:Mol. Cell. Biol
2710:
2667:
2647:(3–4): 219–21.
2638:
2604:Electrophoresis
2600:
2596:
2594:Further reading
2591:
2555:
2554:
2550:
2501:(3): e0150288.
2488:
2487:
2483:
2444:
2443:
2439:
2387:
2386:
2382:
2330:
2329:
2325:
2281:
2280:
2276:
2267:
2266:
2262:
2216:
2215:
2206:
2173:(6): e1004465.
2160:
2159:
2144:
2098:
2097:
2093:
2049:
2048:
2041:
2012:(7–8): 721–35.
2003:
2002:
1995:
1965:
1964:
1960:
1924:
1923:
1916:
1856:
1855:
1851:
1813:
1812:
1793:
1763:
1762:
1753:
1724:(7–8): 721–35.
1715:
1714:
1705:
1669:
1668:
1661:
1607:
1606:
1602:
1589:
1588:
1584:
1571:
1570:
1566:
1554:
1545:
1533:
1524:
1520:
1464:
1408:
1347:
1339:innate immunity
1251:
1207:
1167:
1147:type I collagen
1109:View/Edit Mouse
1104:View/Edit Human
1067:
1060:
1057:Location (UCSC)
1043:
1022:
996:
975:
888:ENSG00000166794
781:
757:protein folding
720:
654:
630:protein binding
575:
565:
564:
537:
528:
523:
519:
515:
511:
507:
503:
499:
495:
491:
477:
466:
461:
457:
453:
449:
445:
441:
437:
433:
429:
415:
359:
346:
338:
328:
327:
326:
319:
299:
276:Gene location (
258:
245:
237:
227:
226:
225:
218:
196:
173:Gene location (
124:
111:
104:
73:
51:
17:
12:
11:
5:
3444:
3442:
3434:
3433:
3423:
3422:
3416:
3415:
3413:
3412:
3406:
3399:
3396:
3393:
3392:
3389:
3387:
3386:
3379:
3372:
3364:
3358:
3357:
3334:
3333:External links
3331:
3329:
3328:
3299:
3279:(8): 1175–86.
3264:
3229:
3209:(9): 5326–32.
3194:
3159:
3116:
3081:
3053:(3): 2107–13.
3038:
3012:(6): 1067–78.
3001:
2966:
2928:(11): 5183–6.
2913:
2860:
2831:
2786:
2751:
2723:(7): 3484–91.
2708:
2665:
2636:
2597:
2595:
2592:
2590:
2589:
2568:(9): 7459–68.
2548:
2481:
2437:
2400:(2): 521–530.
2380:
2323:
2274:
2260:
2204:
2142:
2091:
2062:(5): 1734–41.
2039:
1993:
1958:
1914:
1869:(3): 423–436.
1849:
1791:
1751:
1703:
1659:
1600:
1582:
1564:
1543:
1521:
1519:
1516:
1515:
1514:
1508:
1502:
1496:
1490:
1484:
1478:
1463:
1460:
1407:
1406:Bacterial PPIB
1404:
1346:
1343:
1250:
1247:
1206:
1203:
1166:
1163:
1159:archaebacteria
1116:
1115:
1112:
1111:
1106:
1096:
1095:
1089:
1088:
1085:
1084:
1082:
1080:
1073:
1072:
1065:
1058:
1054:
1053:
1050:
1049:
1039:
1038:
1032:
1029:
1028:
1018:
1017:
1011:
1007:
1006:
1003:
1002:
992:
991:
985:
982:
981:
971:
970:
964:
960:
959:
956:
955:
947:
946:
940:
937:
936:
928:
927:
921:
915:
914:
911:
910:
902:
901:
895:
892:
891:
883:
882:
876:
870:
869:
866:
865:
857:
856:
850:
847:
846:
838:
837:
831:
825:
824:
819:
814:
810:
809:
799:
798:
795:
794:
783:
782:
780:
779:
774:
769:
764:
759:
754:
749:
744:
739:
734:
728:
726:
722:
721:
719:
718:
713:
708:
703:
698:
693:
688:
683:
681:focal adhesion
678:
673:
668:
662:
660:
656:
655:
653:
652:
647:
642:
637:
632:
627:
622:
617:
612:
606:
604:
600:
599:
589:
588:
585:
584:
581:
580:
572:
571:
553:
547:
546:
543:
542:
534:
533:
530:
529:
527:
526:
522:
518:
514:
510:
