26:
373:
518:
In realgar's crystal structure, each arsenic atom is bonded to two sulphur atoms and one other arsenic atom. The As-As bonds are 30% weaker than the As-S bonds and certain wavelengths of lights interact with the crystal structure of realgar, breaking the weaker bonds between arsenic atoms. The free
514:
Pararealgar is an alteration product of realgar resulting from exposure to light. The process of alteration is dependent on the wavelength of light, with alteration only occurring at wavelengths longer than approximately 500 nm, in the visible light spectrum.
519:
As formed as a result of this process destabilises the realgar structure, causing the realgar to become powdery pararealgar without changing overall chemical composition.
353:. It forms upon exposure of the symmetrical isomer to light. Its name derives from the fact that its elemental composition is identical to realgar,
729:
637:
Paola
Bonazzi, Silvio Menchetti, Giovanni Pratesi "The crystal structure of pararealgar, As4S4" American Mineralogist, 1995, vol.80 400.
79:
498:
It was first described in 1980 for an occurrence in the Grey Rock Mine, Truax Creek, Bridge River area, Lillooet Mining
Division,
647:
Jason, M. E.; Ngo, T.; Rahman, S. (1997). "Products and
Mechanisms in the Oxidation of Phosphorus by Sulfur at Low Temperature".
419:), which is polymeric. In pararealgar, there are three kinds of As centres (and three kinds of S centres). The molecule has C
195:
228:
724:
583:
719:
714:
309:
under exposure to light. Its name derives from the fact that its elemental composition is identical to realgar,
69:
185:
175:
548:
218:
208:
25:
566:
431:
472:
136:
657:
556:
499:
328:
238:
46:
626:
291:
463:
typically as a result of exposure to light. It occurs associated with realgar, stibnite,
552:
460:
423:
89:
708:
570:
488:
484:
321:
256:
165:
101:
426:. In realgar, the four As (and four S) centres are equivalent and the molecule has D
676:
649:
468:
464:
430:
symmetry. An analogous pair of isomers is also recognized for the corresponding
266:
119:
610:
480:
325:
110:
94:
160:
Bright yellow when powdery, to yellow-orange and orange-brown when granular
453:
405:
561:
536:
476:
335:
306:
288:
661:
599:
372:
503:
492:
457:
339:
506:. It has since been reported from a variety of locations worldwide.
371:
404:
are molecular, in contrast to the other main sulfide of arsenic,
452:
Pararealgar occurs as an alteration product of realgar in
271:
High: x = orange yellow, y = bright yellow, z = orange red
677:"The light-induced alteration of realgar to pararealgar"
331:
crystals are very brittle, easily crumbling to powder.
675:Douglass, D. L.; Shing, Chichang; Wang, Ge (1992).
305:, also represented as AsS. It forms gradually from
275:
265:
255:
247:
237:
227:
217:
207:
194:
184:
174:
164:
156:
151:
135:
118:
100:
88:
78:
68:
45:
37:
32:
18:
324:of 1 - 1.5, is yellow orange in colour, and its
611:Webmineral.com Mineralogy Database: Pararealgar
8:
24:
560:
595:
593:
591:
440:
436:
415:
411:
400:
396:
383:
379:
360:
356:
349:
345:
316:
312:
301:
297:
61:
57:
527:
15:
622:
620:
618:
7:
537:"IMA–CNMNC approved mineral symbols"
14:
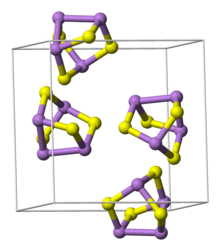
389:realgar (left) and pararealgar.
1:
338:of arsenic and is one of two
147:β = 97.20°; Z = 16
746:
730:Minerals in space group 14
294:with the chemical formula
23:
170:Fine powder to granular
627:Handbook of Mineralogy
541:Mineralogical Magazine
390:
684:American Mineralogist
375:
80:Strunz classification
320:. It is soft with a
213:Vitreous to resinous
725:Monoclinic minerals
562:10.1180/mgm.2021.43
553:2021MinM...85..291W
535:Warr, L.N. (2021).
432:phosphorus sulfides
376:The two isomers of
391:
248:Optical properties
145:c = 8.502 Å;
143:b = 9.655 Å,
141:a = 9.909 Å,
662:10.1021/ic9614879
656:(12): 2633–2640.
