530:
24:
540:
498:
of malignant cancer cells. The exact mechanism of this inhibition is highly complex and not entirely elucidated, but could involve multiple processes, including phosphatase inhibition, copper mediated cleavage of double stranded DNA, or disrupting the pH gradient through transmembrane transport of H+
411:. Because it is easy to detect, it has been used as a model system to study secondary metabolism. Prodigiosin production has long been known to be enhanced by phosphate limitation. In low phosphate conditions, pigmented strains have been shown to grow to a higher density than unpigmented strains.
700:
of prodigiosin were published in 1962, confirming the chemical structure. As with the biosynthesis, the key intermediate was the A-B aldehyde shown in Figure 5. This aldehyde has subsequently been prepared by other methods and used to make prodigiosin and related natural products.
493:
Prodigiosin received renewed attention for its wide range of biological activities, including activities as antimalarial, antifungal, immunosuppressant, and antibiotic agents. It is perhaps best known for its capacity to trigger
617:(PLP) mediated decarboxylation of L-serine, which cyclizes in a dehydration reaction to yield the second pyrrole ring. This intermediate is then modified by oxidation of the primary alcohol to the aldehyde, catalysed by
499:
and Cl- ions. As a result, prodigiosin is a highly promising drug lead, and is currently in preclinical phase study for pancreatic cancer treatment. Prodigiosin has recently been found to have excellent activity against
600:
Ring A is then expanded via the polyketide synthase pathway to incorporate L-serine into ring B (figure 3). Ring A fragment is transferred from the peptidyl carrier protein (PCP) to the acyl carrier protein (ACP) by a
686:
1019:
Magae, J., Miller, M. W., Nagai, K. & Shearer, G. M. Effect of metacycloprodigiosin, an inhibitor of killer T cells on murine skin and heart transplants. J. Antibiot. (Tokyo) 49, 86–90 (1996).
595:
638:
901:
1078:
Rastogi, S.; et al. (2013). "Synthetic prodigiosenes and the influence of C-ring substitution on DNA cleavage, transmembrane chloride transport and basicity".
668:
559:
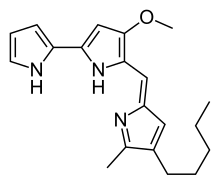
involves the convergent coupling of three pyrrole type rings (labeled A, B, and C in figure 1) from L-proline, L-serine, L-methionine, pyruvate, and 2-octenal.
375:. It is responsible for the pink tint occasionally found in grime that accumulates on porcelain surfaces such as bathtubs, sinks, and toilet bowls. It is in the
262:
925:
Williamson NR, Fineran PC, Gristwood T, Chawrai SR, Leeper FJ, Salmond GP (2007). "Anticancer and immunosuppressive properties of bacterial prodiginines".
467:. During the Mass, the eucharist appeared to bleed and each time the priest wiped away the blood, more would appear. This event is celebrated in a
685:
94:
681:(ATP) in a dehydration reaction which establishes a conjugated system across all three rings and completes the synthesis of prodigiosin.
379:
family of compounds which are produced in some Gram-negative gamma proteobacteria, as well as select Gram-positive
Actinobacteria (e.g.
771:
227:
1150:"Identification of new compounds with high activity against stationary phase Borrelia burgdorferi from the NCI compound collection"
594:
236:
InChI=1S/C20H25N3O/c1-4-5-6-8-15-11-16(22-14(15)2)12-19-20(24-3)13-18(23-19)17-9-7-10-21-17/h7,9-13,21,23H,4-6,8H2,1-3H3/b16-12+
246:
InChI=1/C20H25N3O/c1-4-5-6-8-15-11-16(22-14(15)2)12-19-20(24-3)13-18(23-19)17-9-7-10-21-17/h7,9-13,21,23H,4-6,8H2,1-3H3/b16-12+
1010:
Berg, G. Diversity of antifungal and plant-associated
Serratia plymuthica strains. J. Appl. Microbiol. 88, 952–960 (2000).
