556:
533:
836:
29:
1475:
944:
rejected P&G's attempted injunction. P&G was criticized for attempting to "preserve its market share by denigrating Boniva". Judge Crotty wrote that "Roche was clearly entitled to respond with its own data, provided that the data was truthfully and accurately presented".
940:. The manufacturers of Boniva, a rival bisphosphonate, were accused in the suit of causing a "serious public health risk" through misrepresentation of scientific findings. In a ruling on September 7, 2006, U.S. District Judge
1216:
184:
1525:
139:
686:
1500:
1209:
1071:
1202:
908:
under the trade names
Actonel, Atelvia, and Benet. It is also available in a preparation that includes a calcium carbonate supplement, as Actonel with Calcium.
51:
642:
1124:
1085:
628:
1422:
1515:
1047:
1022:
883:
662:
857:
985:
948:
In 2006, P&G faced controversy over its handling of clinical research involving risedronate (News
Reports and discussion).
295:
169:
69:
861:
905:
1465:
1106:
1510:
1505:
1327:
417:
512:
88:
846:
1319:
719:
865:
850:
1495:
956:
366:
1360:
125:
551:
1132:
241:
670:
InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)4-6-2-1-3-8-5-6/h1-3,5,9H,4H2,(H2,10,11,12)(H2,13,14,15)
357:
473:
261:
1183:
917:
723:
528:
312:
132:
1370:
1171:
1065:
99:
494:
1425:
1053:
1043:
1018:
1012:
929:
484:
210:
197:
41:
1194:
568:
321:
223:
426:
1520:
1479:
1332:
1307:
1287:
1277:
1272:
1267:
1262:
1257:
1252:
937:
933:
897:
809:
791:
781:
771:
761:
406:
251:
231:
61:
555:
532:
1302:
1297:
1239:
952:
941:
921:
901:
751:
711:
1489:
1430:
544:
1435:
1230:
1226:
715:
152:
147:
1146:
346:
1408:
1400:
1353:
835:
20:
714:. It slows down the cells which break down bone. It's used to treat or prevent
1442:
1415:
1393:
925:
604:
397:
1057:
1452:
1385:
274:
55:
1247:
377:
83:
386:
28:
1447:
332:
955:
drugs, risedronate appears to be associated with the rare side effect
464:
279:
959:, often preceded by dental procedures inducing trauma to the bone.
453:
446:
1378:
627:
618:
500:
437:
191:
1198:
204:
78:
829:
729:
It was patented in 1984 and approved for medical use in 1998.
517:
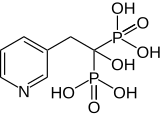
303:(1-hydroxy-1-phosphono-2-pyridin-3-yl-ethyl)phosphonic acid
178:
110:
693:
1463:
1341:
1318:
1238:
1107:"Boniva advertising 'not misleading' says US judge"
980:
978:
976:
974:
972:
616:
603:
567:
562:
543:
511:
483:
463:
436:
416:
396:
376:
365:
356:
331:
311:
286:
273:
260:
250:
240:
230:
222:
168:
163:
138:
124:
98:
68:
50:
40:
35:
986:"Actonel- risedronate sodium tablet, film coated"
1526:Drugs developed by Takeda Pharmaceutical Company
345:
320:
928:false claims lawsuit against rival drugmakers
1210:
8:
87:
19:
864:. Unsourced material may be challenged and
215:In general: ℞ (Prescription only)
1501:Farnesyl pyrophosphate synthase inhibitors
1217:
1203:
1195:
1070:: CS1 maint: location missing publisher (
554:
531:
405:
884:Learn how and when to remove this message
425:
736:
1470:
968:
667:
647:
527:
385:
300:
1063:
1017:. John Wiley & Sons. p. 523.
545:
18:
493:
472:
60:
7:
1190:. U.S. National Library of Medicine.
1178:. U.S. National Library of Medicine.
1149:. Scientific-misconduct.blogspot.com
862:adding citations to reliable sources
499:
151:
1423:Parathyroid hormone-related protein
452:
445:
336:
1040:Essentials of medical pharmacology
594:
14:
1038:Tripathi KD (30 September 2013).
936:claiming false advertising about
650:OC(Cc1cccnc1)(P(=O)(O)O)P(=O)(O)O
1473:
834:
706:, often used as its sodium salt
585:
579:
27:
1042:(Seventh ed.). New Delhi.
1011:Fischer J, Ganellin CR (2006).