506:
502:
498:
494:
490:
486:
483:
482:
471:
468:
467:
465:
464:
460:
456:
452:
448:
444:
440:
436:
432:
428:
424:
421:
420:
408:
407:
399:
388:
382:
381:
378:RNA expression
370:
369:
366:
365:
357:
353:
352:
344:
341:
336:
330:
329:
320:
313:
307:
303:
302:
297:
291:
290:
282:
281:
269:
268:
265:
264:
256:
252:
251:
243:
240:
235:
229:
228:
219:
212:
206:
202:
201:
194:
188:
187:
179:
178:
166:
165:
122:
118:
117:
109:
101:
100:
96:
95:
92:
91:
88:
87:
69:
68:
60:
59:
48:
42:
41:
33:
32:
24:
23:
15:
13:
10:
9:
6:
4:
3:
2:
3443:
3432:
3429:
3428:
3426:
3409:
3403:
3398:
3394:
3385:
3380:
3378:
3373:
3371:
3366:
3365:
3362:
3355:
3351:
3350:
3345:
3341:
3337:
3336:
3332:
3325:
3321:
3317:
3313:
3309:
3305:
3300:
3296:
3292:
3287:
3282:
3278:
3274:
3270:
3265:
3261:
3257:
3252:
3247:
3243:
3239:
3235:
3230:
3226:
3222:
3217:
3212:
3208:
3204:
3203:J. Biol. Chem
3200:
3195:
3191:
3187:
3182:
3177:
3173:
3169:
3165:
3160:
3156:
3152:
3147:
3142:
3138:
3134:
3131:(4): 609–17.
3130:
3126:
3122:
3117:
3113:
3109:
3104:
3099:
3095:
3091:
3090:J. Biol. Chem
3087:
3082:
3078:
3074:
3069:
3064:
3060:
3056:
3052:
3048:
3044:
3039:
3035:
3031:
3027:
3023:
3019:
3015:
3011:
3007:
3002:
2998:
2994:
2989:
2984:
2980:
2976:
2975:J. Biol. Chem
2972:
2967:
2963:
2959:
2954:
2949:
2944:
2939:
2935:
2931:
2927:
2923:
2919:
2914:
2910:
2906:
2901:
2896:
2891:
2886:
2882:
2878:
2875:(9): 3931–5.
2874:
2870:
2866:
2861:
2857:
2853:
2849:
2845:
2842:(1): 113–20.
2841:
2837:
2832:
2828:
2824:
2820:
2816:
2812:
2808:
2804:
2800:
2796:
2792:
2787:
2783:
2779:
2774:
2769:
2765:
2761:
2760:J. Biol. Chem
2757:
2752:
2748:
2744:
2739:
2734:
2730:
2726:
2722:
2718:
2714:
2709:
2705:
2701:
2696:
2691:
2687:
2683:
2680:(1): 113–25.
2679:
2675:
2671:
2666:
2662:
2658:
2654:
2650:
2646:
2642:
2637:
2633:
2629:
2625:
2621:
2617:
2613:
2610:(12): 960–9.
2609:
2605:
2599:
2598:
2593:
2585:
2581:
2576:
2571:
2567:
2563:
2562:J. Biol. Chem
2559:
2552:
2549:
2544:
2540:
2535:
2530:
2526:
2522:
2517:
2512:
2508:
2504:
2500:
2496:
2492:
2485:
2482:
2477:
2473:
2469:
2465:
2461:
2457:
2453:
2449:
2441:
2438:
2433:
2429:
2424:
2419:
2415:
2411:
2407:
2403:
2399:
2395:
2391:
2384:
2381:
2376:
2372:
2367:
2362:
2358:
2354:
2350:
2346:
2342:
2338:
2334:
2327:
2324:
2319:
2315:
2311:
2307:
2302:
2297:
2293:
2289:
2285:
2278:
2275:
2270:
2264:
2261:
2256:
2252:
2247:
2242:
2237:
2232:
2228:
2224:
2220:
2213:
2211:
2209:
2205:
2200:
2196:
2191:
2186:
2181:
2176:
2172:
2168:
2167:PLOS Genetics
2164:
2157:
2155:
2153:
2151:
2149:
2147:
2143:
2138:
2134:
2129:
2124:
2119:
2114:
2110:
2106:
2102:
2095:
2092:
2087:
2083:
2078:
2073:
2069:
2065:
2061:
2057:
2053:
2046:
2044:
2040:
2035:
2031:
2027:
2023:
2019:
2015:
2011:
2007:
2000:
1998:
1994:
1989:
1985:
1981:
1977:
1973:
1969:
1962:
1959:
1954:
1950:
1945:
1940:
1937:(1): 109–14.
