31:
488:
1544:
94:
different concentrations of oxygen present in various inhaled breathing gases or dissolved in blood; consequently, mixture ratios, like that of breathable 20% oxygen and 80% Nitrogen, are determined by volume instead of by weight or mass. Furthermore, the partial pressures of oxygen and carbon dioxide are important parameters in tests of
93:
in gas mixtures or liquids. This general property of gases is also true in chemical reactions of gases in biology. For example, the necessary amount of oxygen for human respiration, and the amount that is toxic, is set by the partial pressure of oxygen alone. This is true across a very wide range of
3123:
Diving Manual recommends a maximum single exposure of 45 minutes at 1.6 bar absolute, of 120 minutes at 1.5 bar absolute, of 150 minutes at 1.4 bar absolute, of 180 minutes at 1.3 bar absolute and of 210 minutes at 1.2 bar absolute. Oxygen toxicity becomes a risk when these oxygen partial pressures
495:
Dalton's law expresses the fact that the total pressure of a mixture of ideal gases is equal to the sum of the partial pressures of the individual gases in the mixture. This equality arises from the fact that in an ideal gas, the molecules are so far apart that they do not interact with each other.
1468:
1249:
1596:
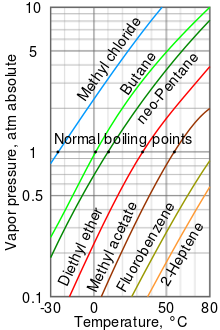
has the highest vapor pressure of any of the liquids in the chart. It also has the lowest normal boiling point (−24.2 °C), which is where the vapor pressure curve of methyl chloride (the blue line) intersects the horizontal pressure line of one atmosphere
1733:
898:
1837:
1588:
The vapor pressure chart displayed has graphs of the vapor pressures versus temperatures for a variety of liquids. As can be seen in the chart, the liquids with the highest vapor pressures have the lowest normal boiling points.
1328:
1129:
3149:
in breathing gas is also related to the partial pressure when breathed. A mixture which may be relatively safe at the surface could be dangerously toxic at the maximum depth of a dive, or a tolerable level of
615:
1621:
for a chemical reaction involving a mixture of gases given the partial pressure of each gas and the overall reaction formula. For a reversible reaction involving gas reactants and gas products, such as:
950:
1322:
The partial volume of a particular gas in a mixture is the volume of one component of the gas mixture. It is useful in gas mixtures, e.g. air, to focus on one particular gas component, e.g. oxygen.
2705:
2805:. Since both may be referred to as the Henry's law constant, readers of the technical literature must be quite careful to note which version of the Henry's law equation is being used.
1625:
2464:
1578:. A liquid's atmospheric pressure boiling point corresponds to the temperature at which its vapor pressure is equal to the surrounding atmospheric pressure and it is often called the
3975:
3480:
3329:
3239:
3820:
3400:
3290:
3200:
3103:
3056:
830:
459:
413:
354:
308:
3009:
2941:
1061:
1026:
991:
814:
769:
724:
679:
260:
221:
1740:
3690:
2325:
2248:
2171:
2094:
2783:
2746:
2589:
2536:
1876:
1601:) of absolute vapor pressure. At higher altitudes, the atmospheric pressure is less than that at sea level, so boiling points of liquids are reduced. At the top of
180:
153:
2969:
2803:
2645:
2609:
2556:
2503:
2367:
2347:
2290:
2270:
2213:
2193:
2136:
2116:
2059:
2037:
2014:
1992:
1969:
1947:
1924:
1902:
1113:
1087:
638:
122:
which may use a subscript to identify the pressure, and gas species are also referred to by subscript. When combined, these subscripts are applied recursively.
3158:
may become intolerable within seconds during descent when the partial pressure rapidly increases, and could lead to panic or incapacitation of the diver.
3755:
525:
3877:(2nd ed.). Silver Spring, Maryland: US Department of Commerce: National Oceanic and Atmospheric Administration, Office of Ocean Engineering.
3839:
3663:
3957:
905:
3897:
3765:
3606:
1463:{\displaystyle V_{\rm {X}}=V_{\rm {tot}}\times {\frac {p_{\rm {X}}}{p_{\rm {tot}}}}=V_{\rm {tot}}\times {\frac {n_{\rm {X}}}{n_{\rm {tot}}}}}
3945:
from The
University of Texas Southwestern Medical Center at Dallas. Used in Interactive Case Study Companion to Pathologic basis of disease.
3111:
The minimum safe lower limit for the partial pressures of oxygen in a breathing gas mixture for diving is 0.16 bars (16 kPa) absolute.
3134:
is a problem when breathing gases at high pressure. Typically, the maximum total partial pressure of narcotic gases used when planning for
3972:
3998:
3817:
2408:
to an extent that is determined by the equilibrium between the undissolved gas and the gas that has dissolved in the liquid (called the
4028:
3982:
at the
Department of Pathology and Laboratory Medicine at the University of British Columbia. By G.P. Bondy. Retrieved November 2011
2386:
may either oppose or enhance the equilibrium shift. In some cases, the reaction kinetics may be the overriding factor to consider.
2808:
Henry's law is an approximation that only applies for dilute, ideal solutions and for solutions where the liquid solvent does not
3443:
2658:
2422:
1244:{\displaystyle {\frac {V_{\rm {X}}}{V_{\rm {tot}}}}={\frac {p_{\rm {X}}}{p_{\rm {tot}}}}={\frac {n_{\rm {X}}}{n_{\rm {tot}}}}}
1123:
The mole fraction of a gas component in a gas mixture is equal to the volumetric fraction of that component in a gas mixture.
4003:
2858:
For example, at 50 metres (164 ft) underwater, the total absolute pressure is 6 bar (600 kPa) (i.e., 1 bar of
3938:
4018:
3622:
2379:
1585:
The higher the vapor pressure of a liquid at a given temperature, the lower the normal boiling point of the liquid.
3115:
and sudden unconsciousness can become a problem with an oxygen partial pressure of less than 0.16 bar absolute.
3139:
2374:
For reversible reactions, changes in the total pressure, temperature or reactant concentrations will shift the
902:
and the partial pressure of an individual gas component in an ideal gas can be obtained using this expression:
3888:
Sawatzky, David (August 2008). "3: Oxygen and its affect on the diver". In Mount, Tom; Dituri, Joseph (eds.).
3366:
3357:
3125:
1558:
in equilibrium with its non-vapor phases (i.e., liquid or solid). Most often the term is used to describe a
3446:
3295:
3205:
3369:
3259:
3169:
3072:
3025:
3795:
Francis L. Smith & Allan H. Harvey (September 2007). "Avoid Common
Pitfalls When Using Henry's Law".
