965:(WHO) functional class II or III) evaluated the safety and tolerability, and the effects on hemodynamics, exercise capacity and functional class. Riociguat was given three times daily for 12 weeks. Doses were titrated at 2-week intervals from 1.0 mg three times daily to a maximum of 2.5 mg three times daily. Riociguat had a favourable safety profile, and also significantly improved exercise capacity and hemodynamic parameters such as pulmonary vascular resistance, cardiac output and pulmonary arterial pressure compared to baseline values.
560:
537:
31:
40:
869:). In patients with pulmonary arterial hypertension eNOS levels are reduced. This results in overall lower levels of endothelial cell-derived NO and reduced vasodilation of smooth muscle cells. NO also reduces pulmonary smooth muscle cell growth and antagonises platelet inhibition, factors which play a key role in the pathogenesis of PAH. In contrast to NO- and haem-independent sGC activators like
3493:
1066:(20 mg thrice daily) in a non-randomized uncontrolled trial. The study showed potentially unfavorable safety signals with sildenafil plus riociguat and no evidence of a positive benefit/risk ratio. Therefore, the concomitant use of riociguat with phosphodiesterase-5 inhibitors is contraindicated.
1038:
rial (PATENT) was a randomized, placebo-controlled trial that investigated the efficacy and safety of riociguat in PAH patients. After a 12-week treatment the patient's exercise capacity was evaluated by measuring the change in the 6-MWT. Patients having completed PATENT-1 were invited to enter the
911:
derivative, was described in 1978. The characterisation 20 years later demonstrated that as well as increasing sGC activity, YC-1 acted in synergy with NO to stimulate sGC. However, YC-1 was a relatively weak vasodilator and had side effects. Therefore, the search began for novel indazole compounds
1013:
rial (CHEST) was a randomized, placebo-controlled trial aimed to analyse the efficacy and safety of riociguat in CTEPH patients. After a 16-week riociguat treatment the patient's exercise capacity were evaluated by measuring the change in the six-minute walk test (6-MWT). Patients having completed
1052:
This randomized, double blind, placebo controlled Phase I study investigated the effect of riociguat, administered as 2.5 mg immediate-release (IR)-tablets twice daily over 14 days, on the bone metabolism. Effects on bone formation had been seen in growing, juvenile and adolescent rats. In
912:
that were more potent and more specific sGC stimulators. The result was the identification of BAY 41-2272 and BAY 41–8543. Both compounds were tested in various preclinical studies on different animal models and appeared to improve systemic arterial oxygenation. To improve the pharmacologic and
885:
stimulates in a dose-dependent manner sGC activity up to 73-fold. In addition, it acts synergistically with diethylamine/NO, the donor of NO, to increase sGC activity in vitro up to 112-fold. A phase I study showed that riociguat is rapidly absorbed, and maximum plasma concentration is reached
980:
The phase III trials on riociguat are multi-center studies. The study program included large randomized, double-blind, placebo-controlled pivotal trial phase (CHEST-1 and PATENT-1), and open-label extensions of these studies (CHEST-2 and PATENT-2). Details of these studies are reported on
940:
of single oral doses of riociguat (0.25–5 mg). 58 healthy male subjects were given riociguat orally (oral solution or immediate-release tablet) in a randomised, placebo-controlled trial. Doses of riociguat were increased stepwise, and riociguat was well tolerated up to 2.5 mg.
1039:
extension trial, PATENT-2. The first interim analysis of PATENT-2 showed that at one year, long-term riociguat was well tolerated in patients with PAH and showed sustained benefits in 6MWD and WHO FC. The safety profile of riociguat in PATENT-2 was similar to that observed in PATENT-1.
886:
between 0.5 and 1.5 h. The mean elimination half-life appears to be 5–10 hours. Riociguat plasma concentrations have been also shown to be quite variable between patients, indicating that for clinical use it is probably necessary to titrate the drug specifically for each individual.
1053:
juvenile rats, the changes consisted of thickening of trabecular bone and hyperostosis and remodeling of metaphyseal and diaphyseal bone, whereas in adolescent rats an overall increase of bone mass was observed. On the other hand, no such effects were observed in adult rats.
1517:
Frey R, Mück W, Unger S, Artmeier-Brandt U, Weimann G, Wensing G (December 2008). "Pharmacokinetics, pharmacodynamics, tolerability, and safety of the soluble guanylate cyclase activator cinaciguat (BAY 58-2667) in healthy male volunteers".
916:
profile an additional 1000 compounds were screened leading to the discovery of riociguat. Riociguat was tested in mouse and rat disease models, where it effectively reduced pulmonary hypertension and reversed the associated
1976:
1563:"[Studies on heterocyclic compounds. XXXVI. Synthesis of furo[3,2-c]pyrazole derivatives. (4) Synthesis of 1,3-diphenylfuro[3,2-c]pyrazole-5-carboxaldehyde and its derivatives (author's transl)]"
1061:
This study investigated safety, tolerability, pharmacokinetics and the impact on pulmonary and systemic haemodynamics of single doses of 0.5 and 1 mg of riociguat in patients with PAH and stable treatment of
957:
Lung Center, was the first small study (in 4 PAH patients) to investigate safety, tolerability, pharmacokinetics and efficacy parameters. The drug was well tolerated and superior to NO in efficacy and duration.
1014:
CHEST-1 were invited to enter the extension trial, CHEST-2. The first interim analysis of CHEST-2 showed that riociguat was well tolerated, with a good long-term safety profile in patients with CTEPH.
1771:
for "Impact of
Multiple Doses of BAY 63-2521 on Safety, Tolerability, Pharmacokinetics and Pharmacodynamics in Patients With Interstitial Lung Disease (ILD) Associated Pulmonary Hypertension" at
203:
2181:
1969:
873:, the sGC stimulator riociguat directly stimulates sGC activity independent of NO and also acts in synergy with NO to produce anti-aggregatory, anti-proliferative, and vasodilatory effects.
1223:
1962:
158:
690:
1748:
807:) increase the hypotensive (blood pressure lowering) effect of riociguat. Combining such drugs is therefore contraindicated. Riociguat levels in the blood are reduced by
2174:
3513:
1602:
Stasch JP, Becker EM, Alonso-Alija C, Apeler H, Dembowsky K, Feurer A, et al. (March 2001). "NO-independent regulatory site on soluble guanylate cyclase".
731:
70:
1112:
646:
632:
3314:
2167:
674:
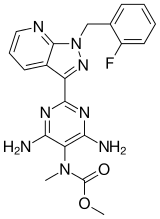
InChI=1S/C20H19FN8O2/c1-28(20(30)31-2)15-16(22)25-18(26-17(15)23)14-12-7-5-9-24-19(12)29(27-14)10-11-6-3-4-8-13(11)21/h3-9H,10H2,1-2H3,(H4,22,23,25,26)
1211:
1785:
1836:
1716:
1653:
1498:
1400:
843:
consisting of a larger alpha-subunit and a smaller haem-binding beta-subunit. The synthesised cGMP acts as a secondary messenger and activates
1433:
928:
Several clinical trials have been undertaken to investigate and evaluate diverse aspects of riociguat, and some of them are still ongoing.