1936:
1932:
1928:
1921:
1919:
1915:
1910:
1906:
1902:
1898:
1893:
1888:
1884:
1880:
1876:
1872:
1868:
1864:
1860:
1853:
1850:
1845:
1841:
1837:
1833:
1829:
1825:
1822:(3): 276–80.
1821:
1817:
1810:
1808:
1806:
1804:
1802:
1800:
1798:
1796:
1792:
1787:
1783:
1779:
1775:
1771:
1767:
1760:
1758:
1756:
1752:
1747:
1743:
1739:
1735:
1731:
1727:
1723:
1719:
1712:
1710:
1708:
1704:
1699:
1695:
1690:
1685:
1682:(1): 109–14.
1681:
1677:
1673:
1666:
1664:
1660:
1655:
1651:
1646:
1641:
1636:
1631:
1627:
1623:
1620:(5): 1903–7.
1619:
1615:
1611:
1604:
1601:
1596:
1592:
1586:
1583:
1578:
1574:
1568:
1565:
1561:
1557:
1552:
1550:
1548:
1544:
1540:
1536:
1531:
1529:
1527:
1523:
1517:
1512:
1509:
1506:
1503:
1500:
1497:
1494:
1491:
1488:
1485:
1482:
1479:
1476:
1473:
1472:
1471:
1469:
1461:
1459:
1457:
1456:
1451:
1450:
1445:
1441:
1437:
1436:
1432:activity. In
1431:
1428:activity and
1427:
1423:
1422:
1417:
1413:
1405:
1403:
1401:
1397:
1393:
1389:
1386:
1382:
1378:
1374:
1370:
1365:
1363:
1359:
1354:
1352:
1344:
1342:
1340:
1336:
1332:
1328:
1322:
1318:
1316:
1312:
1308:
1304:
1300:
1296:
1292:
1291:mitochondrial
1288:
1284:
1280:
1276:
1272:
1268:
1264:
1260:
1256:
1248:
1246:
1244:
1243:cyclosporin A
1240:
1236:
1232:
1228:
1224:
1220:
1216:
1212:
1204:
1202:
1200:
1196:
1192:
1188:
1184:
1180:
1176:
1172:
1164:
1162:
1160:
1156:
1152:
1148:
1144:
1143:peptide bonds
1140:
1139:isomerization
1137:
1133:
1130:
1126:
1122:
1110:
1105:
1101:
1097:
1094:
1090:
1083:
1081:
1078:
1074:
1070:
1066:
1063:
1059:
1055:
1048:
1046:
1040:
1036:
1033:
1027:
1025:
1019:
1015:
1012:
1008:
1001:
999:
993:
989:
986:
980:
978:
972:
968:
965:
963:RefSeq (mRNA)
961:
954:
953:
948:
944:
941:
935:
934:
929:
925:
922:
920:
916:
909:
908:
903:
899:
896:
890:
889:
884:
880:
877:
875:
871:
864:
863:
858:
854:
851:
845:
844:
839:
835:
832:
830:
826:
823:
820:
818:
815:
811:
808:
804:
800:
793:
789:
784:
778:
775:
773:
770:
768:
765:
763:
760:
758:
755:
753:
750:
748:
745:
743:
740:
738:
735:
733:
730:
729:
727:
724:
723:
717:
714:
712:
709:
707:
704:
702:
699:
697:
694:
692:
689:
687:
684:
682:
679:
677:
674:
672:
669:
667:
664:
663:
661:
658:
657:
651:
648:
646:
643:
641:
638:
636:
633:
631:
628:
626:
623:
621:
618:
616:
613:
611:
608:
607:
605:
602:
601:
598:
597:Gene ontology
594:
590:
578:
573:
569:
562:
557:
554:
552:
548:
540:
535:
524:
520:
516:
512:
508:
504:
500:
496:
492:
488:
487:
484:
480:
475:
472:
462:
458:
455:parotid gland
454:
450:
446:
442:
438:
434:
430:
426:
425:
422:
418:
413:
410:
409:
406:
404:
400:
398:
397:
393:
392:
389:
387:
383:
379:
375:
371:
363:
358:
354:
350:
345:
335:
331:
324:
317:
311:
304:
296:
292:
288:
283:
279:
274:
270:
262:
257:
253:
249:
244:
234:
230:
223:
216:
210:
203:
199:
193:
189:
185:
180:
176:
171:
167:
163:
159:
155:
151:
147:
143:
139:
135:
131:
127:
119:
114:
107:
102:
97:
86:
84:
80:
76:
70:
65:
62:
61:
57:
54:
47:
43:
38:
34:
30:
25:
20:
3407:
3347:
3310:(4): 786–8.