4033:
1605:, the atmospheric pressure is approximately 0.333 atm, so by using the graph, the boiling point of
30:
2863:
496:
Most actual real-world gases come very close to this ideal. For example, given an ideal gas mixture of
487:
418:
372:
313:
267:
2985:
2917:
1037:
1002:
967:
790:
3652:
2859:
2559:
2375:
1618:
1579:
89:. Gases dissolve, diffuse, and react according to their partial pressures but not according to their
35:
3843:
735:
3892:(1st ed.). Miami Shores, Florida: International Association of Nitrox Divers. pp. 41–50.
3351:
3246:
1728:{\displaystyle {\ce {{{\mathit {a}}A}+{{\mathit {b}}B}<=>{{\mathit {c}}C}+{{\mathit {d}}D}}}}
1516:
1301:
690:
645:
226:
187:
99:
3340:
3242:
2621:
gas in a solution is directly proportional to the partial pressure of that gas above the solution
784:
Ideally the ratio of partial pressures equals the ratio of the number of molecules. That is, the
95:
2298:
2221:
2144:
2067:
4023:
4008:
3893:
3800:
3761:
3602:
3526:
3155:
3131:
3112:
2821:
2809:
2383:
1598:
3707:
3135:
893:{\displaystyle x_{\mathrm {i} }={\frac {p_{\mathrm {i} }}{p}}={\frac {n_{\mathrm {i} }}{n}}}
2567:
2514:
1854:
158:
131:
3979:
3942:
3824:
3722:
3253:
3146:
3119:, involving convulsions, becomes a problem when oxygen partial pressure is too high. The
3116:
1593:
38:
is roughly equal to the sum of partial pressures of constituent gases – oxygen, nitrogen,
3958:
The
Medical Education Division of the Brookside Associates--> ABG (Arterial Blood Gas)
482:
79:
2763:
2748:
is also referred to as the Henry's law constant. As can be seen by comparing equations (
2726:
4013:
3538:
3151:
2954:
2788:
2630:
2624:
2594:
2541:
2488:
2395:
2352:
2332:
2275:
2255:
2198:
2178:
2121:
2101:
2044:
2022:
1999:
1977:
1954:
1932:
1909:
1887:
1551:
1538:
1098:
1072:
623:
3733:
3992:
3558:
3550:
3532:
2825:
1832:{\displaystyle K_{\mathrm {p} }={\frac {p_{C}^{c}\,p_{D}^{d}}{p_{A}^{a}\,p_{B}^{b}}}}
1606:
1602:
785:
90:
3700:
1543:
3639:
3575: – Substances in the gas phase at a temperature lower than its critical point
1609:
would be approximately 7.5 °C versus 34.6 °C at sea level (1 atm).
85:
The partial pressure of a gas is a measure of thermodynamic activity of the gas's
2836:
partial pressure = (total absolute pressure) × (volume fraction of gas component)
17:
3935:
3695:
3564:
2378:
so as to favor either the right or left side of the reaction in accordance with
824:
67:
43:
3124:
and exposures are exceeded. The partial pressure of oxygen also determines the
2862:+ 5 bar of water pressure) and the partial pressures of the main components of
3595:
3804:
3699:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
3711:
3544:
2506:
2401:
1563:
817:
75:
3818:
Introductory
University Chemistry, Henry's Law and the Solubility of Gases
1126:
The ratio of partial pressures relies on the following isotherm relation:
3547: – Mathematical model which approximates the behavior of real gases
2871:
1567:
505:
497:
86:
78:
mixture is the sum of the partial pressures of the gases in the mixture (
59:
3744:
Page 200 in: Medical biophysics. Flemming
Cornelius. 6th Edition, 2008.
2410:
820:
513:
1325:
It can be approximated both from partial pressure and molar fraction:
2867:
2618:
2405:
1559:
1479:
is the partial volume of an individual gas component X in the mixture
823:
can be expressed in terms of the component's partial pressure or the
63:
3873:
NOAA Diving
Program (U.S.) (December 1979). Miller, James W. (ed.).
1030:= partial pressure of any individual gas component in a gas mixture
98:. That said, these pressures can also be measured in, for example,
3660:
Respiratory
Physiology & Neurobiology : Guide for Authors
3572:
2982:= volume fraction of gas component i = mole fraction,
1575:
1555:
1542:
486:
39:
29:
3784:
An extensive list of Henry's law constants, and a conversion tool
995:= mole fraction of any individual gas component in a gas mixture
3424:
3345:
3120:
1571:
1715:
1700:
1648:
1633:
610:{\displaystyle p=p_{{\ce {N2}}}+p_{{\ce {H2}}}+p_{{\ce {NH3}}}}
3405:
51:
3783:
62:
of that constituent gas as if it alone occupied the entire
2824:
the physiological effects of individual component gases of
3868:
3866:
3864:
3862:
3860:
3541: – Gas law regarding proportionality of dissolved gas
3553: – Equation of the state of a hypothetical ideal gas
1613:
Equilibrium constants of reactions involving gas mixtures
1260:
is the partial volume of any individual gas component (X)
1065:= moles of any individual gas component in a gas mixture
755:
710:
665:
601:
576:
551:
443:
397:
338:
292:
246:
207:
2647:
is quite often referred to as the Henry's law constant.
945:{\displaystyle p_{\mathrm {i} }=x_{\mathrm {i} }\cdot p}
3734:
Frostberg State
University's "General Chemistry Online"
3679:
All symbols referring to gas species are in subscript,
2831:
Using diving terms, partial pressure is calculated as:
3251:
1678:
3875:
NOAA Diving Manual, Diving for
Science and Technology
3449:
3372:
3298:
3262:
3208:
3172:
3075:
3028:
2988:
2957:
2920:
2791:
2766:
2729:
2661:
2633:
2597:
2570:
2544:
2517:
2491:
2425:
2414:). The equilibrium constant for that equilibrium is:
2355:
2335:
2301:
2278:
2258:
2224:
2201:
2181:
2147:
2124:
2104:
2070:
2047:
2025:
2002:
1980:
1957:
1935:
1912:
1890:
1857:
1743:
1628:
1331:
1132:
1101:
1075:
1040:
1005:
970:
908:
833:
793:
738:
693:
648:
626:
528:
421:
375:
316:
270:
229:
190:
161:
134:
1737:the equilibrium constant of the reaction would be:
1528:
is the total amount of substance in the gas mixture
3594:
3474:
3394:
3323:
3284:
3233:
3194:
3097:
3050:
3003:
2963:
2935:
2797:
2777:
2740:
2699:
2639:
2603:
2583:
2550:
2530:
2497:
2458:
2361:
2341:
2319:
2284:
2264:
2242:
2207:
2187:
2165:
2130:
2110:
2088:
2053:
2031:
2008:
1986:
1963:
1941:
1918:
1896:
1870:
1831:
1727:
1547:A log-lin vapor pressure chart for various liquids
1462:
1243:
1107:
1081:
1055:
1020:
985:
944:
892:
808:
763:
718:
673:
632:
609:
453:
407:
348:
302:
254:
215:
174:
147:
3561: – Proportion of a constituent in a mixture
3138:may be around 4.5 bar absolute, based on an
1880:= the equilibrium constant of the reaction
1686:
1685:
1668:
1667:
3923:Derived from mmHg values using 0.133322 kPa/mmHg
2615:The form of the equilibrium constant shows that
1318:Partial volume (Amagat's law of additive volume)
2389:
1313:is the total amount of substance in gas mixture
3779:
3777:
3166:The partial pressures of particularly oxygen (
2914:= partial pressure of gas component i =
491:Schematic showing the concept of Dalton's Law.