896:It is produced and marketed by
675:Key:IIDJRNMFWXDHID-UHFFFAOYSA-N
46:Actonel, Atelvia, Benet, others
588:
573:
1:
1014:Analogue-based Drug Discovery
1147:"Scientific Misconduct Blog"
1125:"Actonel Case Media Reports"
1320:Bone morphogenetic proteins
1542:
1129:Scientific Misconduct Wiki
920:and its marketing partner
563:Chemical and physical data
1516:Drugs developed by AbbVie
1086:"P&G Press statement"
683:
658:
638:
291:
26:
957:osteonecrosis of the jaw
1361:Aluminium chlorohydrate
1225:Drugs for treatment of
1188:Drug Information Portal
1176:Drug Information Portal
720:Paget's disease of bone
1366:Dual action bone agent
951:In common with other
1506:Procter & Gamble
1349:Resorption inhibitor
1184:"Risedronate sodium"
858:improve this section
1511:3-Pyridyl compounds
1135:on 2 February 2009.
1113:. 8 September 2006.
821:Society and culture
739:
200:(Prescription only)
187:(Prescription only)
23:
1371:Strontium ranelate
904:, and in Japan by
737:
708:risedronate sodium
1461:
1460:
1172:"Risedronic acid"
992:. 1 November 2019
894:
893:
886:
818:
817:
746:Relative potency
738:Relative potency
701:
700:
629:Interactive image
513:CompTox Dashboard
208:
195:
182:
114:
81:
16:Chemical compound
1533:
1478:
1477:
1476:
1469:
1294:Non-nitrogenous
1219:
1212:
1205:
1196:
1191:
1179:
1158:
1157:
1155:
1154:
1143:
1137:
1136:
1131:. Archived from
1121:
1115:
1114:
1103:
1097:
1096:
1094:
1093:
1082:
1076:
1075:
1069:
1061:
1035:
1029:
1028:
1008:
1002:
1001:
999:
997:
982:
916:In January 2006
889:
882:
878:
875:
869:
838:
830:
740:
697:
696:
689:
631:
611:
596:
590:
587:
581:
575:
558:
547:
536:
535:
521:
519:
503:
497:
476:
456:
449:
429:
409:
389:
369:
349:
339:
338:
324:
265:
206:
203:
193:
190:
180:
177:
155:
112:
109:
91:
80:
77:
64:
31:
24:
22:
1541:
1540:
1536:
1535:
1534:
1532:
1531:
1530:
1496:Bisphosphonates
1486:
1485:
1484:
1474:
1472:
1464:
1462:
1457:
1337:
1333:Eptotermin alfa
1328:Dibotermin alfa
1314:
1308:Tiludronic acid
1288:Zoledronic acid
1283:Risedronic acid
1278:Pamidronic acid
1273:Neridronic acid
1268:Minodronic acid
1263:Incadronic acid
1258:Ibandronic acid
1253:Alendronic acid
1240:Bisphosphonates
1234:
1223:
1182:
1170:
1167:
1162:
1161:
1152:
1150:
1145:
1144:
1140:
1123:
1122:
1118:
1105:
1104:
1100:
1091:
1089:
1084:
1083:
1079:
1062:
1050:
1037:
1036:
1032:
1025:
1010:
1009:
1005:
995:
993:
984:
983:
970:
965:
934:GlaxoSmithKline
914:
898:Warner Chilcott
890:
879:
873:
870:
855:
839:
828:
823:
743:Bisphosphonate
735:
704:Risedronic acid
692:
690:
687:(what is this?)