3307:
3303:
3276:
3272:
3244:(4): 334–6.
3241:
3237:
3206:
3202:
3174:(3): 441–6.
3171:
3167:
3128:
3124:
3093:
3089:
3050:
3046:
3009:
3005:
2978:
2974:
2925:
2921:
2872:
2868:
2839:
2835:
2794:
2790:
2763:
2759:
2720:
2716:
2677:
2674:J. Cell Biol
2673:
2644:
2640:
2607:
2603:
2565:
2561:
2551:
2498:
2494:
2484:
2451:
2448:Biochemistry
2447:
2440:
2397:
2393:
2383:
2340:
2336:
2326:
2294:(1): 32–44.
2291:
2287:
2277:
2263:
2226:
2222:
2170:
2166:
2108:
2104:
2094:
2059:
2055:
2009:
2005:
1974:(3): 845–9.
1971:
1967:
1961:
1934:
1930:
1866:
1862:
1852:
1819:
1815:
1772:(3): 845–9.
1769:
1765:
1721:
1717:
1679:
1675:
1617:
1613:
1603:
1594:
1585:
1576:
1567:
1511:calreticulin
1465:
1462:Interactions
1453:
1447:
1443:
1433:
1419:
1409:
1366:
1355:
1348:
1323:
1319:
1299:inflammation
1293:metabolism,
1271:inflammation
1252:
1226:
1222:
1218:
1208:
1193:, while its
1171:cyclophilins
1168:
1128:
1120:
1119:
1042:
1021:
995:
974:
950:
931:
905:
886:
860:
841:
821:
816:
505:vas deferens
401:
394:
121:External IDs
72:
3390:PDB gallery
1400:lung cancer
1381:influenza A
1373:hepatitis C
1351:calcineurin
1303:cell growth
1231:cyclophilin
1175:hydrophobic
1169:Like other
640:RNA binding
517:Paneth cell
463:pericardium
360:65,973,905
347:65,967,504
259:64,163,134
246:64,155,740
99:Identifiers
3125:Immunology
1562:, May 2017
1541:, May 2017
1518:References
1327:chemotaxis
1259:metabolism
1249:Human PPIB
1199:substrates
1195:N-terminal
1187:C-terminal
1155:eukaryotes
1151:eubacteria
686:melanosome
405:(ortholog)
339:9|9 C
142:HomoloGene
2525:1932-6203
2468:0006-2960
2414:0019-9567
2357:1098-5530
2310:1365-2443
1883:1420-682X
1385:antigenic
1295:apoptosis
1287:chaperone
1263:apoptosis
1191:organelle
1183:α-helices
1179:β-strands
1165:Structure
1136:cis-trans
1045:NP_035279
1024:NP_000933
998:NM_011149
977:NM_000942
807:Orthologs
150:GeneCards
3425:Category
3324:11688976
3295:10935542
3260:10096576
3225:10026140
3047:J. Virol
3034:38546328
2632:41855774
2584:12397072
2543:26981873
2495:PLOS ONE
2432:23230297
2375:27795319
2318:27868330
2255:24043621
2199:24968150
2137:24990953
2086:22484812
2034:32395688
2026:24713575
1988:15963461
1909:24868224
1901:10228556
1892:11146858
1844:11678319
1836:15706440
1786:15963461
1746:32395688
1738:24713575
1558:–
1537:–
1468:interact
1396:vaccines
1388:epitopes