3890:Exploration and Mixed Gas Diving Encyclopedia
3840:"University of Arizona chemistry class notes"
8:
3953:
3951:
3931:
3929:
3245:, but can also be measured in, for example,
3834:
3832:
2896:
1840:
953:
3145:The effect of a toxic contaminant such as
3463:
3455:
3454:
3448:
3383:
3378:
3377:
3371:
3312:
3304:
3303:
3297:
3273:
3268:
3267:
3261:
3222:
3214:
3213:
3207:
3183:
3178:
3177:
3171:
3086:
3081:
3080:
3074:
3039:
3034:
3033:
3027:
2994:
2993:
2987:
2956:
2926:
2925:
2919:
2790:
2765:
2728:
2700:{\displaystyle k'={\frac {C_{x}}{p_{x}}}}
2689:
2679:
2673:
2660:
2632:
2596:
2575:
2569:
2543:
2522:
2516:
2505:= the equilibrium constant for the
2490:
2448:
2438:
2432:
2424:
2354:
2334:
2311:
2306:
2300:
2277:
2257:
2234:
2229:
2223:
2200:
2180:
2157:
2152:
2146:
2123:
2103:
2080:
2075:
2069:
2046:
2024:
2001:
1979:
1956:
1934:
1911:
1889:
1862:
1856:
1820:
1815:
1810:
1804:
1799:
1787:
1782:
1777:
1771:
1766:
1759:
1749:
1748:
1742:
1714:
1713:
1712:
1699:
1698:
1697:
1687:
1680:
1679:
1677:
1669:
1662:
1660:
1659:
1657:
1647:
1646:
1645:
1632:
1631:
1630:
1629:
1627:
1445:
1444:
1433:
1432:
1426:
1410:
1409:
1387:
1386:
1375:
1374:
1368:
1352:
1351:
1337:
1336:
1330:
1226:
1225:
1214:
1213:
1207:
1189:
1188:
1177:
1176:
1170:
1152:
1151:
1140:
1139:
1133:
1131:
1100:
1074:
1046:
1045:
1039:
1011:
1010:
1004:
976:
975:
969:
929:
928:
914:
913:
907:
878:
877:
871:
856:
855:
849:
839:
838:
832:
799:
798:
792:
754:
749:
744:
743:
737:
709:
704:
699:
698:
692:
664:
659:
654:
653:
647:
625:
600:
595:
590:
589:
575:
570:
565:
564:
550:
545:
540:
539:
527:
442:
437:
432:
431:
426:
420:
396:
391:
386:
385:
380:
374:
337:
332:
327:
326:
321:
315:
291:
286:
281:
280:
275:
269:
245:
240:
235:
234:
228:
206:
201:
196:
195:
189:
166:
160:
139:
133:
2459:{\displaystyle k={\frac {p_{x}}{C_{x}}}}
1506:is the total pressure of the gas mixture
1291:is the total pressure of the gas mixture
476:
27:Pressure of a component gas in a mixture
3919:
3917:
3915:
3913:
3911:
3909:
3754:Perry, R.H.; Green, D.W., eds. (1997).
3585:
3529: – Partial pressure of blood gases
3241:) are important parameters in tests of
3022:= partial pressure of nitrogen =
2390:Henry's law and the solubility of gases
1661:
1592:For example, at any given temperature,
1317:
3968:
3966:
3567: – SI unit of amount of substance
3535: – Gas used for human respiration
1566:. It is a measure of the tendency of
1488:is the total volume of the gas mixture
1269:is the total volume of the gas mixture
3475:{\displaystyle p_{\mathrm {CO_{2}} }}
3324:{\displaystyle p_{\mathrm {CO_{2}} }}
3234:{\displaystyle p_{\mathrm {CO_{2}} }}
3069:= partial pressure of oxygen =
2650:Henry's law is sometimes written as:
816:of an individual gas component in an
367:= arterial partial pressure of oxygen
7:
3757:Perry's Chemical Engineers' Handbook
3395:{\displaystyle p_{\mathrm {O_{2}} }}
3285:{\displaystyle p_{\mathrm {O_{2}} }}
3195:{\displaystyle p_{\mathrm {O_{2}} }}
3098:{\displaystyle P_{\mathrm {O_{2}} }}
3051:{\displaystyle P_{\mathrm {N_{2}} }}
3011:, in the terms used in this article
2828:are a function of partial pressure.
2652:
2416:
1117:= total pressure of the gas mixture
66:of the original mixture at the same
640:= total pressure of the gas mixture
472:= venous partial pressure of oxygen
110:The symbol for pressure is usually
3973:Pathology 425 Cerebrospinal Fluid
3696:Compendium of Chemical Terminology
3460:
3456:
3380:
3309:
3305:
3270:
3219:
3215:
3180:
3105:in the terms used in this article
3083:
3058:in the terms used in this article
3036:
2995:
2971:in the terms used in this article
2943:in the terms used in this article
2927:
1750:
1452:
1449:
1446:
1434:
1417:
1414:
1411:
1394:
1391:
1388:
1376:
1359:
1356:
1353:
1338:
1233:
1230:
1227:
1215:
1196:
1193:
1190:
1178:
1159:
1156:
1153:
1141:
1047:
1012:
977:
930:
915:
879:
857:
840:
800:
454:{\displaystyle p_{v_{{\ce {O2}}}}}
408:{\displaystyle P_{v_{{\ce {O2}}}}}
349:{\displaystyle p_{a_{{\ce {O2}}}}}
303:{\displaystyle P_{a_{{\ce {O2}}}}}
25:
3723:Dalton's Law of Partial Pressures
2893:= 6 bar × 0.21 = 1.3 bar absolute
2884:= 6 bar × 0.79 = 4.7 bar absolute
2874:approximately 79% by volume are:
2591:= the concentration of gas
1091:= total moles of the gas mixture
771:= partial pressure of ammonia (NH
726:= partial pressure of hydrogen (H
681:= partial pressure of nitrogen (N
477:Dalton's law of partial pressures
3004:{\displaystyle x_{\mathrm {i} }}
2936:{\displaystyle P_{\mathrm {i} }}
2538:= partial pressure of gas
2329:= the partial pressure of
2252:= the partial pressure of
2175:= the partial pressure of
2098:= the partial pressure of
1951:= coefficient of reactant
1906:= coefficient of reactant
1497:is the partial pressure of gas X
1056:{\displaystyle n_{\mathrm {i} }}
1021:{\displaystyle p_{\mathrm {i} }}
986:{\displaystyle x_{\mathrm {i} }}
809:{\displaystyle x_{\mathrm {i} }}
3669:from the original on 2015-07-23
2041:= coefficient of product
1996:= coefficient of product
1617:It is possible to work out the
764:{\displaystyle p_{{\ce {NH3}}}}
3623:"Gas Pressure and Respiration"
2812:with the gas being dissolved.