718:
563:
1031:"Prodigiosin 25-C uncouples vacuolar type H+-ATPase, inhibits vacuolar acidification and affects glycoprotein processing"
637:
337:
1481:
1343:
621:, and methylation (which incorporates a methyl group from L-methionine onto the alcohol at the 6-position) catalysed by
204:
571:
500:
1486:
1255:; Howard-Jones, Annaleise R. (2006). "Biological formation of pyrroles: Nature's logic and enzymatic machinery".
1252:
165:
656:
448:
381:
578:
to yield pyrrole ring A. In the first step, proline is attached to a peptidyl carrier protein (PCP) called
1202:"Biosynthesis and Bioactivity of Prodiginine Analogs in Marine Bacteria, Pseudoalteromonas: A Mini Review"
678:
644:
171:
667:
134:
36:
1397:
Rapoport, Henry.; Willson, Clyde D. (1962). "The
Preparation and Properties of Some Methoxypyrroles".
969:
606:
504:
614:
481:
358:
60:
1453:
1364:
Brass, Hannah U. C.; Klein, Andreas S.; Nyholt, Silke; Classen, Thomas; Pietruszka, Jörg (2019).
1113:
Perez-Tomas, R.; Vinas, M. (2010). "New
Insights on the Antitumoral Properties of Prodiginines".
1060:
993:
870:
552:
464:
371:
1476:
1445:
1324:
1272:
1233:
1179:
1130:
1095:
1052:
985:
942:
862:
816:
777:
767:
629:. This yields the core A-B ring structure ready for further transformations, including to the
154:
1437:
1406:
1377:
1314:
1306:
1264:
1223:
1213:
1169:
1161:
1122:
1087:
1042:
977:
934:
854:
808:
759:
285:
213:
1200:
Sakai-Kawada, Francis E.; Ip, Courtney G.; Hagiwara, Kehau A.; Awaya, Jonathan D. (2019).
697:
529:
1426:"Recent advancements in high-level synthesis of the promising clinical drug, prodigiosin"
973:
114:
70:
1319:
1294:
1228:
1201:
1174:
1149:
452:
436:
331:
763:
1470:
1047:
1030:
602:
444:
1457:
1064:
874:
193:
1424:
Yip, Chee-Hoo; Yarkoni, Orr; Ajioka, James; Wan, Kiew-Lian; Nathan, Sheila (2019).
997:
842:
655:
then converts the intermediate to an amine (using an amino-acid and PLP) ready for
509:
472:
1295:"Structure, Chemical Synthesis, and Biosynthesis of Prodiginine Natural Products"
1293:
Hu, Dennis X.; Withall, David M.; Challis, Gregory L.; Thomson, Regan J. (2016).
533:
Chemical transformations and gene clusters for prodiginine biosynthetic pathways
1310:
812:
613:. This fragment is then able to react with the masked carbanion formed from the
567:
556:
391:
376:
1441:
1126:
887:
M. Todd-Guay and P.H. Demchick. 1995. Role of prodigiosin in phosphate-starved
647:(TPP) mediated decarboxylative addition of pyruvate to 2-octenal, catalysed by
543:
Figure 1: Structure of
Prodigiosin 1 highlighting the A, B, and C pyrrole rings
630:
316:
145:
1218:
938:
730:
495:
432:
1449:
1382:
1365:
1328:
1276:
1237:
1183:
1134:
1099:
946:
866:
781:
539:
1056:
989:
820:
710:
575:
428:
1410:
1165:
858:
663:
oxidises the resulting ring using oxygen and FAD, yielding the pyrrole.
23:
1091:
476:
460:
456:
440:
180:
841:
Williamson NR, Fineran PC, Gristwood T, Leeper FJ, Salmond GP (2006).
750:
Bennett JW, Bentley R (2000). "Seeing red: The story of prodigiosin".
1268:
981:
605:(KS) domain, followed by transfer to malonyl-ACP via decarboxylative
468:
364:
125:
1425:
891:. Abstract of the Annual Meeting, American Society for Microbiology.
330:
Except where otherwise noted, data are given for materials in their
1148:
Feng, Jie; Shi, Wanliang; Zhang, Shuo; Zhang, Ying (3 June 2015).