1669:"Discovery of riociguat (BAY 63-2521): a potent, oral stimulator of soluble guanylate cyclase for the treatment of pulmonary hypertension"
768:(PH-IIP). A clinical trial testing riociguat for this purpose was prematurely terminated because it increased severe adverse effects and
2943:
1245:
3369:
3528:
2628:
765:
738:(PAH). Riociguat constitutes the first drug of the class of sGC stimulators. The drug has a half-life of 12 hours and will decrease
666:
2794:
1954:
2981:
2307:
1752:
2598:
735:
347:
188:
88:
2703:
3383:
2042:
836:
1185:
1891:
for "A Study to
Evaluate Efficacy and Safety of Oral BAY63-2521 in Patients With Pulmonary Arterial Hypertension (PAH)" at
3065:
2933:
986:
844:
3483:
3471:
2633:
2457:
1217:
456:
3319:
3130:
2603:
2343:
961:
An open-label, non-controlled phase II trial of riociguat in 75 adult patients (42 with CTEPH and 33 with PAH, all in
800:
516:
107:
3533:
3202:
3088:
3021:
2888:
2467:
968:
In addition, a phase II study of riociguat is underway in patients with other forms of PH such as associated with
2353:
2241:
1249:
969:
962:
785:
723:
3160:
3034:
2951:
2593:
1945:
for "Interaction Study in
Patients With Pulmonary Hypertension and Stable Treatment of Sildenafil 20 mg TID" at
3538:
2098:
1873:
for "BAY63-2521 - Long-term
Extension Study in Patients With Chronic Thromboembolic Pulmonary Hypertension" at
2848:
1302:
1300:
1265:"Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension"
784:
Serious adverse effects in clinical trials included bleeding. Hypotension (low blood pressure), headache, and
405:
3140:
2971:
2838:
1309:"NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential"
3400:
3011:
2928:
2660:
2472:
2348:
2128:
1361:"First acute haemodynamic study of soluble guanylate cyclase stimulator riociguat in pulmonary hypertension"
954:
922:
918:
144:
3300:
3207:
2956:
2688:
1457:
Schermuly RT, Stasch JP, Pullamsetti SS, Middendorff R, Müller D, Schlüter KD, et al. (October 2008).
555:
3397:
3101:
3047:
2910:
2897:
2655:
2358:
1986:
796:
727:
256:
3016:
2618:
2588:
1246:"Adempas not for use in patients with pulmonary hypertension caused by idiopathic interstitial pneumonia"
1084:"FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)"
3324:
3006:
2821:
2608:
1830:
1710:
1647:
1492:
1394:
396:
2961:
2665:
2613:
2437:
2432:
3246:
2843:
2583:
2452:
2442:
3523:
2858:
2853:
2683:
2670:
2623:
2447:
2373:
2363:
1611:
1083:
772:
in patients with pulmonary hypertension caused by idiopathic interstitial pneumonia when compared to
307:
1414:
Stasch JP, Hobbs AJ (2009). "NO-independent, haem-dependent soluble guanylate cyclase stimulators".
835:. NO binds to soluble guanylate cyclase (sGC) and mediates the synthesis of the secondary messenger
532:
3518:
3334:
3264:
865:, which results in vasodilation. NO is produced by the enzyme endothelial nitric oxide synthetase (
376:
151:
2282:
1667:
Mittendorf J, Weigand S, Alonso-Alija C, Bischoff E, Feurer A, Gerisch M, et al. (May 2009).
3001:
2986:
2764:
2744:
2713:
2565:
2522:
2287:
2267:
2262:
2257:
2196:
1946:
1928:
1910:
1892:
1874:
1856:
1772:
1732:
1635:
1543:
982:
746:
118:
3269:
3060:
2991:
2739:
2718:
3145:
2878:
2272:
2252:
1909:
for "BAY63-2521:Long-term
Extension Study in Patients With Pulmonary Arterial Hypertension" at
3180:
3175:
2789:
2784:
2708:
2542:
2403:
2398:
2393:
2388:
2232:
1818:
1698:
1627:
1584:
1535:
1480:
1439:
1429:
1382:
1338:
1286:
505:
52:
3170:
3165:
3155:
3135:
2868:
2383:
1784:
Ghofrani HA, D'Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, et al. (July 2013).
445:
3216:
2810:
2779:
2693:
2644:
2537:
2488:
2368:
1808:
1800:
1688:
1680:
1619:
1574:
1527:
1470:
1419:
1372:
1359:
Grimminger F, Weimann G, Frey R, Voswinckel R, Thamm M, Bölkow D, et al. (April 2009).
1328:
1320:
1276:
1138:
950:
937:
572:
385:
238:
2966:
2578:
1855:
for "A Study to
Evaluate Efficacy and Safety of Oral BAY63-2521 in Patients With CTEPH" at
465:
3497:
3404:
3075:
2863:
2698:
2462:
913:
866:
808:
266:
246:
1459:"Expression and function of soluble guanylate cyclase in pulmonary arterial hypertension"
559:
536:
1736:
1615:
3424:
3083:
2976:
2873:
2573:
2560:
2547:
2193:
2143:
2075:
1693:
1668:
1333:
1308:
936:
One of the first studies was designed to test the safety profile, pharmacokinetics and
804:
3507:
3365:
3114:
2923:
2552:
2532:
2527:
2512:
1547:
862:
548:
216:
2159:
764:
The substance is also contraindicated in pulmonary hypertension in combination with
3442:
3418:
2749:
2517:
2217:
2204:
2190:
2032:
2017:
2003:
1994:
1990:
1639:
1307:
Evgenov OV, Pacher P, Schmidt PM, Haskó G, Schmidt HH, Stasch JP (September 2006).
900:
840:
832:
828:
171:
166:
30:
1941:
1923:
1905:
1887:
1869:
1851:
1767:
1113:"Prescription medicines: registration of new chemical entities in Australia, 2014"
39:
1579:
1562:
1424:
1189:
1172:
80:
3455:
3226:
3150:
2759:
2754:
2639:
2480:
2378:
2222:
2050:
1281:
1264:
299:
3428:
3408:
3342:
2996:
2413:
2408:
2297:
2277:
2112:
2083:
2065:
2060:
1786:"Riociguat for the treatment of chronic thromboembolic pulmonary hypertension"
1475:
1458:
1377:
1360:
1063:
882:
870:
816:
715:
608:
436:
290:
1531:
3414:
3373:
3236:
3231:
2088:
2027:
2012:
848:
831:(NO) acts as a signaling molecule on vascular smooth muscle cells to induce
332:
320:
74:
1822:
1813:
1702:
1684:
1631:
1539:
1484:
1443:
1386:
1342:
3448:
cGMP preferring PDE inhibitors (e.g., sildenafil, paraxanthine, tadalafil)
1804:
1418:. Handbook of Experimental Pharmacology. Vol. 191. pp. 277–308.