1331:integrin
1239:parvulin
1205:Function
1093:Wikidata
786:Sources:
676:membrane
238:15q22.31
3354:PDBe-KB
3344:UniProt
3190:9546659
3155:9378502
3146:1363883
3112:9045699
3077:9032343
3026:8513493
2997:8206968
2962:8197205
2930:Bibcode
2909:7909608
2877:Bibcode
2856:7829860
2827:4318545
2819:7522304
2799:Bibcode
2782:2040592
2747:1710767
2704:1530944
2695:2289259
2661:1505219
2624:1286667
2534:4794191
2503:Bibcode
2476:9748346
2423:3553818
2366:5165095
2246:3814740
2190:4072593
2128:4132807
2077:3336995
1953:1530810
1698:1530810
1654:2000394
1622:Bibcode
1560:Ensembl
1539:Ensembl
1377:measles
1358:hypoxia
1237:), and
1227:in vivo
919:UniProt
874:Ensembl
813:Species
792:QuickGO
716:nucleus
521:utricle
509:condyle
380:pattern
106:Aliases
3349:P23284
3322:
3293:
3258:
3223:
3188:
3153:
3143:
3110:
3075:
3068:191305
3065:
3032:
3024:
2995:
2960:
2950:
2907:
2897:
2854:
2825:
2817:
2791:Nature
2780:
2745:
2738:361082
2735:
2702:
2692:
2659:
2630:
2622:
2582:
2541:
2531:
2523:
2474:
2466:
2430:
2420:
2412:
2373:
2363:
2355:
2316:
2308:
2253:
2243:
2197:
2187:
2135:
2125:
2084:
2074:
2032:
2024:
1986:
1951:
1907:
1899:
1889:
1881:
1842:
1834:
1784:
1744:
1736:
1696:
1652:
1642:
1470:with:
1426:PPIase
1379:, and
1301:, and
1283:cancer
1281:, and
1269:, and
1211:PPIase
1125:enzyme
1123:is an
1079:search
1077:PubMed
952:P24369
933:P23284
829:Entrez
551:BioGPS
130:123841
3030:S2CID
2953:43956
2900:43696
2823:S2CID
2628:S2CID
2343:(1).
2030:S2CID
1905:S2CID
1840:S2CID
1742:S2CID
1645:51134
1507:, and
1499:GRP94
1487:CRTAP
1315:redox
1267:redox
1223:trans
862:19035
822:Mouse
817:Human
788:Amigo
501:fossa
403:Mouse
396:Human
343:Start
278:Mouse
242:Start
175:Human
138:97750
3408:1cyn
3342:for
3320:PMID
3291:PMID
3256:PMID
3221:PMID
3186:PMID
3151:PMID
3108:PMID
3073:PMID
3022:PMID
3006:Cell
2993:PMID
2958:PMID
2905:PMID
2852:PMID
2815:PMID
2778:PMID
2743:PMID
2700:PMID
2657:PMID
2620:PMID
2580:PMID
2539:PMID
2521:ISSN
2472:PMID
2464:ISSN
2428:PMID
2410:ISSN
2371:PMID
2353:ISSN
2314:PMID
2306:ISSN
2251:PMID
2195:PMID
2133:PMID
2082:PMID
2022:PMID
1984:PMID
1949:PMID
1897:PMID
1879:ISSN
1832:PMID
1782:PMID
1734:PMID
1694:PMID
1650:PMID
1481:P3H1
1414:and
1369:AIDS
1329:and
1307:PPIC
1279:AIDS
1235:FKBP
1221:and
1153:and
1132:gene
1129:PPIB
843:5479
386:Bgee
334:Band
295:Chr.
233:Band
192:Chr.