538:
528:
424:
105:
93:
83:
362:, as well as other Gram-negative, gamma proteobacteria such as
960:
Castro, A. J. (1967). "Antimalarial
Activity of Prodigiosin".
709:
Potential pharmaceutical uses of prodigiosin, or its use as a
353:
843:"The biosynthesis and regulation of bacterial prodiginines"
733:, an experimental drug with related chemical structure
562:Ring A is synthesized from L-proline through the
1195:
1193:
192:
799:— Historical Perspective and Clinical Review".
69:
713:, have led to studies of its production from
8:
836:
834:
832:
830:
551:The biosynthesis of prodigiosin and related
356:produced by many strains of the bacterium
153:
15:
1381:
1318:
1227:
1217:
1173:
1046:
407:Prodigiosin is a secondary metabolite of
212:
1399:Journal of the American Chemical Society
673:Finally, the two pieces are combined by
451:in 1264. This followed celebration of a
742:
566:(NRPS) pathway (figure 2), wherein the
267:
232:
170:
1430:Applied Microbiology and Biotechnology
431:transubstantiation miracles, in which
1288:
1286:
427:has led to a possible explanation of
239:Key: SZXDNGVQRDTJSD-FOWTUZBSSA-N
133:
113:
7:
1346:. MetaCyc Metabolic Pathway Database
754:. Advances in Applied Microbiology.
419:The ability of pigmented strains of
1344:"Pathway: prodigiosin biosynthesis"
249:Key: SZXDNGVQRDTJSD-FOWTUZBSBS
183:
1374:Advanced Synthesis & Catalysis
1154:Emerging Microbes & Infections
14:
1029:Kataoka, T.; et al. (1995).
463:priest who had doubts concerning
270:CCCCCc3cc(=Cc2c(c1ccc1)cc2OC)nc3C
902:"The Mass at Bolsena by Raphael"
684:
666:
636:
593:
471:in the Pontifical Palace in the
303:
297:
22:
801:New England Journal of Medicine
334:(at 25 °C , 100 kPa).
309:
291:
1:
764:10.1016/S0065-2164(00)47000-0
633:as well as the prodiginines.
564:nonribosomal peptide synthase
1048:10.1016/0014-5793(94)01446-8
582:by the action of the enzyme
435:bread is converted into the
1311:10.1021/acs.chemrev.6b00024
847:Nature Reviews Microbiology
813:10.1056/NEJM197904193001604
572:flavin adenine dinucleotide
1503:
1442:10.1007/s00253-018-09611-z
1370:for Prodiginine Synthesis"
1127:10.2174/092986710791331103
643:Ring C is formed from the
1366:"Condensing Enzymes from
1253:Garneau-Tsodikova, Sylvie
1206:Frontiers in Microbiology
508:, the causative agent of
328:
278:
258:
223:
53:
35:
30:
21:
1219:10.3389/fmicb.2019.01715
939:10.2217/17460913.2.6.605
609:catalysed by the enzyme
590:performs the oxidation.
1342:R. Caspi (2014-08-14).
1257:Natural Product Reports
1251:Walsh, Christopher T.;
795:Yu, Victor L. (1979). "
570:ring is oxidized, with
449:Feast of Corpus Christi
382:Streptomyces coelicolor
1383:10.1002/adsc.201900183
1368:Pseudoalteromonadaceae
679:adenosine triphosphate
645:thiamine pyrophosphate
544:
534:
399:something marvelous).
696:Details of the first
542:
532:
719:genetic modification
607:Claisen condensation
586:and then the enzyme
505:Borrelia burgdorferi
403:Secondary metabolite
1482:Biological pigments
1411:10.1021/ja00863a025
1166:10.1038/emi.2015.31
974:1967Natur.213..903C
889:Serratia marcescens
859:10.1038/nrmicro1531
797:Serratia marcescens
715:Serratia marcescens
615:pyridoxal phosphate
489:Biological activity
482:The Mass at Bolsena
421:Serratia marcescens
409:Serratia marcescens
359:Serratia marcescens
324: g·mol
18:
1092:10.1039/c3ob40477c
752:Adv Appl Microbiol
545:
535:
465:transubstantiation
459:in 1263, led by a
415:Religious function
372:Hahella chejuensis
338:Infobox references
16:
1305:(14): 7818–7853.