1290:
22:
3435:
3391:
3377:
3292:
3194:
2883:
2774:
2321:
2212:
2148:
2133:
2055:
2022:
1588:
908:
416:
102:
425:
3355:
3221:
2734:
2729:
2678:
2497:
2492:
2418:
2338:
2327:
2138:
851:
773:
739:
3451:
3347:
3241:
2804:
1623:
859:
812:
326:
283:
279:
275:
271:
1324:
485:
855:
769:
758:
719:
631:
622:
496:
3329:
3256:
2830:
904:
476:
225:
2163:
1958:
210:
97:
1091:
521:
197:
129:
761:
harm and is therefore contraindicated in pregnant women.
1416:
CGMP: Generators, Effectors and
Therapeutic Implications
697:
2213:
Nitroxyl anion (NO; oxonitrate(1-), hyponitrite anion)
3481:
881:
Riociguat at concentration between 0.1 and 100
3282:
3255:
3193:
3123:
3074:
2942:
2829:
2820:
2803:
2704:
Methylamine hexamethylene methylamine/NO (MAHMA/NO)
2320:
2240:
2231:
2203:
2121:
2097:
2074:
2041:
2002:
907:-dependent sGC stimulator, YC-1, a synthetic benzyl
654:
c14ncccc4c(-c(nc2N)nc(N)c2N(C)C(=O)OC)nn1Cc3ccccc3F
620:
607:
571:
566:
547:
515:
495:
475:
455:
435:
415:
404:
395:
375:
338:
319:
306:
289:
265:
255:
245:
237:
187:
182:
157:
143:
117:
87:
69:
61:
51:
46:
2518:Amyl nitrite (isoamyl nitrite, isopentyl nitrite)
1751:. American Thoracic Society. 2009. Archived from
1728:
1726:
742:associated with pulmonary arterial hypertension.
1927:for "Effect of Riociguat on Bone Metabolism" at
2379:Naproxcinod (nitronaproxen; AZD-3582, HCT-3012)
384:
2795:N-Acetyl-N-acetoxy-4-chlorobenzenesulfonamide
2354:Ethylene glycol dinitrate (EGDN; nitroglycol)
2175:
1970:
1354:
1352:
732:chronic thromboembolic pulmonary hypertension
8:
106:
21:
1166:
1164:
1162:
1160:
2826:
2817:
2237:
2182:
2168:
2160:
1977:
1963:
1955:
1561:Yoshina S, Tanaka A, Kuo SC (March 1978).
985:, a register of studies maintained by the
558:
535:
444:
2538:Isobutyl nitrite (2-methylpropyl nitrite)
2448:Nitroglycerin (glyceryl trinitrate (GTN))
1812:
1692:
1578:
1474:
1423:
1376:
1332:
1280:
464:
811:and strong inducers of the liver enzyme
726:(sGC). It is used to treat two forms of
3488:
2614:S-Nitrosocysteine (SNC, CysNO, SNO-Cys)
2604:S-Nitroso-N-valerylpenicillamine (SNVP)
1075:
671:
651:
531:
424:
352:
2599:S-Nitroso-N-acetylpenicillamine (SNAP)
2473:Sodium trioxodinitrate (Angeli's salt)
1985:Medications used in the management of
1828:
1708:
1645:
1512:
1510:
1508:
1490:
1392:
1117:Therapeutic Goods Administration (TGA)
1048:Effect of riociguat on bone metabolism
549:
363:-pyrazolopyridin-3-yl]-5-pyrimidinyl]-
20:
3514:Soluble guanylate cyclase stimulators
1171:
504:
315:12 h (patients); 7 h (healthy people)
79:
7:
3434:non-selective PDE inhibitors (e.g.,
2218:Nitric oxide (NO; nitrogen monoxide)
854:ion concentration. This changes the
170:
2458:Pentaerithrityl tetranitrate (PETN)
2344:Diethylene glycol dinitrate (DEGDN)
1793:The New England Journal of Medicine
1269:The New England Journal of Medicine
1212:"2022 First Generic Drug Approvals"
484:
3363:Indirect/downstream NO modulators:
3131:Asymmetric dimethylarginine (ADMA)
1835:: CS1 maint: overridden setting (
1715:: CS1 maint: overridden setting (
1652:: CS1 maint: overridden setting (
1497:: CS1 maint: overridden setting (
1399:: CS1 maint: overridden setting (
1188:. Bayer HealthCare. Archived from
1005:mbolic Pulmonary Hypertension sGC-
14:
3161:Nitroarginine methyl ester (NAME)
2624:S-Nitrosoglutathione (GSNO, SNOG)
2594:S-Nitroso-N-acetylcysteine (SNAC)
2468:Propylene glycol dinitrate (PGDN)
2223:Nitrosonium (NO; nitrosyl cation)
1226:from the original on 30 June 2023
1173:FDA Professional Drug Information
1057:Interaction study with sildenafil
823:Chemistry and mechanism of action
766:idiopathic interstitial pneumonia
3491:
1520:Journal of Clinical Pharmacology
1463:The European Respiratory Journal
1365:The European Respiratory Journal
592:
589:
583:
38:
29:
2689:Diethylenetriamine/NO (DETA/NO)
2043:Endothelin receptor antagonists
1987:pulmonary arterial hypertension
1143:European Medicines Agency (EMA)
847:(protein kinase G) to regulate
736:pulmonary arterial hypertension
679:Key:WXXSNCNJFUAIDG-UHFFFAOYSA-N
2889:N-(1-Iminoethyl)-L-ornithine (
1749:"ATS International conference"
1313:Nature Reviews. Drug Discovery
1263:Giaid A, Saleh D (July 1995).