1688:
1663:
719:{\displaystyle p_{{\ce {H2}}}}
674:{\displaystyle p_{{\ce {N2}}}}
262:= partial pressure of hydrogen
255:{\displaystyle p_{{\ce {H2}}}}
216:{\displaystyle P_{{\ce {H2}}}}
1:
3797:Chemical Engineering Progress
3760:(7th ed.). McGraw-Hill.
2627:and the equilibrium constant
2623:. This statement is known as
1574:to escape from a liquid or a
54:, each constituent gas has a
3936:Normal Reference Range Table
3500:
3441:
3422:
3364:
3142:of 35 metres (115 ft).
3593:Charles Henrickson (2005).
3154:in the breathing loop of a
3062:
3015:
2975:
2947:
2907:
2899:
2841:For the component gas "i":
2756:
2750:
2295:
2218:
2141:
2064:
2019:
1974:
1929:
1884:
1851:
1843:
1095:
1069:
1034:
999:
964:
956:
4050:
3999:Engineering thermodynamics
3068:
3021:
2981:
2950:
2913:
2562:containing some of the gas
2393:
2328:
2251:
2174:
2097:
2040:
1995:
1950:
1905:
1879:
1536:
1116:
1090:
1064:
1029:
994:
480:
4029:Underwater diving physics
3442:
3365:
3140:equivalent narcotic depth
2816:In diving breathing gases
2320:{\displaystyle p_{B}^{b}}
2243:{\displaystyle p_{A}^{a}}
2166:{\displaystyle p_{D}^{d}}
2089:{\displaystyle p_{C}^{c}}
3960:Retrieved on Dec 6, 2009
2380:Le Chatelier's Principle
3712:10.1351/goldbook.P04819
3662:. Elsevier. p. 1.
3126:maximum operating depth
2617:the concentration of a
2349:raised to the power of
2272:raised to the power of
2195:raised to the power of
2118:raised to the power of
3476:
3396:
3325:
3286:
3235:
3202:) and carbon dioxide (
3196:
3099:
3052:
3005:
2965:
2937:
2799:
2779:
2742:
2701:
2641:
2611:in the liquid solution
2605:
2585:
2558:in equilibrium with a
2552:
2532:
2499:
2460:
2363:
2343:
2321:
2286:
2266:
2244:
2209:
2189:
2167:
2132:
2112:
2090:
2055:
2033:
2010:
1988:
1965:
1943:
1920:
1898:
1872:
1833:
1729:
1548:
1464:
1245:
1109:
1083:
1057:
1022:
987:
946:
894:
810:
765:
720:
675:
634:
611:
492:
455:
409:
350:
304:
256:
217:
176:
149:
58:which is the notional
47:
46:, carbon dioxide, etc.
4004:Equilibrium chemistry
3477:
3397:
3326:
3287:
3236:
3197:
3100:
3053:
3006:
2966:
2938:
2800:
2785:is the reciprocal of
2780:
2743:
2702:
2642:
2606:
2586:
2584:{\displaystyle C_{x}}
2553:
2533:
2531:{\displaystyle p_{x}}
2500:
2461:
2364:
2344:
2322:
2287:
2267:
2245:
2210:
2190:
2168:
2133:
2113:
2091:
2056:
2034:
2011:
1989:
1966:
1944:
1921:
1899:
1873:
1871:{\displaystyle K_{p}}
1834:
1730:
1554:is the pressure of a
1546:
1465:
1246:
1110:
1084:
1058:
1023:
988:
947:
895:
811:
766:
721:
676:
635:
612:
490:
456:
410:
351:
305:
257:
218:
177:
175:{\displaystyle p_{1}}
150:
148:{\displaystyle P_{1}}
33:
3447:
3370:
3296:
3260:
3243:arterial blood gases
3206:
3170:
3073:
3026:
2986:
2955:
2918:
2860:atmospheric pressure
2789:
2764:
2727:
2659:
2631:
2595:
2568:
2542:
2515:
2489:
2423:
2353:
2333:
2299:
2276:
2256:
2222:
2199:
2179:
2145:
2122:
2102:
2068:
2045:
2023:
2000:
1978:
1955:
1933:
1910:
1888:
1855:
1741:
1626:
1619:equilibrium constant
1580:normal boiling point
1329:
1130:
1099:
1073:
1038:
1003:
968:
906:
831:
791:
736:
691:
646:
624:
526:
419:
373:
314:
268:
227:
188:
182:= pressure at time 1
159:
132:
96:arterial blood gases
36:atmospheric pressure
3653:"Symbols and Units"
3352:Cerebrospinal fluid
3331:
3247:cerebrospinal fluid
2951:= total pressure =
2316:
2239:
2162:
2085:
1825:
1809:
1792:
1776:
1674:
1517:amount of substance
1302:amount of substance
757:
712:
667:
603:
578:
553:
445:
399:
340:
294:
248:
209:
100:cerebrospinal fluid
4019:Physical chemistry
3978:2012-02-22 at the
3941:2011-12-25 at the
3823:2012-05-04 at the
3472:
3392:
3341:Arterial blood gas
3321:
3282:
3252:
3231:
3192:
3128:of a gas mixture.