1121:(21): 2222–2231.
1086:(23): 3834–3845.
1080:Org. Biomol. Chem
968:(5079): 903–904.
717:, possibly after
677:and its cofactor
549:
548:
346:Chemical compound
344:
343:
95:Interactive image
1494:
1487:Pentyl compounds
1462:
1461:
1436:(4): 1667–1680.
1421:
1415:
1414:
1394:
1388:
1387:
1385:
1361:
1355:
1354:
1352:
1351:
1339:
1333:
1332:
1322:
1299:Chemical Reviews
1290:
1281:
1280:
1269:10.1039/B605245M
1248:
1242:
1241:
1231:
1221:
1197:
1188:
1187:
1177:
1145:
1139:
1138:
1110:
1104:
1103:
1075:
1069:
1068:
1050:
1026:
1020:
1017:
1011:
1008:
1002:
1001:
982:10.1038/213903a0
957:
951:
950:
927:Future Microbiol
922:
916:
915:
913:
912:
898:
892:
885:
879:
878:
838:
825:
824:
792:
786:
785:
747:
688:
670:
640:
597:
525:
524:
501:stationary phase
447:instituting the
389:is derived from
323:
311:
305:
299:
293:
286:Chemical formula
216:
196:
185:
174:
157:
137:
117:
97:
73:
26:
19:
1502:
1501:
1497:
1496:
1495:
1493:
1492:
1491:
1467:
1466:
1465:
1423:
1422:
1418:
1396:
1395:
1391:
1363:
1362:
1358:
1349:
1347:
1341:
1340:
1336:
1292:
1291:
1284:
1250:
1249:
1245:
1199:
1198:
1191:
1147:
1146:
1142:
1115:Curr. Med. Chem
1112:
1111:
1107:
1077:
1076:
1072:
1028:
1027:
1023:
1018:
1014:
1009:
1005:
959:
958:
954:
924:
923:
919:
910:
908:
906:Vatican Museums
900:
899:
895:
886:
882:
853:(12): 887–899.
840:
839:
828:
807:(16): 887–893.
794:
793:
789:
774:
749:
748:
744:
740:
727:
707:
698:total synthesis
694:
523:
518:
491:
417:
405:
367:psychroerythrus
347:
340:
335:
321:
308:
302:
296:
288:
274:
271:
266:
265:
254:
251:
250:
247:
241:
240:
237:
231:
230:
219:
199:
186:
160:
140:
120:
100:
87:
76:
63:
49:
48:-2,2′-bipyrrole
12:
11:
5:
1500:
1498:
1490:
1489:
1484:
1479:
1469:
1468:
1464:
1463:
1416:
1405:(4): 630–635.
1389:
1356:
1334:
1282:
1243:
1189:
1140:
1105:
1070:
1021:
1012:
1003:
952:
933:(6): 605–618.
917:
893:
880:
826:
787:
772:
741:
739:
736:
735:
734:
726:
723:
706:
703:
693:
690:
659:condensation.
657:intramolecular
547:
546:
536:
522:
519:
517:
514:
490:
487:
437:Body of Christ
416:
413:
404:
401:
345:
342:
341:
336:
332:standard state
329:
326:
325:
319:
313:
312:
306:
300:
294:
289:
284:
281:
280:
276:
275:
273:
272:
269:
261:
260:
259:
256:
255:
253:
252:
248:
245:
244:
242:
238:
235:
234:
226:
225:
224:
221:
220:
218:
217:
209:
207:
201:
200:
198:
197:
189:
187:
179:
176:
175:
168:
162:
161:
159:
158:
150:
148:
142:
141:
139:
138:
130:
128:
122:
121:
119:
118:
110:
108:
102:
101:
99:
98:
90:
88:
81:
78:
77:
75:
74:
66:
64:
59:
56:
55:
51:
50:
40:4-Methoxy-5--1
39:
33:
32:
28:
27:
13:
10:
9:
6:
4:
3:
2:
1499:
1488:
1485:
1483:
1480:
1478:
1475:
1474:
1472:
1459:
1455:
1451:
1447:
1443:
1439:
1435:
1431:
1427:
1420:
1417:
1412:
1408:
1404:
1400:
1393:
1390:
1384:
1379:
1375:
1371:
1369:
1360:
1357:
1345:
1338:
1335:
1330:
1326:
1321:
1316:
1312:
1308:
1304:
1300:
1296:
1289:
1287:
1283:
1278:
1274:
1270:
1266:
1263:(4): 517–31.