837:cyclic guanosine monophosphate
598:
577:
298:-desmethylriociguat (active),
1:
3472:Receptor/signaling modulators
3141:Guanidinoethyldisulfide (GED)
2359:Isosorbide mononitrate (ISMN)
2349:Erythritol tetranitrate (ETN)
987:National Institutes of Health
845:cGMP-dependent protein kinase
2609:S-Nitrosocaptopril (SNO-Cap)
2438:Nitroflurbiprofen (HCT-1026)
2433:Nitroatorvastatin (NCX-6560)
1580:10.1248/yakushi1947.98.3_272
1425:10.1007/978-3-540-68964-5_13
1218:Food and Drug Administration
801:phosphodiesterase inhibitors
710:, sold under the brand name
3084:Aminoguanidine (pimagedine)
2977:Aminoguanidine (pimagedine)
2874:Aminoguanidine (pimagedine)
2638:N-Nitroso compounds (e.g.,
2453:Nitropravastatin (NCX-6550)
2364:Isosorbide dinitrate (ISDN)
1282:10.1056/NEJM199507273330403
3555:
3370:AT-II receptor antagonists
3115:Nitroarginine (NNA, NOARG)
2952:1-Amino-2-hydroxyguanidine
2924:Nitroarginine (NNA, NOARG)
2671:Sodium nitroprusside (SNP)
815:, and increased by strong
786:gastrointestinal disorders
567:Chemical and physical data
3465:
1476:10.1183/09031936.00114407
1378:10.1183/09031936.00039808
1250:European Medicines Agency
976:Phase III clinical trials
970:interstitial lung disease
963:World Health Organization
724:soluble guanylate cyclase
687:
662:
642:
343:
37:
28:
3529:Drugs developed by Bayer
2849:3-Chloro-5-nitroindazole
2684:Diethylamine/NO (DEA/NO)
2656:Metal nitrosyl complexes
2584:S-Nitrosoalbumin (SNALB)
2278:Cinaciguat (BAY 58-2667)
2129:Calcium channel blockers
1532:10.1177/0091270008322906
945:Phase II clinical trials
827:In healthy individuals,
722:that is a stimulator of
3441:PDE9 inhibitors (e.g.,
2929:Pentamidine isethionate
2839:3-Bromo-7-nitroindazole
2726:Heterocyclic compounds:
2250:Activators/stimulators:
953:study, reported by the
932:Phase I clinical trials
923:ventricular remodelling
919:right heart hypertrophy
3398:L-Type calcium channel
1939:Clinical trial number
1921:Clinical trial number
1903:Clinical trial number
1885:Clinical trial number
1867:Clinical trial number
1849:Clinical trial number
1765:Clinical trial number
1685:10.1002/cmdc.200900014
1186:"Background Riociguat"
728:pulmonary hypertension
3002:Cindunistat (SD-6010)
2957:2-Ethylaminoguanidine
2714:Spermine/NO (SPER/NO)
2511:O-Nitroso compounds (
1805:10.1056/NEJMoa1209657
745:It is available as a
3388:receptor antagonists
3061:Ronopterin (VAS-203)
2760:Molsidomine (SIN-10)
2681:(diazeniumdiolates):
2661:Roussin's black salt
2374:Mannitol hexanitrate
1755:on 30 December 2009.
1034:sion sGC-Stimulator
955:University of Gießen
757:Riociguat can cause
2755:Linsidomine (SIN-1)
2652:Nitrosyl compounds:
2619:S-Nitrosodiclofenac
2589:S-Nitrosated AR545C
2574:S-Nitroso compounds
1616:2001Natur.410..212S
367:-methyl-carbaminate
206:(Prescription only)
134: X (High risk)
25:
2666:Roussin's red salt
2523:Cyclohexyl nitrite
2414:Nipradilol (K-351)
2122:Adjunctive therapy
1947:ClinicalTrials.gov
1929:ClinicalTrials.gov
1911:ClinicalTrials.gov
1893:ClinicalTrials.gov
1875:ClinicalTrials.gov
1857:ClinicalTrials.gov
1773:ClinicalTrials.gov
1733:ClinicalTrials.gov
1145:. 20 December 2007
983:ClinicalTrials.gov
903:(NO) independent,
839:(cGMP). sGC forms
747:generic medication
3534:Pyrazolopyridines
3479:
3478:
3304:
3295:
3278:
3277:
3211:
3189:
3188:
3109:
3105:
3096:
3092:
3089:N-(1-Iminoethyl)-
3055:
3051:
3042:
3038:
3035:N-(1-Iminoethyl)-
3029:
3025:
3022:N-(1-Iminoethyl)-
2962:2-Iminopiperidine
2918:
2914:
2905:
2901:
2892:
2814:
2543:Isopropyl nitrite
2489:Nitroso compounds
2331:
2316:
2315:
2157:
2156:
1435:978-3-540-68960-7
753:Contraindications
705:
704:
633:Interactive image
517:CompTox Dashboard
229:
214:
201:
133:
100:
16:Chemical compound
3546:
3496:
3495:
3494:
3487:
3405:dihydropyridines
3305:-arginine (NOHA)
3302:
3293:
3247:alpha aminoacids
3217:chlorogenic acid
3212:-arginine (NOHA)
3209:
3107:
3103:
3094:
3090:
3053:
3049:
3040:
3036:
3027:
3023:
2916:
2912:
2903:
2899:
2890:
2844:3-Chloroindazole
2827:
2818:
2808:
2506:
2505:
2443:Nitrofluvastatin
2427:
2426:
2369:Itramin tosilate
2325:
2238:
2184:
2177:
2170:
2161:
1979:
1972:
1965:
1956:
1949:
1937:
1931:
1919:
1913:
1901:
1895:
1883:
1877:
1865:
1859:
1847:
1841:
1840:
1834:
1826:
1816:
1790:
1781:
1775:
1763:
1757:
1756:
1745:
1739:
1730:
1721:
1720:
1714:
1706:
1696:
1664:
1658:
1657:
1651:
1643:
1624:10.1038/35065611
1599:
1593:
1592:
1582:
1558:
1552:
1551:
1514:
1503:
1502:
1496:
1488:
1478:
1454:
1448:
1447:
1427:
1411:
1405:
1404:
1398:
1390:
1380:
1356:
1347:
1346:
1336:
1304:
1295:
1294:
1284:
1260:
1254:
1253:
1242:
1236:
1235:
1233:
1231:
1222:. 3 March 2023.
1208:
1202:
1201:
1199:
1197:
1182:
1176:
1175:
1168:
1155:
1154:
1152:
1150:
1135:
1129:
1128:
1126:
1124:
1109:
1103:
1102:
1100:
1098:
1088:nctr-crs.fda.gov
1080:
951:proof-of-concept
938:pharmacodynamics
701:
700:
693:
635:
615:
600:
594:
591:
585:
579:
562:
551:
540:
539:
525:
523:
508:
488:
468:
448:
428:
408:
388:
311:
227:
224:
219:
212:
209:
199:
196:
174:
131:
128:
110:
99:
96:
83:
42:
33:
26:
24:
3554:
3553:
3549:
3548:
3547:
3545:
3544:
3543:
3539:Organofluorides
3504:
3503:
3502:
3492:
3490:
3482:
3480:
3475:
3461:
3425:PDE5 inhibitors
3387:
3351:
3338:
3274:
3251:
3185:
3119:
3070:
2938:
2864:7-Nitroindazole
2859:6-Nitroindazole
2854:5-Nitroindazole
2807:
2799:
2576:(thionitrites):
2504:
2501:
2500:
2499:
2463:Propatylnitrate
2425:
2422:
2421:
2420:
2324:
2312:
2227:
2199:
2188:
2158:
2153:
2117:
2093:
2076:PDE5 inhibitors
2070:
2037:
1998:
1983:
1953:
1952:
1938:
1934:
1920:
1916:
1902:
1898:
1884:
1880:
1866:
1862:
1848:
1844:
1827:
1788:
1783:
1782:
1778:
1764:
1760:
1747:
1746:
1742:
1731:
1724:
1707:
1666:
1665:
1661:
1644:
1610:(6825): 212–5.