3095:
3048:
3001:
2961:
2933:
2870:21% by volume and
2795:
2778:{\displaystyle k'}
2775:
2741:{\displaystyle k'}
2738:
2697:
2637:
2601:
2581:
2548:
2528:
2495:
2456:
2359:
2339:
2317:
2302:
2282:
2262:
2240:
2225:
2205:
2185:
2163:
2148:
2128:
2108:
2086:
2071:
2051:
2029:
2006:
1984:
1961:
1939:
1916:
1894:
1868:
1829:
1811:
1795:
1778:
1762:
1725:
1693:
1549:
1460:
1241:
1105:
1079:
1053:
1018:
983:
942:
890:
827:of the component:
806:
780:Ideal gas mixtures
761:
745:
716:
700:
671:
655:
630:
607:
591:
566:
541:
493:
451:
433:
405:
387:
346:
328:
300:
282:
252:
236:
213:
197:
172:
145:
48:
3899:978-0-915539-10-9
3767:978-0-07-049841-9
3608:978-0-7645-7419-1
3527:Blood gas tension
3518:
3517:
3156:diving rebreather
3109:
3108:
2964:{\displaystyle P}
2822:underwater diving
2798:{\displaystyle k}
2721:
2720:
2695:
2640:{\displaystyle k}
2604:{\displaystyle x}
2551:{\displaystyle x}
2498:{\displaystyle k}
2480:
2479:
2454:
2384:reaction kinetics
2372:
2371:
2362:{\displaystyle b}
2342:{\displaystyle B}
2285:{\displaystyle a}
2265:{\displaystyle A}
2208:{\displaystyle d}
2188:{\displaystyle D}
2131:{\displaystyle c}
2111:{\displaystyle C}
2054:{\displaystyle D}
2032:{\displaystyle d}
2009:{\displaystyle C}
1987:{\displaystyle c}
1964:{\displaystyle B}
1942:{\displaystyle b}
1919:{\displaystyle A}
1897:{\displaystyle a}
1827:
1722:
1717:
1707:
1702:
1695:
1655:
1650:
1640:
1635:
1458:
1400:
1239:
1202:
1165:
1121:
1120:
1108:{\displaystyle p}
1082:{\displaystyle n}
888:
866:
748:
703:
658:
633:{\displaystyle p}
594:
569:
544:
436:
390:
331:
285:
239:
200:
18:Partial pressures
16:(Redirected from
4041:
3983:
3970:
3961:
3955:
3946:
3933:
3924:
3921:
3904:
3903:
3885:
3879:
3878:
3870:
3855:
3854:
3852:
3851:
3842:. Archived from
3836:
3827:
3815:
3809:
3808:
3792:
3786:
3781:
3772:
3771:
3751:
3745:
3742:
3736:
3731:
3725:
3720:
3714:
3688:
3682:
3681:
3676:
3674:
3668:
3657:
3648:
3642:
3637:
3631:
3630:
3619:
3613:
3612:
3601:. Cliffs Notes.
3600:
3590:
3481:
3479:
3478:
3473:
3471:
3470:
3469:
3468:
3467:
3401:
3399:
3398:
3393:
3391:
3390:
3389:
3388:
3387:
3332:
3330:
3328:
3327:
3322:
3320:
3319:
3318:
3317:
3316:
3291:
3289:
3288:
3283:
3281:
3280:
3279:
3278:
3277:
3254:Reference ranges
3240:
3238:
3237:
3232:
3230:
3229:
3228:
3227:
3226:
3201:
3199:
3198:
3193:
3191:
3190:
3189:
3188:
3187:
3136:technical diving
3104:
3102:
3101:
3096:
3094:
3093:
3092:
3091:
3090:
3057:
3055:
3054:
3049:
3047:
3046:
3045:
3044:
3043:
3010:
3008:
3007:
3002:
3000:
2999:
2998:
2970:
2968:
2967:
2962:
2942:
2940:
2939:
2934:
2932:
2931:
2930:
2897:
2810:react chemically
2804:
2802:
2801:
2796:
2784:
2782:
2781:
2776:
2774:
2747:
2745:
2744:
2739:
2737:
2715:
2706:
2704:
2703:
2698:
2696:
2694:
2693:
2684:
2683:
2674:
2669:
2653:
2646:
2644:
2643:
2638:
2610:
2608:
2607:
2602:
2590:
2588:
2587:
2582:
2580:
2579:
2557:
2555:
2554:
2549:
2537:
2535:
2534:
2529:
2527:
2526:
2504:
2502:
2501:
2496:
2474:
2465:
2463:
2462:
2457:
2455:
2453:
2452:
2443:
2442:
2433:
2417:
2368:
2366:
2365:
2360:
2348:
2346:
2345:
2340:
2326:
2324:
2323:
2318:
2315:
2310:
2291:
2289:
2288:
2283:
2271:
2269:
2268:
2263:
2249:
2247:
2246:
2241:
2238:
2233:
2214:
2212:
2211:
2206:
2194:
2192:
2191:
2186:
2172:
2170:
2169:
2164:
2161:
2156:
2137:
2135:
2134:
2129:
2117:
2115:
2114:
2109:
2095:
2093:
2092:
2087:
2084:
2079:
2060:
2058:
2057:
2052:
2038:
2036:
2035:
2030:
2015:
2013:
2012:
2007:
1993:
1991:
1990:
1985:
1970:
1968:
1967:
1962:
1948:
1946:
1945:
1940:
1925:
1923:
1922:
1917:
1903:
1901:
1900:
1895:
1877:
1875:
1874:
1869:
1867:
1866:
1841:
1838:
1836:
1835:
1830:
1828:
1826:
1824:
1819:
1808:
1803:
1793:
1791:
1786:
1775:
1770:
1760:
1755:
1754:
1753:
1734:
1732:
1731:
1726:
1724:
1723:
1720:
1719:
1718:
1708:
1705:
1704:
1703:
1696:
1694:
1692:
1691:
1684:
1676:
1675:
1673:
1666:
1658:
1656:
1653:
1652:
1651:
1641:
1638:
1637:
1636:
1469:
1467:
1466:
1461:
1459:
1457:
1456:
1455:
1439:
1438:
1437:
1427:
1422:
1421:
1420:
1401:
1399:
1398:
1397:
1381:
1380:
1379:
1369:
1364:
1363:
1362:
1343:
1342:
1341:
1280:partial pressure
1250:
1248:
1247:
1242:
1240:
1238:
1237:
1236:
1220:
1219:
1218:
1208:
1203:
1201:
1200:
1199:
1183:
1182:
1181:
1171:
1166:
1164:
1163:
1162:
1146:
1145:
1144:
1134:
1114:
1112:
1111:
1106:
1088:
1086:
1085:
1080:
1062:
1060:
1059:
1054:
1052:
1051:
1050:
1027:
1025:
1024:
1019:
1017:
1016:
1015:
992:
990:
989:
984:
982:
981:
980:
954:
951:
949:
948:
943:
935:
934:
933:
920:
919:
918:
899:
897:
896:
891:
889:
884:
883:
882:
872:
867:
862:
861:
860:
850:
845:
844:
843:
815:
813:
812:
807:
805:
804:
803:
770:
768:
767:
762:
760:
759:
758:
756:
753:
746:
725:
723:
722:
717:
715:
714:
713:
711:
708:
701:
680:
678:
677:
672:
670:
669:
668:
666:
663:
656:
639:
637:
636:
631:
616:
614:
613:
608:
606:
605:
604:
602:
599:
592:
581:
580:
579:
577:
574:
567:
556:
555:
554:
552:
549:
542:
460:
458:
457:
452:
450:
449:
448:
447:
446:
444:
441:
434:
414:
412:
411:
406:
404:
403:
402:
401:
400:
398:
395:
388:
355:
353:
352:
347:
345:
344:
343:
342:
341:
339:
336:
329:
309:
307:
306:
301:
299:
298:
297:
296:
295:
293:
290:
283:
261:
259:
258:
253:
251:
250:
249:
247:
244:
237:
222:
220:
219:
214:
212:
211:
210:
208:
205:
198:
181:
179:
178:
173:
171:
170:
154:
152:
151:
146:
144:
143:
121:
115:
56:partial pressure
50:In a mixture of
21:
4049:
4048:
4044:
4043:
4042:
4040:
4039:
4038:
3989:
3988:
3987:
3986:
3980:Wayback Machine
3971:
3964:
3956:
3949:
3943:Wayback Machine
3934:
3927:
3922:
3907:
3900:
3887:
3886:
3882:
3872:
3871:
3858:
3849:
3847:
3838:
3837:
3830:
3825:Wayback Machine
3816:
3812:
3794:
3793:
3789:
3782:
3775:
3768:
3753:
3752:
3748:
3743:
3739:
3732:
3728:
3721:
3717:
3689:
3685:
3672:
3670:
3666:
3655:
3650:
3649:
3645:
3638:
3634:
3621:
3620:
3616:
3609:
3592:
3591:
3587:
3582:
3523:
3459:
3450:
3445:
3444:
3379:
3373:
3368:
3367:
3359:
3308:
3299:
3294:
3293:
3269:
3263:
3258:
3257:
3218:
3209:
3204:
3203:
3179:
3173:
3168:
3167:
3164:
3147:carbon monoxide
3117:Oxygen toxicity
3082:
3076:
3071:
3070:
3066:
3035:
3029:
3024:
3023:
3019:
2989:
2984:
2983:
2979:
2953:
2952:
2921:
2916:
2915:
2911:
2891:
2882:
2853:
2849:
2826:breathing gases
2818:
2787:
2786:
2767:
2762:
2761:
2730:
2725:
2724:
2713:
2685:
2675:
2662:
2657:
2656:
2629:
2628:
2593:
2592:
2571:
2566:
2565:
2540:
2539:
2518:
2513:
2512:
2487:
2486:
2472:
2444:
2434:
2421:
2420:
2398:
2392:
2382:. However, the
2351:
2350:
2331:
2330:
2297:
2296:
2274:
2273:
2254:
2253:
2220:
2219:
2197:
2196:
2177:
2176:
2143:
2142:
2120:
2119:
2100:
2099:
2066:
2065:
2043:
2042:
2021:
2020:
1998:
1997:
1976:
1975:
1953:
1952:
1931:
1930:
1908:
1907:
1886:
1885:
1858:
1853:
1852:
1794:
1761:
1744:
1739:
1738:
1624:
1623:
1615:
1594:methyl chloride
1562:'s tendency to
1541:
1535:
1527:
1514:
1505:
1496:
1487:
1478:
1440:
1428:
1405:
1382:
1370:
1347:
1332:
1327:
1326:
1320:
1312:
1299:
1290:
1277:
1268:
1259:
1221:
1209:
1184:
1172:
1147:
1135:
1128:
1127:
1097:
1096:
1071:
1070:
1041:
1036:
1035:
1006:
1001:
1000:
971:
966:
965:
924:
909:
904:
903:
873:
851:
834:
829:
828:
794:
789:
788:
782:
774:
739:
734:
733:
729:
694:
689:
688:
684:
649:
644:
643:
622:
621:
585:
560:
535:
524:
523:
519:
511:
503:
485:
479:
470:
466:
427:
422:
417:
416:
381:
376:
371:
370:
365:
361:
322:
317:
312:
311:
276:
271:
266:
265:
230:
225:
224:
191:
186:
185:
162:
157:
156:
135:
130:
129:
117:
111:
108:
28:
23:
22:
15:
12:
11:
5:
4047:
4045:
4037:
4036:
4031:
4026:
4021:
4016:
4011:
4006:
4001:
3991:
3990:
3985:
3984:
3962:
3947:
3925:
3905:
3898:
3880:
3856:
3828:
3810:
3787:
3773:
3766:
3746:
3737:
3726:
3715:
3683:
3643:
3632:
3627:Lumen Learning
3614:
3607:
3584:
3583:
3581:
3578:
3577:
3576:
3570:
3569:
3568:
3556:
3555:
3554:
3542:
3536:
3530:
3522:
3519:
3516:
3515:
3512:
3509:
3506:
3503:
3499:
3498:
3495:
3492:
3489:
3486:
3483:
3466:
3462:
3458:
3453:
3440:
3439:
3436:
3433:
3430:
3427:
3421:
3420:
3417:
3414:
3411:
3408:
3403:
3386:
3382:
3376:
3363:
3362:
3354:
3349:
3343:
3338:
3335:
3315:
3311:
3307:
3302:
3276:
3272:
3266:
3225:
3221:
3217:
3212:
3186:
3182:
3176:
3163:
3160:
3152:carbon dioxide
3107:
3106:
3089:
3085:
3079:
3067:
3064:
3060:
3059:
3042:
3038:
3032:
3020:
3017:
3013:
3012:
2997:
2992:
2980:
2977:
2973:
2972:
2960:
2949:
2945:
2944:
2929:
2924:
2912:
2909:
2905:
2904:
2901:
2895:
2894:
2889:
2885:
2880:
2856:
2855:
2851:
2847:
2839:
2838:
2817:
2814:
2794:
2773:
2770:
2736:
2733:
2719:
2718:
2709:
2707:
2692:
2688:
2682:
2678:
2672:
2668:
2665:
2636:
2613:
2612:
2600:
2578:
2574:
2563:
2547:
2525:
2521:
2510:
2494:
2478:
2477:
2468:
2466:
2451:
2447:
2441:
2437:
2431:
2428:
2394:Main article:
2391:
2388:
2370:
2369:
2358:
2338:
2327:
2314:
2309:
2305:
2293:
2292:
2281:
2261:
2250:
2237:
2232:
2228:
2216:
2215:
2204:
2184:
2173:
2160:
2155:
2151:
2139:
2138:
2127:
2107:
2096:
2083:
2078:
2074:
2062:
2061:
2050:
2039:
2028:
2017:
2016:
2005:
1994:
1983:
1972:
1971:
1960:
1949:
1938:
1927:
1926:
1915:
1904:
1893:
1882:
1881:
1878:
1865:
1861:
1849:
1848:
1845:
1823:
1818:
1814:
1807:
1802:
1798:
1790:
1785:
1781:
1774:
1769:
1765:
1758:
1752:
1747:
1711:
1690:
1683:
1672:
1665:
1644:
1614:
1611:
1552:Vapor pressure
1539:Vapor pressure
1537:Main article:
1534:
1533:Vapor pressure
1531:
1530:
1529:
1525:
1520:
1512:
1507:
1503:
1498:
1494:
1489:
1485:
1480:
1476:
1454:
1451:
1448:
1443:
1436:
1431:
1425:
1419:
1416:
1413:
1408:
1404:
1396:
1393:
1390:
1385:
1378:
1373:
1367:
1361:
1358:
1355:
1350:
1346:
1340:
1335:
1319:
1316:
1315:
1314:
1310:
1305:
1297:
1292:
1288:
1283:
1275:
1270:
1266:
1261:
1257:
1235:
1232:
1229:
1224:
1217:
1212:
1206:
1198:
1195:
1192:
1187:
1180:
1175:
1169:
1161:
1158:
1155:
1150:
1143:
1138:
1119:
1118:
1115:
1104:
1093:
1092:
1089:
1078:
1067:
1066:
1063:
1049:
1044:
1032:
1031:
1028:
1014:
1009:
997:
996:
993:
979:
974:
962:
961:
958:
941:
938:
932:
927:
923:
917:
912:
887:
881:
876:
870:
865:
859:
854:
848:
842:
837:
802:
797:
781:
778:
777:
776:
772:
752:
742:
731:
727:
707:
697:
686:
682:
662:
652:
641:
629:
598:
588:
584:
573:
563:
559:
548:
538:
534:
531:
517:
509:
501:
481:Main article:
478:
475:
474:
473:
468:
464:
440:
430:
425:
394:
384:
379:
368:
363:
359:
335:
325:
320:
289:
279:
274:
263:
243:
233:
204:
194:
183:
169:
165:
142:
138:
107:
104:
91:concentrations
72:total pressure
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
4046:
4035:
4032:
4030:
4027:
4025:
4022:
4020:
4017:
4015:
4012:
4010:
4007:
4005:
4002:
4000:
3997:
3996:
3994:
3981:
3977:
3974:
3969:
3967:
3963:
3959:
3954:
3952:
3948:
3944:
3940:
3937:
3932:
3930:
3926:
3920:
3918:
3916:
3914:
3912:
3910:
3906:
3901:
3895:
3891:
3884:
3881:
3876:
3869:
3867:
3865:
3863:
3861:
3857:
3846:on 2012-03-07
3845:
3841:
3835:
3833:
3829:
3826:
3822:
3819:
3814:
3811:
3806:
3802:
3798:
3791:
3788:
3785:
3780:
3778:
3774:
3769:
3763:
3759:
3758:
3750:
3747:
3741:
3738:
3735:
3730:
3727:
3724:
3719:
3716:
3713:
3709:
3705:
3704:
3698:
3697:
3692:
3687:
3684:
3680:
3665:
3661:
3654:
3647:
3644:
3641:
3636:
3633:
3628:
3624:
3618:
3615:
3610:
3604:
3599:
3598:
3589:
3586:
3579:
3574:
3571:
3566:
3563:
3562:
3560:
3559:Mole fraction
3557:
3552:
3551:Ideal gas law
3549:
3548:
3546:
3543:
3540:
3537:
3534:
3533:Breathing gas
3531:
3528:
3525:
3524:
3520:
3513:
3510:
3507:
3504:
3501:
3496:
3493:
3490:
3487:
3484:
3482:
3464:
3451:
3437:
3434:
3431:
3428:
3426:
3423:
3418:
3415:
3412:
3409:
3407:
3404:
3402:
3384:
3374:
3361:
3360:gas pressures
3355:
3353:
3350:
3347:
3344:
3342:
3339:
3336:
3334:
3333:
3313:
3300:
3274:
3264:
3255:
3250:
3248:
3244:
3223:
3210:
3184:
3174:
3161:
3159:
3157:
3153:
3148:
3143:
3141:
3137:
3133:
3129:
3127:
3122:
3118:
3114:
3087:
3077:
3061:
3040:
3030:
3014:
2990:
2974:
2958:
2946:
2922:
2906:
2902:
2898:
2892:
2886:
2883:
2877:
2876:
2875:
2873:
2869:
2865:
2861:
2854:
2844:
2843:
2842:
2837:
2834:
2833:
2832:
2829:
2827:
2823:
2815:
2813:
2811:
2806:
2792:
2771:
2768:
2759:
2758:
2753:
2752:
2734:
2731:
2717:
2710:
2708:
2690:
2686:
2680:
2676:
2670:
2666:
2663:
2655:
2654:
2651:
2648:
2634:
2626:
2622:
2620:
2598:
2576:
2572:
2564:
2561:
2545:
2523:
2519:
2511:
2508:
2492:
2485:
2484:
2483:
2476:
2469:
2467:
2449:
2445:
2439:
2435:
2429:
2426:
2419:
2418:
2415:
2413:
2412:
2407:
2403:
2397:
2387:
2385:
2381:
2377:
2356:
2336:
2312:
2307:
2303:
2294:
2279:
2259:
2235:
2230:
2226:
2217:
2202:
2182:
2158:
2153:
2149:
2140:
2125:
2105:
2081:
2076:
2072:
2063:
2048:
2026:
2018:
2003:
1981:
1973:
1958:
1936:
1928:
1913:
1891:
1883:
1863:
1859:
1850:
1846:
1842:
1839:
1821:
1816:
1812:
1805:
1800:
1796:
1788:
1783:
1779:
1772:
1767:
1763:
1756:
1745:
1735:
1709:
1681:
1670:
1642:
1620:
1612:
1610:
1608:
1607:diethyl ether
1604:
1603:Mount Everest
1600:
1595:
1590:
1586:
1583:
1581:
1577:
1573:
1569:
1565:
1561:
1557:
1553:
1545:
1540:
1532:
1524:
1521:
1518:
1511:
1508:
1502:
1499:
1493:
1490:
1484:
1481:
1475:
1472:
1471:
1470:
1441:
1429:
1423:
1406:
1402:
1383:
1371:
1365:
1348:
1344:
1333:
1323:
1309:
1306:
1303:
1296:
1293:
1287:
1284:
1281:
1274:
1271:
1265:
1262:
1256:
1253:
1252:
1251:
1222:
1210:
1204:
1185:
1173:
1167:
1148:
1136:
1124:
1102:
1094:
1076:
1068:
1042:
1033:
1007:
998:
972:
963:
959:
955:
952:
939:
936:
925:
921:
910:
900:
885:
874:
868:
863:
852:
846:
835:
826:
822:
819:
795:
787:
786:mole fraction
779:
750:
740:
732:
705:
695:
687:
660:
650:
642:
627:
620:
619:
618:
596:
586:
582:
571:
561:
557:
546:
536:
532:
529:
521:
515:
507:
499:
489:
484:
471:
438:
428:
423:
392:
382:
377:
369:
366:
333:
323:
318:
287:
277:
272:
264:
241:
231:
202:
192:
184:
167:
163:
140:
136:
128:
127:
126:
123:
120:
114:
105:
103:
101:
97:
92:
88:
83:
81:
77:
73:
69:
65:
61:
57:
53:
45:
41:
37:
32:
19:
4034:Distillation
3889:
3883:
3874:
3848:. Retrieved
3844:the original
3813:
3796:
3790:
3756:
3749:
3740:
3729:
3718:
3702:
3694:
3686:
3678:
3671:. Retrieved
3659:
3646:
3640:Gas blending
3635:
3626:
3617:
3596:
3588:
3165:
3144:
3130:
3110:
2887:
2878:
2857:
2845:
2840:
2835:
2830:
2819:
2807:
2755:
2749:
2722:
2711:
2649:
2616:
2614:
2481:
2470:
2409:
2399:
2373:
1736:
1616:
1591:
1587:
1584:
1550:
1522:
1509:
1500:
1491:
1482:
1473:
1324:
1321:
1307:
1294:
1285:
1279:
1272:
1263:
1254:
1125:
1122:
901:
783:
522:
494:
483:Dalton's law
462:
357:
124:
118:
112:
109:
84:
80:Dalton's Law
71:
55:
49:
3701:pressure,
3565:Mole (unit)
3539:Henry's law
3162:In medicine
2625:Henry's law
2400:Gases will
2396:Henry's law
2376:equilibrium
68:temperature
44:water vapor
3993:Categories
3850:2006-05-26
3580:References
1304:of gas (X)
125:Examples:
3805:0360-7275
3597:Chemistry
3545:Ideal gas
3358:pulmonary
3356:Alveolar
3348:blood gas
2760:) above,
2507:solvation
1689:⇀
1682:−
1671:−
1664:↽
1568:molecules
1564:evaporate
1424:×
1366:×
937:⋅
818:ideal gas
87:molecules
76:ideal gas
4024:Pressure
4009:Gas laws
3976:Archived
3939:Archived
3821:Archived
3664:Archived
3521:See also
3132:Narcosis
2872:nitrogen
2772:′
2735:′
2667:′
2560:solution
2402:dissolve
1519:of gas X
1282:of gas X
506:hydrogen
498:nitrogen
60:pressure
3651:Staff.