154:PPIB
126:OMIM
113:PPIB
83:3ICI
79:3ICH
75:1CYN
56:RCSB
53:PDBe
22:PPIB
3340:PDB
3312:doi
3308:288
3281:doi
3246:doi
3211:doi
3207:274
3176:doi
3172:252
3141:PMC
3133:doi
3098:doi
3094:272
3063:PMC
3055:doi
3014:doi
2983:doi
2979:269
2948:PMC
2938:doi
2895:PMC
2885:doi
2844:doi
2840:178
2807:doi
2795:371
2768:doi
2764:266
2733:PMC
2725:doi
2690:PMC
2682:doi
2678:116
2649:doi
2612:doi
2570:doi
2566:278
2529:PMC
2511:doi
2456:doi
2418:PMC
2402:doi
2361:PMC
2345:doi
2341:199
2296:doi
2241:PMC
2231:doi
2227:288
2185:PMC
2175:doi
2123:PMC
2113:doi
2109:289
2072:PMC
2064:doi
2060:122
2014:doi
2010:395
1976:doi
1972:333
1939:doi
1887:PMC
1871:doi
1824:doi
1774:doi
1770:333
1726:doi
1722:395
1684:doi
1640:PMC
1630:doi
1505:PDI
1493:BiP
1219:cis
356:End
255:End
158:OMA
146:726
134:MGI
46:PDB
3427::
3346::
3318:.
3306:.
3289:.
3277:14
3275:.
3271:.
3254:.
3242:20
3240:.
3236:.
3219:.
3205:.
3201:.
3184:.
3170:.
3166:.
3149:.
3139:.
3129:91
3127:.
3123:.
3106:.
3092:.
3088:.
3071:.
3061:.
3051:71
3049:.
3045:.
3028:.
3020:.
3010:73
3008:.
2991:.
2977:.
2973:.
2956:.
2946:.
2936:.
2926:91
2924:.
2920:.
2903:.
2893:.
2883:.
2873:91
2871:.
2867:.
2850:.
2838:.
2821:.
2813:.
2805:.
2793:.
2776:.
2762:.
2758:.
2741:.
2731:.
2721:11
2719:.
2715:.
2698:.
2688:.
2676:.
2672:.
2655:.
2645:60
2643:.
2626:.
2618:.
2608:13
2606:.
2578:.
2564:.
2560:.
2537:.
2527:.
2519:.
2509:.
2499:11
2497:.
2493:.
2470:.
2462:.
2452:37
2450:.
2426:.
2416:.
2408:.
2398:81
2396:.
2392:.
2369:.
2359:.
2351:.
2339:.
2335:.
2312:.
2304:.
2292:22
2290:.
2286:.
2249:.
2239:.
2225:.
2221:.
2207:^
2193:.
2183:.
2171:10
2169:.
2165:.
2145:^
2131:.
2121:.
2107:.
2103:.
2080:.
2070:.
2058:.
2054:.
2042:^
2028:.
2020:.
2008:.
1996:^
1982:.
1970:.
1947:.
1935:53
1933:.
1929:.
1917:^
1903:.
1895:.
1885:.
1877:.
1867:55
1865:.
1861:.
1838:.
1830:.
1820:29
1818:.
1794:^
1780:.
1768:.
1754:^
1740:.
1732:.
1720:.
1706:^
1692:.
1680:53
1678:.
1674:.
1662:^
1648:.
1638:.
1628:.
1618:88
1616:.
1612:.
1593:.
1575:.
1546:^
1525:^
1452:,
1446:,
1375:,
1371:,
1277:,
1265:,
1261:,
1201:.
790:/
362:bp
349:bp
261:bp
248:bp
156:;
152::
148:;
144::
140:;
136::
132:;
128::
81:,
77:,
3383:e
3376:t
3369:v
3356:.
3326:.
3314::
3297:.
3283::
3262:.
3248::
3227:.
3213::
3192:.
3178::
3157:.
3135::
3114:.
3100::
3079:.
3057::
3036:.
3016::
2999:.
2985::
2964:.
2940::
2932::
2911:.
2887::
2879::
2858:.
2846::
2829:.
2809::
2801::
2784:.
2770::
2749:.
2727::
2706:.
2684::
2663:.
2651::
2634:.
2614::
2586:.
2572::
2545:.
2513::
2505::
2478:.
2458::
2434:.
2404::
2377:.
2347::
2320:.
2298::
2271:.
2257:.
2233::
2201:.
2177::
2139:.
2115::
2088:.
2066::
2036:.
2016::
1990:.
1978::
1955:.
1941::
1911:.
1873::
1846:.
1826::
1788:.
1776::
1748:.
1728::
1700:.
1686::
1656:.
1632::
1624::
1597:.
1579:.
1513:.
1501:,
1495:,
1489:,
1483:,
1477:.
280:)
177:)
160::
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.