1262:
1258:
1254:
1247:
1244:
1239:
1235:
1230:
1225:
1220:
1215:
1211:
1207:
1203:
1196:
1194:
1190:
1185:
1181:
1176:
1171:
1167:
1163:
1159:
1155:
1151:
1144:
1141:
1136:
1132:
1128:
1124:
1120:
1116:
1109:
1106:
1101:
1097:
1093:
1089:
1085:
1081:
1074:
1071:
1066:
1062:
1058:
1054:
1049:
1044:
1040:
1036:
1032:
1025:
1022:
1016:
1013:
1007:
1004:
999:
995:
991:
987:
983:
979:
975:
971:
967:
963:
956:
953:
948:
944:
940:
936:
932:
928:
921:
918:
907:
903:
897:
894:
890:
884:
881:
876:
872:
868:
864:
860:
856:
852:
848:
844:
837:
835:
833:
831:
827:
822:
818:
814:
810:
806:
802:
798:
791:
788:
783:
779:
775:
773:9780120026470
769:
765:
761:
757:
753:
746:
743:
737:
732:
729:
728:
724:
722:
720:
716:
712:
704:
702:
699:
691:
689:
687:
682:
680:
676:
671:
669:
664:
662:
658:
654:
650:
646:
641:
639:
634:
632:
628:
624:
620:
616:
612:
608:
604:
603:keto-synthase
598:
596:
591:
589:
585:
581:
577:
574:(FAD) as the
573:
569:
565:
560:
558:
554:
541:
537:
531:
527:
526:
520:
515:
513:
511:
507:
506:
502:
497:
488:
486:
484:
483:
478:
475:, painted by
474:
470:
466:
462:
458:
454:
450:
446:
445:Pope Urban IV
442:
438:
434:
430:
426:
422:
414:
412:
410:
402:
400:
398:
394:
393:
388:
384:
383:
378:
374:
373:
368:
366:
361:
360:
355:
351:
339:
333:
327:
320:
318:
315:
314:
290:
287:
283:
282:
277:
268:
264:
257:
243:
233:
229:
222:
215:
211:
210:
208:
206:
203:
202:
195:
191:
190:
188:
182:
178:
177:
173:
169:
167:
164:
163:
156:
152:
151:
149:
147:
144:
143:
136:
132:
131:
129:
127:
124:
123:
116:
112:
111:
109:
107:
104:
103:
96:
92:
91:
89:
85:
80:
79:
72:
68:
67:
65:
62:
58:
57:
52:
47:
43:
38:
34:
29:
25:
20:
1433:
1429:
1419:
1402:
1398:
1392:
1373:
1367:
1359:
1348:. Retrieved
1337:
1302:
1298:
1260:
1256:
1246:
1209:
1205:
1157:
1153:
1143:
1118:
1114:
1108:
1083:
1079:
1073:
1041:(1): 53–59.
1038:
1034:
1024:
1015:
1006:
965:
961:
955:
930:
926:
920:
909:. Retrieved
905:
896:
888:
883:
850:
846:
804:
800:
796:
790:
755:
751:
745:
714:
708:
695:
683:
674:
672:
665:
660:
652:
648:
642:
635:
626:
622:
618:
610:
599:
592:
587:
583:
579:
561:
557:prodiginines
550:
521:Biosynthesis
510:Lyme disease
503:
492:
480:
473:Vatican City
420:
418:
408:
406:
396:
390:
386:
385:). The name
380:
370:
363:
357:
349:
348:
135:ChEMBL275787
54:Identifiers
45:
41:
17:Prodigiosin
1160:(5): e31–.