3494:5.9–6.7
3491:5.5–6.8
3488:4.7–6.0
3416:5.3–5.9
3413:4.0–5.3
3113:Hypoxia
2903:
2900:where:
2850:= P × F
2754:) and (
2509:process
2482:where:
2411:solvent
2406:liquids
1847:
1844:where:
1515:is the
1300:is the
1278:is the
960:
957:where:
821:mixture
617:where:
514:ammonia
512:) and
3896:
3803:
3764:
3673:3 June
3605:
3429:75–100
3346:Venous
2868:oxygen
2723:where
2619:solute
1560:liquid
106:Symbol
74:of an
70:. The
64:volume
4014:Gases
3691:IUPAC
3667:(PDF)
3656:(PDF)
3573:Vapor
3511:44–50
3508:41–51
3505:35–45
3435:40–44
3432:30–40
3419:14.2
3410:11–13
1576:solid
1572:atoms
1556:vapor
825:moles
52:gases
40:argon
3894:ISBN
3801:ISSN
3762:ISBN
3675:2017
3603:ISBN
3502:mmHg
3497:4.8
3438:107
3425:mmHg
3337:Unit
3292:and
3256:for
3121:NOAA
1570:and
34:The
3708:doi
3706:".
3514:36
3485:kPa
3406:kPa
3249:.
2864:air
2820:In
2404:in
1599:atm
1526:tot
1504:tot
1486:tot
1311:tot
1289:tot
1267:tot
520:):
516:(NH
504:),
461:or
415:or
356:or
310:or
223:or
155:or
116:or
82:).
3995::
3965:^
3950:^
3928:^
3908:^
3859:^
3831:^
3799:.
3776:^
3693:,
3677:.
3658:.
3625:.
3063:pO
3016:pN
2948:P
2888:pO
2879:pN
2866:,
1582:.
747:NH
593:NH
508:(H
500:(N
119:pp
102:.
42:,
3902:.
3853:.
3807:.
3770:.
3710::
3703:p
3629:.
3611:.
3465:2
3461:O
3457:C
3452:p
3385:2
3381:O
3375:p
3314:2
3310:O
3306:C
3301:p
3275:2
3271:O
3265:p
3224:2
3220:O
3216:C
3211:p
3185:2
3181:O
3175:p
3088:2
3084:O
3078:P
3065:2
3041:2
3037:N
3031:P
3018:2
2996:i
2991:x
2978:i
2976:F
2959:P
2928:i
2923:P
2910:i
2908:p
2890:2
2881:2
2852:i
2848:i
2846:p
2793:k
2769:k
2757:2
2751:1
2732:k
2716:)
2714:2
2712:(
2691:x
2687:p
2681:x
2677:C
2671:=
2664:k
2635:k
2599:x
2577:x
2573:C
2546:x
2524:x
2520:p
2493:k
2475:)
2473:1
2471:(
2450:x
2446:C
2440:x
2436:p
2430:=
2427:k
2357:b
2337:B
2313:b
2308:B
2304:p
2280:a
2260:A
2236:a
2231:A
2227:p
2203:d
2183:D
2159:d
2154:D
2150:p
2126:c
2106:C
2082:c
2077:C
2073:p
2049:D
2027:d
2004:C
1982:c
1959:B
1937:b
1914:A
1892:a
1864:p
1860:K
1822:b
1817:B
1813:p
1806:a
1801:A
1797:p
1789:d
1784:D
1780:p
1773:c
1768:C
1764:p
1757:=
1751:p
1746:K
1721:D
1716:d
1710:+
1706:C
1701:c
1654:B
1649:b
1643:+
1639:A
1634:a
1597:(
1523:n
1513:X
1510:n
1501:p
1495:X
1492:p
1483:V
1477:X
1474:V
1453:t
1450:o
1447:t
1442:n
1435:X
1430:n
1418:t
1415:o
1412:t
1407:V
1403:=
1395:t
1392:o
1389:t
1384:p
1377:X
1372:p
1360:t
1357:o
1354:t
1349:V
1345:=
1339:X
1334:V
1308:n
1298:X
1295:n
1286:p
1276:X
1273:p
1264:V
1258:X
1255:V
1234:t
1231:o
1228:t
1223:n
1216:X
1211:n
1205:=
1197:t
1194:o
1191:t
1186:p
1179:X
1174:p
1168:=
1160:t
1157:o
1154:t
1149:V
1142:X
1137:V
1103:p
1077:n
1048:i
1043:n
1013:i
1008:p
978:i
973:x
940:p
931:i
926:x
922:=
916:i
911:p
886:n
880:i
875:n
869:=
864:p
858:i
853:p
847:=
841:i
836:x
801:i
796:x
775:)
773:3
751:3
741:p
730:)
728:2
706:2
702:H
696:p
685:)
683:2
661:2
657:N
651:p
628:p
597:3
587:p
583:+
572:2
568:H
562:p
558:+
547:2
543:N
537:p
533:=
530:p
518:3
510:2
502:2
469:2
467:O
465:v
463:P
439:2
435:O
429:v
424:p
393:2
389:O
383:v
378:P
364:2
362:O
360:a
358:P
334:2
330:O
324:a
319:p
288:2
284:O
278:a
273:P
242:2
238:H
232:p
203:2
199:H
193:P
168:1
164:p
141:1
137:P
113:p
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.