1601:
1600:
1596:
1569:(in Japanese).
1567:Yakugaku Zasshi
1560:
1559:
1555:
1526:(12): 1400–10.
1516:
1515:
1506:
1489:
1456:
1455:
1451:
1436:
1413:
1412:
1408:
1391:
1358:
1357:
1350:
1325:10.1038/nrd2038
1306:
1305:
1298:
1262:
1261:
1257:
1252:. 24 June 2016.
1244:
1243:
1239:
1229:
1227:
1210:
1209:
1205:
1195:
1193:
1192:on 18 July 2011
1184:
1183:
1179:
1169:
1158:
1148:
1146:
1137:
1136:
1132:
1122:
1120:
1111:
1110:
1106:
1096:
1094:
1082:
1081:
1077:
1072:
1059:
1050:
1045:
1020:
995:
978:
947:
934:
914:pharmacokinetic
897:
892:
879:
825:
809:tobacco smoking
805:PDE5 inhibitors
794:
788:also occurred.
782:
780:Adverse effects
755:
696:
694:
691:(what is this?)
688:
683:
680:
675:
670:
669:
658:
655:
650:
649:
638:
613:
603:
597:
588:
582:
543:
519:
511:
491:
471:
451:
431:
411:
391:
371:
368:
351:
350:
330:
309:
257:Protein binding
247:Bioavailability
239:Pharmacokinetic
233:
217:
178:
146:
139:
120:
113:
17:
12:
11:
5:
3552:
3550:
3542:
3541:
3536:
3531:
3526:
3521:
3516:
3506:
3505:
3501:
3500:
3477:
3476:
3466:
3463:
3462:
3460:
3459:
3449:
3446:
3439:
3432:
3422:
3412:
3395:
3385:
3381:
3366:ACE inhibitors
3359:
3358:
3353:
3349:
3345:
3340:
3336:
3332:
3327:
3322:
3317:
3308:
3307:
3298:
3286:
3284:
3280:
3279:
3276:
3275:
3273:
3272:
3267:
3261:
3259:
3253:
3252:
3250:
3249:
3244:
3239:
3234:
3229:
3224:
3219:
3214:
3205:
3199:
3197:
3191:
3190:
3187:
3186:
3184:
3183:
3178:
3173:
3168:
3163:
3158:
3153:
3148:
3143:
3138:
3133:
3127:
3125:
3121:
3120:
3118:
3117:
3112:
3099:
3086:
3080:
3078:
3072:
3071:
3069:
3068:
3063:
3058:
3045:
3032:
3019:
3014:
3009:
3004:
2999:
2994:
2989:
2984:
2979:
2974:
2969:
2964:
2959:
2954:
2948:
2946:
2940:
2939:
2937:
2936:
2931:
2926:
2921:
2908:
2895:
2886:
2881:
2876:
2871:
2866:
2861:
2856:
2851:
2846:
2841:
2835:
2833:
2824:
2815:
2801:
2800:
2798:
2797:
2792:
2787:
2782:
2777:
2768:
2767:
2762:
2757:
2752:
2737:
2722:
2721:
2716:
2711:
2706:
2701:
2696:
2691:
2686:
2674:
2673:
2668:
2663:
2648:
2647:
2631:
2626:
2621:
2616:
2611:
2606:
2601:
2596:
2591:
2586:
2581:
2569:-Butyl nitrite
2563:
2561:Pentyl nitrite
2558:
2556:-Butyl nitrite
2550:
2548:Methyl nitrite
2545:
2540:
2535:
2530:
2525:
2520:
2513:alkyl nitrites
2502:
2484:
2483:
2478:
2475:
2470:
2465:
2460:
2455:
2450:
2445:
2440:
2435:
2430:
2423:
2416:
2411:
2406:
2401:
2396:
2391:
2386:
2381:
2376:
2371:
2366:
2361:
2356:
2351:
2346:
2334:
2332:
2318:
2317:
2314:
2313:
2311:
2310:
2301:
2300:
2295:
2290:
2285:
2280:
2275:
2270:
2265:
2260:
2255:
2246:
2244:
2235:
2229:
2228:
2226:
2225:
2220:
2215:
2209:
2207:
2201:
2200:
2189:
2187:
2186:
2179:
2172:
2164:
2155:
2154:
2152:
2151:
2146:
2144:Oxygen therapy
2141:
2136:
2131:
2125:
2123:
2119:
2118:
2116:
2115:
2110:
2104:
2102:
2095:
2094:
2092:
2091:
2086:
2080:
2078:
2072:
2071:
2069:
2068:
2063:
2058:
2053:
2047:
2045:
2039:
2038:
2036:
2035:
2030:
2025:
2020:
2015:
2009:
2007:
2000:
1999:
1984:
1982:
1981:
1974:
1967:
1959:
1951:
1950:
1932:
1914:
1896:
1878:
1860:
1842:
1776:
1758:
1740:
1722:
1659:
1594:
1553:
1504:
1449:
1434:
1406:
1348:
1296:
1255:
1237:
1203:
1177:
1156:
1139:"Adempas EPAR"
1130:
1119:. 21 June 2022
1104:
1074:
1073:
1071:
1068:
1058:
1055:
1049:
1046:
1044:
1041:
1019:
1016:
994:
991:
977:
974:
946:
943:
933:
930:
896:
893:
891:
888:
878:
875:
824:
821:
793:
790:
781:
778:
754:
751:
703:
702:
685:
684:
682:
681:
678:
676:
673:
665:
664:
663:
660:
659:
657:
656:
653:
645:
644:
643:
640:
639:
637:
636:
628:
626:
618:
617:
611:
605:
604:
601:
595:
586:
580:
575:
569:
568:
564:
563:
553:
545:
544:
542:
541:
533:DTXSID50978109
528:
526:
513:
512:
510:
509:
501:
499:
493:
492:
490:
489:
481:
479:
473:
472:
470:
469:
461:
459:
453:
452:
450:
449:
441:
439:
433:
432:
430:
429:
421:
419:
413:
412:
410:
409:
401:
399:
393:
392:
390:
389:
381:
379:
373:
372:
370:
369:
354:
346:
345:
344:
341:
340:
336:
335:
323:
317:
316:
313:
304:
303:
293:
287:
286:
269:
263:
262:
259:
253:
252:
249:
243:
242:
235:
234:
232:
231:
222:
207:
193:
191:
185:
184:
180:
179:
177:
176:
163:
161:
155:
154:
149:
147:administration
141:
140:
138:
137:
135:
125:
123:
115:
114:
112:
111:
93:
91:
85:
84:
77:
67:
66:
63:
59:
58:
55:
49:
48:
44:
43:
35:
34:
15:
13:
10:
9:
6:
4:
3:
2:
3551:
3540:
3537:
3535:
3532:
3530:
3527:
3525:
3522:
3520:
3517:
3515:
3512:
3511:
3509:
3499:
3489:
3485:
3474:
3473:
3470:
3464:
3457:
3453:
3450:
3447:
3444:
3440:
3437:
3433:
3430:
3426:
3423:
3420:
3416:
3413:
3410:
3406:
3402:
3399:
3396:
3393:
3389:
3382:
3379:
3375:
3371:
3367:
3364:
3361:
3360:
3357:
3354:
3352:
3346:
3344:
3341:
3339:
3333:
3331:
3328:
3326:
3323:
3321:
3318:
3316:
3313:
3310:
3309:
3306:
3299:
3297:
3291:
3288:
3287:
3285:
3281:
3271:
3268:
3266:
3265:Calmidazolium
3263:
3262:
3260:
3258:
3254:
3248:
3245:
3243:
3240:
3238:
3235:
3233:
3230:
3228:
3225:
3223:
3220:
3218:
3215:
3213:
3206:
3204:
3201:
3200:
3198:
3196:
3192:
3182:
3179:
3177:
3174:
3172:
3169:
3167:
3164:
3162:
3159:
3157:
3154:
3152:
3149:
3147:
3144:
3142:
3139:
3137:
3134:
3132:
3129:
3128:
3126:
3122:
3116:
3113:
3111:
3100:
3098:
3087:
3085:
3082:
3081:
3079:
3077:
3073:
3067:
3064:
3062:
3059:
3057:
3046:
3044:
3033:
3031:
3020:
3018:
3015:
3013:
3010:
3008:
3005:
3003:
3000:
2998:
2995:
2993:
2990:
2988:
2985:
2983:
2980:
2978:
2975:
2973:
2970:
2968:
2965:
2963:
2960:
2958:
2955:
2953:
2950:
2949:
2947:
2945:
2941:
2935:
2932:
2930:
2927:
2925:
2922:
2920:
2909:
2907:
2896:
2894:
2887:
2885:
2882:
2880:
2877:
2875:
2872:
2870:
2867:
2865:
2862:
2860:
2857:
2855:
2852:
2850:
2847:
2845:
2842:
2840:
2837:
2836:
2834:
2832:
2828:
2825:
2823:
2819:
2816:
2812:
2806:
2802:
2796:
2793:
2791:
2788:
2786:
2783:
2781:
2778:
2776:
2773:
2770:
2769:
2766:
2763:
2761:
2758:
2756:
2753:
2751:
2748:
2746:
2741:
2738:
2736:
2733:
2731:
2727:
2724:
2723:
2720:
2717:
2715:
2712:
2710:
2707:
2705:
2702:
2700:
2697:
2695:
2692:
2690:
2687:
2685:
2682:
2680:
2676:
2675:
2672:
2669:
2667:
2664:
2662:
2659:
2657:
2653:
2650:
2649:
2646:
2643:
2641:
2635:
2632:
2630:
2627:
2625:
2622:
2620:
2617:
2615:
2612:
2610:
2607:
2605:
2602:
2600:
2597:
2595:
2592:
2590:
2587:
2585:
2582:
2580:
2577:
2575:
2570:
2568:
2564:
2562:
2559:
2557:
2555:
2551:
2549:
2546:
2544:
2541:
2539:
2536:
2534:
2533:Hexyl nitrite
2531:
2529:
2528:Ethyl nitrite
2526:
2524:
2521:
2519:
2516:
2514:
2508:
2496:
2494:
2490:
2486:
2485:
2482:
2479:
2476:
2474:
2471:
2469:
2466:
2464:
2461:
2459:
2456:
2454:
2451:
2449:
2446:
2444:
2441:
2439:
2436:
2434:
2431:
2429:
2417:
2415:
2412:
2410:
2407:
2405:
2402:
2400:
2397:
2395:
2392:
2390:
2387:
2385:
2382:
2380:
2377:
2375:
2372:
2370:
2367:
2365:
2362:
2360:
2357:
2355:
2352:
2350:
2347:
2345:
2342:
2340:
2336:
2335:
2333:
2329:
2323:
2319:
2309:
2306:
2303:
2302:
2299:
2296:
2294:
2291:
2289:
2286:
2284:
2281:
2279:
2276:
2274:
2271:
2269:
2266:
2264:
2261:
2259:
2256:
2254:
2251:
2248:
2247:
2245:
2243:
2239:
2236:
2234:
2230:
2224:
2221:
2219:
2216:
2214:
2211:
2210:
2208:
2206:
2202:
2198:
2195:
2192:
2185:
2180:
2178:
2173:
2171:
2166:
2165:
2162:
2150:
2147:
2145:
2142:
2140:
2137:
2135:
2132:
2130:
2127:
2126:
2124:
2120:
2114:
2111:
2109:
2106:
2105:
2103:
2100:
2096:
2090:
2087:
2085:
2082:
2081:
2079:
2077:
2073:
2067:
2064:
2062:
2059:
2057:
2054:
2052:
2049:
2048:
2046:
2044:
2040:
2034:
2031:
2029:
2026:
2024:
2021:
2019:
2016:
2014:
2011:
2010:
2008:
2005:
2001:
1996:
1992:
1988:
1980:
1975:
1973:
1968:
1966:
1961:
1960:
1957:
1948:
1944:
1943:
1936:
1933:
1930:
1926:
1925:
1918:
1915:
1912:
1908:
1907:
1900:
1897:
1894:
1890:
1889:
1882:
1879:
1876:
1872:
1871:
1864:
1861:
1858:
1854:
1853:
1846:
1843:
1838:
1832:
1824:
1820:
1815:
1814:10044/1/19669
1810:
1806:
1802:
1799:(4): 319–29.
1798:
1794:
1787:
1780:
1777:
1774:
1770:
1769:
1762:
1759:
1754:
1750:
1744:
1741:
1738:
1734:
1729:
1727:
1723:
1718:
1712:
1704:
1700:
1695:
1690:
1686:
1682:
1679:(5): 853–65.
1678:
1674:
1670:
1663:
1660:
1655:
1649:
1641:
1637:
1633:
1629:
1625:
1621:
1617:
1613:
1609:
1605:
1598:
1595:
1590:
1586:
1581:
1576:
1572:
1568:
1564:
1557:
1554:
1549:
1545:
1541:
1537:
1533:
1529:
1525:
1521:
1513:
1511:
1509:
1505:
1500:
1494:
1486:
1482:
1477:
1472:
1469:(4): 881–91.
1468:
1464:
1460:
1453:
1450:
1445:
1441:
1437:
1431:
1426:
1421:
1417:
1410:
1407:
1402:
1396:
1388:
1384:
1379:
1374:
1371:(4): 785–92.
1370:
1366:
1362:
1355:
1353:
1349:
1344:
1340:
1335:
1330:
1326:
1322:
1319:(9): 755–68.