334:It is one of the
282:
281:
114:
737:
720:Sulfide minerals
715:Arsenic minerals
700:
699:
697:
695:
681:
672:
666:
665:
644:
638:
635:
629:
624:
613:
608:
602:
597:
586:
581:
575:
574:
564:
532:
500:British Columbia
443:
418:
403:
393:Both isomers of
386:
363:
352:
319:
304:
239:Specific gravity
200:
108:
106:Prismatic (2/m)
64:
52:
51:(repeating unit)
28:
16:
745:
744:
740:
739:
738:
736:
735:
734:
705:
704:
703:
693:
691:
679:
674:
673:
669:
646:
645:
641:
636:
632:
625:
616:
609:
605:
598:
589:
584:mineralienatlas
582:
578:
534:
533:
529:
525:
512:
450:
442:
438:
434:
429:
422:
417:
413:
409:
402:
398:
394:
388:
385:
381:
377:
370:
362:
358:
354:
351:
347:
343:
318:
314:
310:
303:
299:
295:
292:sulfide mineral
198:
146:
144:
142:
130:
107:
63:
59:
55:
50:
49:
41:Sulfide mineral
12:
11:
5:
743:
741:
733:
732:
727:
722:
717:
707:
706:
702:
701:
667:
639:
630:
614:
603:
587:
576:
547:(3): 291–320.
526:
524:
521:
511:
508:
449:
446:
427:
420:
369:
366:
280:
279:
277:
273:
272:
269:
263:
262:
259:
253:
252:
249:
245:
244:
241:
235:
234:
231:
225:
224:
221:
215:
214:
211:
205:
204:
201:
192:
191:
188:
182:
181:
178:
172:
171:
168:
162:
161:
158:
154:
153:
152:Identification
149:
148:
139:
133:
132:
128:
122:
116:
115:
104:
98:
97:
92:
90:Crystal system
86:
85:
82:
76:
75:
72:
66:
65:
53:
43:
42:
39:
35:
34:
30:
29:
21:
20:
13:
10:
9:
6:
4:
3:
2:
742:
731:
728:
726:
723:
721:
718:
716:
713:
712:
710:
689:
685:
678:
671:
668:
663:
659:
655:
652:
651:
643:
640:
634:
631:
628:
623:
621:
619:
615:
612:
607:
604:
601:
596:
594:
592:
588:
585:
580:
577:
572:
568:
563:
558:
554:
550:
546:
542:
538:
531:
528:
522:
520:
516:
509:
507:
505:
501:
496:
494:
490:
489:lepidocrocite
486:
485:native sulfur
482:
478:
474:
470:
466:
462:
459:
455:
447:
445:
433:
425:
407:
374:
367:
365:
341:
337:
332:
330:
327:
323:
322:Mohs hardness
308:
293:
290:
286:
278:
274:
270:
268:
264:
260:
258:
257:Birefringence
254:
250:
246:
242:
240:
236:
232:
230:
226:
223:Bright yellow
222:
220:
216:
212:
210:
206:
202:
197:
193:
189:
187:
183:
179:
177:
173:
169:
167:
166:Crystal habit
163:
159:
155:
150:
140:
138:
134:
126:
123:
121:
117:
112:
105:
103:
102:Crystal class
99:
96:
93:
91:
87:
83:
81:
77:
73:
71:
67:
54:
48:
44:
40:
36:
31:
27:
22:
17:
692:. Retrieved
687:
683:
670:
653:
650:Inorg. Chem.
648:
642:
633:
606:
579:
544:
540:
530:
517:
513:
497:
469:arsenopyrite
465:tetrahedrite
451:
392:
333:
284:
283:
203:1 – 1.5
124:
690:: 1266–1274
285:Pararealgar
267:Pleochroism
251:Biaxial (?)
233:Translucent
229:Diaphaneity
120:Space group
19:Pararealgar
709:Categories
600:Mindat.org
523:References
481:arsenolite
473:duranusite
448:Occurrence
326:monoclinic
276:References
196:Mohs scale
111:H-M symbol
95:Monoclinic
70:IMA symbol
694:11 August
571:235729616
510:Formation
475:, native
456:-bearing
368:Structure
329:prismatic
137:Unit cell
454:stibnite
424:symmetry
406:orpiment
336:sulfides
199:hardness
186:Tenacity
176:Fracture
84:2.FA.15b
38:Category
549:Bibcode
477:arsenic
340:isomers
307:realgar
289:arsenic
190:Brittle
47:Formula
33:General
569:
504:Canada
493:pyrite
458:quartz
287:is an
219:Streak
209:Lustre
180:Uneven
157:Colour
109:(same
680:(PDF)
567:S2CID
461:veins
696:2014
491:and
261:2.02
243:3.52
74:Prlg
658:doi
557:doi
342:of
711::
688:77
686:.
682:.
654:36
617:^
590:^
565:.
555:.
545:85
543:.
539:.
502:,
495:.
487:,
483:,
479:,
471:,
467:,
444:.
428:2d
410:As
395:As
378:As
364:.
355:As
344:As
311:As
296:As
131:/c
56:As
698:.
664:.
660::
573:.
559::
551::
441:4
439:S
437:4
435:P
421:s
416:3
414:S
412:2
408:(
401:4
399:S
397:4
387::
384:4
382:S
380:4
361:4
359:S
357:4
350:4
348:S
346:4
317:4
315:S
313:4
302:4
300:S
298:4
129:1
127:2
125:P
113:)
62:4
60:S
58:4
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.