684:
679:
676:
671:
666:
665:
654:
651:
646:
645:
634:
609:
599:
593:
584:
578:
539:
515:
507:
479:
459:
432:
412:
392:
372:
352:
335:
327:
307:
304:
299:
298:
263:
242:Protein binding
232:Bioavailability
224:Pharmacokinetic
218:
159:
127:
120:
101:
94:
21:Risedronic acid
17:
12:
11:
5:
1539:
1537:
1529:
1528:
1523:
1518:
1513:
1508:
1503:
1498:
1488:
1487:
1483:
1482:
1459:
1458:
1456:
1455:
1450:
1445:
1440:
1439:
1438:
1433:
1420:
1419:
1418:
1405:
1404:
1403:
1390:
1389:
1388:
1375:
1374:
1373:
1363:
1358:
1357:
1356:
1345:
1343:
1339:
1338:
1336:
1335:
1330:
1324:
1322:
1316:
1315:
1313:
1312:
1311:
1310:
1305:
1303:Clodronic acid
1300:
1298:Etidronic acid
1292:
1291:
1290:
1285:
1280:
1275:
1270:
1265:
1260:
1255:
1244:
1242:
1236:
1235:
1224:
1222:
1221:
1214:
1207:
1199:
1193:
1192:
1180:
1166:
1165:External links
1163:
1160:
1159:
1138:
1116:
1098:
1077:
1048:
1030:
1023:
1003:
967:
966:
964:
961:
953:bisphosphonate
942:Paul A. Crotty
922:Sanofi-Aventis
913:
910:
902:Sanofi-Aventis
892:
891:
874:September 2023
842:
840:
833:
827:
824:
822:
819:
816:
815:
812:
806:
805:
802:
798:
797:
794:
788:
787:
784:
778:
777:
774:
768:
767:
764:
758:
757:
754:
748:
747:
744:
734:
731:
722:. It is taken
712:bisphosphonate
699:
698:
681:
680:
678:
677:
674:
672:
669:
661:
660:
659:
656:
655:
653:
652:
649:
641:
640:
639:
636:
635:
633:
632:
624:
622:
614:
613:
607:
601:
600:
597:
591:
582:
576:
571:
565:
564:
560:
559:
549:
541:
540:
538:
537:
524:
522:
509:
508:
506:
505:
489:
487:
481:
480:
478:
477:
469:
467:
461:
460:
458:
457:
450:
442:
440:
434:
433:
431:
430:
422:
420:
414:
413:
411:
410:
402:
400:
394:
393:
391:
390:
382:
380:
374:
373:
371:
370:
362:
360:
354:
353:
351:
350:
342:
340:
329:
328:
326:
325:
317:
315:
309:
308:
306:
305:
302:
294:
293:
292:
289:
288:
284:
283:
277:
271:
270:
267:
258:
257:
254:
248:
247:
244:
238:
237:
234:
228:
227:
220:
219:
217:
216:
213:
201:
188:
174:
172:
166:
165:
161:
160:
158:
157:
144:
142:
136:
135:
130:
128:administration
122:
121:
119:
118:
116:
106:
104:
96:
95:
93:
92:
74:
72:
66:
65:
58:
48:
47:
44:
38:
37:
33:
32:
15:
13:
10:
9:
6:
4:
3:
2:
1538:
1527:
1524:
1522:
1519:
1517:
1514:
1512:
1509:
1507:
1504:
1502:
1499:
1497:
1494:
1493:
1491:
1481:
1471:
1467:
1454:
1451:
1449:
1446:
1444:
1441:
1437:
1434:
1432:
1431:Abaloparatide
1429:
1428:
1427:
1424:
1421:
1417:
1414:
1413:
1412:
1410:
1406:
1402:
1399:
1398:
1397:
1395:
1391:
1387:
1384:
1383:
1382:
1380:
1376:
1372:
1369:
1368:
1367:
1364:
1362:
1359:
1355:
1352:
1351:
1350:
1347:
1346:
1344:
1340:
1334:
1331:
1329:
1326:
1325:
1323:
1321:
1317:
1309:
1306:
1304:
1301:
1299:
1296:
1295:
1293:
1289:
1286:
1284:
1281:
1279:
1276:
1274:
1271:
1269:
1266:
1264:
1261:
1259:
1256:
1254:
1251:
1250:
1249:
1246:
1245:
1243:
1241:
1237:
1232:
1228:
1227:bone diseases
1220:
1215:
1213:
1208:
1206:
1201:
1200:
1197:
1189:
1185:
1181:
1177:
1173:
1169:
1168:
1164:
1148:
1142:
1139:
1134:
1130:
1126:
1120:
1117:
1112:
1108:
1102:
1099:
1087:
1081:
1078:
1073:
1067:
1059:
1055:
1051:
1049:9789350259375
1045:
1041:
1034:
1031:
1026:
1024:9783527607495
1020:
1016:
1015:
1007:
1004:
991:
987:
981:
979:
977:
975:
973:
969:
962:
960:
958:
954:
949:
946:
943:
939:
935:
931:
927:
923:
919:
912:Controversies
911:
909:
907:
903:
899:
888:
885:
877:
867:
863:
859:
853:
852:
848:
843:This section
841:
837:
832:
831:
825:
820:
813:
811:
808:
807:
803:
800:
799:
795:
793:
790:
789:
785:
783:
780:
779:
775:
773:
770:
769:
765:
763:
760:
759:
755:
753:
750:
749:
745:
742:
741:
732:
730:
727:
725:
721:
717:
713:
709:
705:
695:
688:
682:
673:
668:
664:
657:
648:
644:
637:
630:
626:
625:
623:
620:
615:
608:
606:
602:
572:
570:
566:
561:
557:
553:
550:
548:
546:ECHA InfoCard
542:
534:
530:
529:DTXSID2023563
526:
525:
523:
514:
510:
502:
501:RCSB PDB
496:
491:
490:
488:
486:
482:
475:
471:
470:
468:
466:
462:
455:
451:
448:
444:
443:
441:
439:
435:
428:
424:
423:
421:
419:
415:
408:
404:
403:
401:
399:
395:
388:
384:
383:
381:
379:
375:
368:
364:
363:
361:
359:
355:
348:
344:
343:
341:
334:
330:
323:
319:
318:
316:
314:
310:
301:
297:
290:
285:
281:
278:
276:
272:
268:
266:
259:
255:
253:
249:
245:
243:
239:
235:
233:
229:
225:
221:
214:
212:
202:
199:
189:
186:
176:
175:
173:
171:
167:
162:
154:
149:
146:
145:
143:
141:
137:
134:
131:
129:
123:
117:
108:
107:
105:
103:
97:
90:
85:
76:
75:
73:
71:
67:
63:
59:
57:
53:
49:
45:
43:
39:
36:Clinical data
34:
30:
25:
1436:Teriparatide
1407:
1392:
1377:
1365:
1348:
1282:
1187:
1175:
1151:. Retrieved
1141:
1133:the original
1128:
1119:
1111:Pharma Times
1110:
1101:
1090:. Retrieved
1080:
1039:
1033:
1013:
1006:
994:. Retrieved
989:
950:
947:
915:
895:
880:
871:
856:Please help
844:
801:Risedronate
733:Pharmacology
728:
718:, and treat
716:osteoporosis
707:
703:
702:
691:
685:
262:Elimination
170:Legal status
164:Legal status
70:License data
1409:Cathepsin K
1401:Romosozumab
1354:Ipriflavone
1248:Nitrogenous
1088:. Uk.pg.com
826:Brand names
810:Zoledronate
792:Ibandronate
782:Alendronate
772:Pamidronate
762:Tiludronate
612: g·mol
552:100.116.436
322:105462-24-6
287:Identifiers
42:Trade names
1490:Categories
1443:Calcitonin
1416:Odanacatib
1394:Sclerostin
1153:2013-03-01
1092:2013-03-01
963:References
926:Lanham Act
752:Etidronate
617:3D model (
605:Molar mass
485:PDB ligand
427:KM2Z91756Z
398:ChemSpider
358:IUPHAR/BPS
313:CAS Number
296:IUPAC name
252:Metabolism
89:Risedronic
1453:Vitamin D
1426:analogues
1411:inhibitor
1396:inhibitor
1386:Denosumab
1381:inhibitor
1066:cite book
1058:868299888
845:does not
796:500-1000
474:ChEMBL923
282:and fecal
275:Excretion
264:half-life
126:Routes of
100:Pregnancy
62:Monograph
56:Drugs.com
1480:Medicine
990:DailyMed
924:filed a
786:100-500
724:by mouth
694:(verify)
378:DrugBank
140:ATC code
133:By mouth
115: B3
102:category
84:DailyMed
1448:Calcium
996:28 June
918:P&G
866:removed
851:sources
710:, is a
610:283.113
569:Formula
387:DB00884
333:PubChem
156:)
150: (
148:M05BA07
86::
1521:Sanofi
1466:Portal
1056:
1046:
1021:
938:Boniva
906:Takeda
643:SMILES
465:ChEMBL
454:D03234
447:D00942
280:Kidney
211:℞-only
209:
196:
183:
82:
1379:RANKL
1342:Other
930:Roche
814:5000
804:1000
663:InChI
619:JSmol
492:RIS (
269:1.5 h
236:0.63%
1072:link
1054:OCLC
1044:ISBN
1019:ISBN
998:2022
932:and
849:any
847:cite
776:100
495:PDBe
438:KEGG
418:UNII
407:5055
367:3176
347:5245
256:None
246:~24%
226:data
52:AHFS
1231:M05
860:by
766:10
518:EPA
337:CID
198:POM
153:WHO
1492::
1186:.
1174:.
1127:.
1109:.
1068:}}
1064:{{
1052:.
988:.
971:^
900:,
756:1
726:.
583:11
498:,
205:US
192:UK
185:S4
179:AU
111:AU
79:US
1468::
1233:)
1229:(
1218:e
1211:t
1204:v
1156:.
1095:.
1074:)
1060:.
1027:.
1000:.
887:)
881:(
876:)
872:(
868:.
854:.
621:)
598:2
595:P
592:7
589:O
586:N
580:H
577:7
574:C
520:)
516:(
504:)
207::
194::
181::
113::
54:/
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.