1318:
1314:
1310:
1303:
1301:
1297:
1292:
1288:
1283:
1278:
1275:(4): 214–21.
1274:
1270:
1266:
1259:
1256:
1251:
1247:
1241:
1238:
1225:
1221:
1219:
1213:
1207:
1204:
1191:
1187:
1181:
1178:
1174:
1167:
1165:
1163:
1161:
1157:
1144:
1140:
1134:
1131:
1118:
1114:
1108:
1105:
1093:
1089:
1085:
1079:
1076:
1069:
1067:
1065:
1056:
1054:
1047:
1043:Other studies
1042:
1040:
1037:
1033:
1030:rterial Hyper
1029:
1025:
1017:
1015:
1012:
1008:
1004:
1001:ronic Thrombo
1000:
992:
990:
988:
984:
975:
973:
971:
966:
964:
959:
956:
952:
944:
942:
939:
931:
929:
926:
924:
920:
915:
910:
906:
902:
894:
889:
887:
884:
876:
874:
872:
868:
864:
863:contractility
861:
857:
853:
850:
846:
842:
838:
834:
830:
822:
820:
818:
814:
810:
806:
802:
798:
791:
789:
787:
779:
777:
775:
771:
767:
762:
760:
752:
750:
748:
743:
741:
737:
733:
729:
725:
721:
717:
713:
709:
699:
692:
686:
677:
672:
668:
661:
652:
648:
641:
634:
630:
629:
627:
624:
619:
612:
610:
606:
576:
574:
570:
565:
561:
557:
554:
552:
550:ECHA InfoCard
546:
538:
534:
530:
529:
527:
518:
514:
507:
503:
502:
500:
498:
494:
487:
483:
482:
480:
478:
474:
467:
463:
462:
460:
458:
454:
447:
443:
442:
440:
438:
434:
427:
423:
422:
420:
418:
414:
407:
403:
402:
400:
398:
394:
387:
383:
382:
380:
378:
374:
366:
362:
358:
353:
349:
342:
337:
334:
328:
324:
322:
318:
314:
312:
305:
301:
297:
294:
292:
288:
285:
281:
277:
273:
270:
268:
264:
260:
258:
254:
250:
248:
244:
240:
236:
230: Rx-only
223:
220:
208:
205:
195:
194:
192:
190:
186:
181:
173:
168:
165:
164:
162:
160:
156:
153:
150:
148:
142:
136:
127:
126:
124:
122:
116:
109:
104:
95:
94:
92:
90:
86:
82:
78:
76:
72:
68:
64:
60:
56:
54:
50:
47:Clinical data
45:
41:
36:
32:
27:
19:
3468:
3467:
3443:paraxanthine
3419:beta blocker
3362:
3311:
3289:
3093:-ornithine (
3026:-ornithine (
2771:
2750:Feprosidnine
2745:Sydnonimines
2743:
2728:
2725:
2677:
2654:
2651:
2640:nitrosamines
2637:
2572:
2566:
2553:
2510:
2487:
2337:
2304:
2292:
2283:GSK-2181236A
2249:
2191:Nitric oxide
2107:
2033:Treprostinil
2018:Epoprostenol
2004:Prostacyclin
1940:
1935:
1922:
1917:
1904:
1899:
1886:
1881:
1868:
1863:
1850:
1845:
1831:cite journal
1796:
1792:
1779:
1766:
1761:
1753:the original
1743:
1711:cite journal
1676:
1672:
1662:
1648:cite journal
1607:
1603:
1597:
1573:(3): 272–9.
1570:
1566:
1556:
1523:
1519:
1493:cite journal
1466:
1462:
1452:
1415:
1409:
1395:cite journal
1368:
1364:
1316:
1312:
1272:
1268:
1258:
1240:
1228:. Retrieved
1215:
1206:
1194:. Retrieved
1190:the original
1180:
1147:. Retrieved
1142:
1133:
1121:. Retrieved
1116:
1107:
1095:. Retrieved
1087:
1078:
1060:
1051:
1035:
1031:
1027:
1023:
1021:
1010:
1006:
1002:
998:
996:
979:
967:
960:
948:
935:
927:
901:nitric oxide
898:
880:
877:Pharmacology
841:heterodimers
833:vasodilation
829:nitric oxide
826:
819:inhibitors.
795:
792:Interactions
783:
763:
756:
744:
734:(CTEPH) and
711:
707:
706:
695:
689:
364:
360:
356:
308:Elimination
295:
189:Legal status
183:Legal status
89:License data
18:
3524:Pyrimidines
3456:simvastatin
3290:Precursors:
3227:epicatechin
3151:Indospicine
3106:-arginine (
3052:-arginine (
2987:AR-C 102222
2915:-arginine (
2902:-arginine (
2765:Sydnonimine
2498:Nitrite (NO
2481:Trolnitrate
2477:Tenitramine
2419:Nitrate (NO
2305:Inhibitors:
2288:Praliciguat
2268:BAY 60-4552
2263:BAY 41-8543
2258:BAY 41-2272
2101:stimulators
2051:Ambrisentan
1942:NCT00680654
1924:NCT00855660
1906:NCT00863681
1888:NCT00810693
1870:NCT00910429
1852:NCT00855465
1768:NCT00694850
1673:ChemMedChem
1196:15 December
803:(including
616: g·mol
556:100.169.606
506:CHEBI:76018
386:625115-55-1
339:Identifiers
331:48–59% via
325:33–45% via
300:glucuronide
291:Metabolites
65:BAY 63-2521
62:Other names
53:Trade names
3519:Carbamates
3508:Categories
3429:sildenafil
3409:nifedipine
3312:Cofactors:
3301:N-Hydroxy-
3208:N-Hydroxy-
2997:Canavanine
2992:BYK-191023
2811:inhibitors
2740:REC15/2739
2719:V-PYRRO/NO
2409:Nicorandil
2298:Vericiguat
2197:modulators
2113:Vericiguat
2084:Sildenafil
2066:Sitaxentan
2061:Macitentan
1170:Riociguat
1097:22 October
1070:References
1064:sildenafil
1009:timulator
972:(PH-ILD).