631:tambjamines
568:pyrrolidine
433:Eucharistic
423:to grow on
387:prodigiosin
377:prodiginine
352:is the red
350:Prodigiosin
279:Properties
172:Prodigiosin
115:CHEBI:82758
1471:Categories
1350:2021-04-01
911:2017-08-18
738:References
692:Laboratory
516:Production
392:prodigious
317:Molar mass
214:OL369FU7CJ
146:ChemSpider
82:3D model (
61:CAS Number
37:IUPAC name
1035:FEBS Lett
731:Obatoclax
496:apoptosis
439:. Such
1477:Pyrroles
1458:58004883
1450:30637495
1329:27314508
1277:16874387
1238:31396200
1212:: 1715.
1184:26954881
1135:20459382
1100:23640568
1065:30504320
947:18041902
875:11649828
867:17109029
782:12876793
758:: 1–32.
725:See also
711:dyestuff
576:coenzyme
461:Bohemian
441:miracles
429:Medieval
155:10577755
1320:5555159
1229:6667630
1175:5176177
1057:7851530
998:4221849
990:6030049
970:Bibcode
553:analogs
477:Raphael
457:Bolsena
443:led to
322:323.440
194:5351169
181:PubChem
71:82-89-3
1456:
1448:
1327:
1317:
1275:
1236:
1226:
1182:
1172:
1133:
1098:
1063:
1055:
996:
988:
962:Nature
945:
873:
865:
821:370597
819:
780:
770:
555:, the
469:fresco
365:Vibrio
263:SMILES
126:ChEMBL
31:Names
1454:S2CID
1061:S2CID
994:S2CID
871:S2CID
425:bread
228:InChI
106:ChEBI
84:JSmol
1446:PMID
1325:PMID
1273:PMID
1234:PMID
1180:PMID
1131:PMID
1096:PMID
1053:PMID
986:PMID
943:PMID
863:PMID
817:PMID
778:PMID
768:ISBN
705:Uses
675:pigC
661:PigB
653:PigE
649:pigD
627:pigN
625:and
623:pigF
619:pigM
611:pigJ
588:pigA
584:pigI
580:pigG
453:Mass
397:i.e.
369:and
205:UNII
166:MeSH
1438:doi
1434:103
1407:doi
1378:doi
1315:PMC
1307:doi
1303:116
1265:doi
1224:PMC
1214:doi
1170:PMC
1162:doi
1123:doi
1088:doi
1043:doi
1039:359
978:doi
966:213
935:doi
855:doi
809:doi
805:300
760:doi
455:at
354:dye
184:CID
44:,1′
1473::
1452:.
1444:.
1432:.
1428:.
1403:84
1401:.
1376:.
1372:.
1323:.
1313:.
1301:.
1297:.
1285:^
1271:.
1261:23
1259:.
1232:.
1222:.
1210:10
1208:.
1204:.
1192:^
1178:.
1168:.
1156:.
1152:.
1129:.
1119:17
1117:.
1094:.
1084:11
1082:.
1059:.
1051:.
1037:.
1033:.
992:.
984:.
976:.
964:.
941:.
929:.
904:.
869:.
861:.
849:.
845:.
829:^
815:.
803:.
776:.
766:.
756:47
721:.
651:.
512:.
485:.
479::
301:25
295:20
1460:.
1440::
1413:.
1409::
1386:.
1380::
1353:.
1331:.
1309::
1279:.
1267::
1240:.
1216::
1186:.
1164::
1158:4
1137:.
1125::
1102:.
1090::
1067:.
1045::
1000:.
980::
972::
949:.
937::
931:2
914:.
877:.
857::
851:4
823:.
811::
784:.
762::
395:(
310:O
307:3
304:N
298:H
292:C
86:)
46:H
42:H
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.