899:The first
871:cinaciguat
817:cytochrome
716:medication
621:3D model (
609:Molar mass
466:RU3FE2Y4XI
437:ChemSpider
397:IUPHAR/BPS
377:CAS Number
348:IUPAC name
302:(inactive)
267:Metabolism
3469:See also:
3415:Nebivolol
3374:captopril
3296:-Arginine
3237:norvaline
3232:ornithine
3146:GW-273629
3102:N-Methyl-
3048:N-Methyl-
3039:-lysine (
2911:N-Propyl-
2898:N-Methyl-
2879:ARL-17477
2772:Unsorted:
2322:NO donors
2293:Riociguat
2273:BI-703704
2253:Ataciguat
2194:signaling
2134:Diuretics
2108:Riociguat
2089:Tadalafil
2028:Selexipag
2013:Beraprost
2006:analogues
1737:Riociguat
1548:206433961
1149:10 August
1026:ulmonary
895:Discovery
849:cytosolic
770:mortality
708:Riociguat
333:bile duct
321:Excretion
310:half-life
145:Routes of
119:Pregnancy
108:Riociguat
81:Monograph
75:Drugs.com
23:Riociguat
3498:Medicine
3436:caffeine
3401:blockers
3392:bosentan
3378:losartan
3195:Arginase
3181:VAS-2381
3176:ONO-1714
3124:Unsorted
2884:Indazole
2790:FR146881
2785:FR144220
2775:Cimlanod
2730:Furoxans
2709:PROLI/NO
2679:NONOates
2629:SNO-t-PA
2493:nitrites
2404:NCX-4215
2399:NCX 4040
2394:NCX-4016
2389:NCX-2216
2339:Nitrates
2328:prodrugs
2149:Warfarin
2056:Bosentan
2023:Iloprost
1823:23883377
1703:19263460
1632:11242081
1540:18779378
1485:18550612
1444:19089334
1387:19129292
1343:16955067
1224:Archived
1123:10 April
909:indazole
797:Nitrates
698:(verify)
417:DrugBank
159:ATC code
152:By mouth
121:category
103:DailyMed
3454:(e.g.,
3452:Statins
3427:(e.g.,
3403:(e.g.,
3390:(e.g.,
3372:(e.g.,
3222:ginseng
3171:NXN-462
3166:NCX-456
3156:KD-7040
3136:CKD-712
2869:A-84643
2735:Furoxan
2634:SNO-vWF
2384:NCX-466
2233:Targets
2139:Digoxin
1694:3313366
1640:4402074
1612:Bibcode
1334:2225477
1291:7540722
1230:30 June
989:(NIH).
890:History
852:calcium
774:placebo
740:dyspnea
714:, is a
712:Adempas
614:422.424
573:Formula
446:9479719
426:DB08931
355:Methyl
221:Rx-only
218:WARNING
175:)
169: (
167:C02KX05
105::
57:Adempas
3484:Portal
3283:Others
3242:lysine
2805:Enzyme
2780:FK-409
2694:GLO/NO
2645:SIN-1A
1821:
1701:
1691:
1638:
1630:
1604:Nature
1589:650406
1587:
1546:
1538:
1483:
1442:
1432:
1385:
1341:
1331:
1289:
1018:PATENT
860:myosin
813:CYP3A4
730:(PH):
647:SMILES
486:D09572
327:kidney
284:CYP2J2
280:CYP2C8
276:CYP3A4
272:CYP1A1
215:
202:
101:
3315:NADPH
3110:-NMA)
3097:-NIO)
3056:-NMA)
3043:-NIL)
3030:-NIO)
2972:AEITU
2967:1400W
2919:-NPA)
2906:-NMA)
2893:-NIO)
2579:LA810
2205:Forms
1789:(PDF)
1636:S2CID
1544:S2CID
1220:(FDA)
1216:U.S.
993:CHEST
856:actin
759:fetal
720:Bayer
667:InChI
623:JSmol
497:ChEBI
3330:Heme
3257:CAMK
3076:eNOS
3066:TRIM
3017:MITU
3012:IPTU
3007:EITU
2944:iNOS
2934:TRIM
2831:nNOS
2699:JS-K
2567:tert
1837:link
1819:PMID
1717:link
1699:PMID
1654:link
1628:PMID
1585:PMID
1536:PMID
1499:link
1481:PMID
1440:PMID
1430:ISBN
1401:link
1383:PMID
1339:PMID
1287:PMID
1232:2023
1198:2009
1151:2024
1125:2023
1099:2023
1022:The
997:The
921:and
905:haem
867:eNOS
799:and
477:KEGG
457:UNII
406:5257
241:data
71:AHFS
3343:CaM
3325:FMN
3320:FAD
3270:W-7
3203:ABH
2982:AMT
2822:NOS
2308:ODQ
2242:sGC
2099:sGC
1995:C02
1991:B01
1809:hdl
1801:doi
1797:369
1689:PMC
1681:doi
1620:doi
1608:410
1575:doi
1528:doi
1471:doi
1420:doi
1373:doi
1329:PMC
1321:doi
1277:doi
1273:333
1092:FDA
1032:ten
718:by
522:EPA
359:--1
261:95%
251:94%
172:WHO
3510::
3407::
3384:ET
3376:,
3356:Ca
3335:BH
2742:;
2642:):
2636:;
2571:;
2515:):
2509:;
1993:,
1833:}}
1829:{{
1817:.
1807:.
1795:.
1791:.
1735::
1725:^
1713:}}
1709:{{
1697:.
1687:.
1675:.
1671:.
1650:}}
1646:{{
1634:.
1626:.
1618:.
1606:.
1583:.
1571:98
1565:.
1542:.
1534:.
1524:48
1522:.
1507:^
1495:}}
1491:{{
1479:.
1467:32
1465:.
1461:.
1438:.
1428:.
1397:}}
1393:{{
1381:.
1369:33
1367:.
1363:.
1351:^
1337:.
1327:.
1315:.
1311:.
1299:^
1285:.
1271:.
1267:.
1248:.
1214:.
1159:^
1141:.
1115:.
1090:.
1086:.
999:Ch
949:A
925:.
883:μM
776:.
749:.
587:19
581:20
282:,
278:,
274:,
226:EU
211:US
204:S4
198:AU
130:AU
98:US
3486::
3458:)
3445:)
3438:)
3431:)
3421:)
3417:(
3411:)
3394:)
3386:B
3380:)
3368:/
3350:2
3348:O
3337:4
3303:L
3294:L
3210:L
3108:L
3104:L
3095:L
3091:L
3054:L
3050:L
3041:L
3037:L
3028:L
3024:L
2917:L
2913:L
2904:L
2900:L
2891:L
2813:)
2809:(
2747::
2732::
2658::
2554:n
2507:)
2503:2
2495::
2491:/
2428:)
2424:3
2341::
2330:)
2326:(
2183:e
2176:t
2169:v
1997:)
1989:(
1978:e
1971:t
1964:v
1839:)
1825:.
1811::
1803::
1719:)
1705:.
1683::
1677:4
1656:)
1642:.
1622::
1614::
1591:.
1577::
1550:.
1530::
1501:)
1487:.
1473::
1446:.
1422::
1403:)
1389:.
1375::
1345:.
1323::
1317:5
1293:.
1279::
1234:.
1200:.
1153:.
1127:.
1101:.
1036:T
1028:A
1024:P
1011:T
1007:S
1003:e
858:–
625:)
602:2
599:O
596:8
593:N
590:F
584:H
578:C
524:)
520:(
365:N
361:H
357:N
329:,
296:N
228::
213::
200::
132::
73:/
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.