271:
310:
401:
234:
427:: nucleophilicity increases with increasing negative charge and decreasing electronegativity. For example, OH is a better nucleophile than water, and I is a better nucleophile than Br (in polar protic solvents). In a polar aprotic solvent, nucleophilicity increases up a column of the periodic table as there is no hydrogen bonding between the solvent and nucleophile; in this case nucleophilicity mirrors basicity. I would therefore be a weaker nucleophile than Br because it is a weaker base. Verdict - A strong/anionic nucleophile always favours S
801:
362:
602:
42:
583:
204:
745:
2 not possible) by
Schleyer and co-workers, the use of azide (an excellent nucleophile but very poor leaving group) by Weiner and Sneen, the development of sulfonate leaving groups (non-nucleophilic good leaving groups), and the demonstration of significant experimental problems in the initial claim
715:
Many reactions studied are solvolysis reactions where a solvent molecule (often an alcohol) is the nucleophile. While still a second order reaction mechanistically, the reaction is kinetically first order as the concentration of the nucleophile–the solvent molecule, is effectively constant during
1427:
and because it requires charged reaction products for detection the nucleophile is fitted with an additional sulfonate anionic group, non-reactive and well separated from the other anion. The product ratio of substitution and elimination product can be measured from the intensity their relative
597:
2 reaction in which the leaving group can also act as a nucleophile. In this reaction, the substrate has a halogen atom exchanged with another halogen. As the negative charge is more-or-less stabilized on both halides, the reaction occurs at equilibrium.
1334:
The 2-Adamantyl System, a
Standard for Limiting Solvolysis in a Secondary Substrate J. L. Fry, C. J. Lancelot, L. K. M. Lam, J. M Harris, R. C. Bingham, D. J. Raber, R. E. Hill, P. v. R. Schleyer, J. Am. Chem. Soc.,; 1970; 92, pp 1240-42 (Article); doi:
740:
1 mechanism invariably involve the use of bromide (or other good nucleophile) as the leaving group have confused the understanding of alkyl nucleophilic substitution reactions at secondary carbons for 80 years. Work with the 2-adamantyl system
306:. For example, 1-bromo-1-fluoroethane can undergo nucleophilic attack to form 1-fluoroethan-1-ol, with the nucleophile being an HO group. In this case, if the reactant is levorotatory, then the product would be dextrorotatory, and vice versa.
349:
at the central carbon, i.e. those that do not have as much sterically hindering substituents nearby. Methyl and primary substrates react the fastest, followed by secondary substrates. Tertiary substrates do not react via the
723:
2 reaction on a substrate molecule. If the substrate is chiral, this inverts the configuration of the substrate before solvolysis, leading to a racemized product–the product that would be expected from an
388:
between the reaction centre and the adjacent pi system stabilizes the transition state. Because they destabilize the positive charge in the carbocation intermediate, electron-withdrawing groups favor the
1311:
W.A. Cowdrey; E.D. Hughes; C.K. Ingold; S. Masterman; A.D. Scott (1937). "Relation of Steric orientation to
Mechanism in Substitution Involving Halogen Atoms and Simple or Substituted Hydroxyl Groups".
750:
1 mechanism in the solvolysis of optically active 2-bromooctane by Hughes et al. have demonstrated conclusively that secondary substrates go exclusively (except in unusual but predictable cases) by the
1344:
A Clarification of the
Mechanism of Solvolysis of 2-Octyl Sulfonates. Stereochemical Considerations; H. Weiner, R. A. Sneen, J. Am. Chem. Soc.,; 1965; 87 pp 287-91; (Article) doi: 10.1021/ja01080a026
645:, furnish a weaker nucleophile. In contrast, polar aprotic solvents can only weakly interact with the nucleophile, and thus, are to a lesser extent able to reduce the strength of the nucleophile.
309:
1353:
A Clarification of the
Mechanism of Solvolysis of 2-Octyl Sulfonates. Kinetic Considerations; H. Weiner, R. A. Sneen, J. Am. Chem. Soc.; 1965; 87 pp 292-96; (Article) doi: 10.1021/ja01080a027
613:
The solvent affects the rate of reaction because solvents may or may not surround a nucleophile, thus hindering or not hindering its approach to the carbon atom. Polar aprotic solvents, like
240:
To achieve optimal orbital overlap, the nucleophile attacks 180° relative to the leaving group, resulting in the leaving group being pushed off the opposite side and the product formed with
641:, etc. In parallel, solvation also has a significant impact on the intrinsic strength of the nucleophile, in which strong interactions between solvent and the nucleophile, found for polar
423:, on the other hand, is a strong base, but a poor nucleophile, because of its three methyl groups hindering its approach to the carbon. Nucleophile strength is also affected by charge and
826:
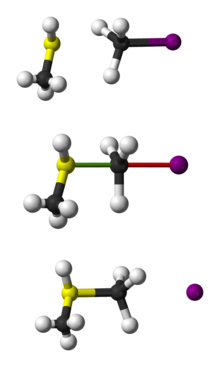
substrate, isopropyl bromide reacts with 55% substitution. In general, gas phase reactions and solution phase reactions of this type follow the same trends, even though in the first,
192:(often denoted X). The formation of the C–Nu bond, due to attack by the nucleophile (denoted Nu), occurs concertedly with the breakage of the C–X bond. The reaction occurs through a
854:. When the chloride ions have sufficient velocity, the initial collision of it with the methyl iodide molecule causes the methyl iodide to spin around once before the actual S
625:
to the nucleophile, hindering it from attacking the carbon with the leaving group. A polar aprotic solvent with low dielectric constant or a hindered dipole end will favour S
1362:
Homogeneous
Hydrolysis and Alcoholysis of β-n-Octyl halides, E. D. Hughes, C. K. Ingold, S. Masterman, J. Chem. Soc.; 1937; pp 1196–1201; (Article) doi: 10.1039/JR9370001196
270:
732:
2 rate constant 100-250 times higher than the rate constant for ethanol. Thus, after only a few percent solvolysis of an enantiospecific substrate, it becomes racemic.
1042:
822:
bromide, substitution is disfavored and elimination is the predominant reaction. Other factors favoring elimination are the strength of the base. With the less basic
521:), serve as good anionic leaving groups because electronegativity stabilizes additional electron density; the fluoride exception is due to its strong bond to carbon.
1523:
1970:
779:. This pathway is favored with sterically hindered nucleophiles. Elimination reactions are usually favoured at elevated temperatures because of increased
218:
between the nucleophile and substrate. The reaction occurs only when the occupied lone pair orbital of the nucleophile donates electrons to the unfilled
1301:
1 Involvement in the
Solvolysis of Secondary Alkyl Compounds, T. J. Murphy, J. Chem. Educ.; 2009; 86(4) pp 519-24; (Article) doi: 10.1021/ed041p678
800:
338:
2 reaction to occur more quickly, the nucleophile must easily access the sigma antibonding orbital between the central carbon and leaving group.
1071:
1018:
951:
711:
1 reaction. There are two factors which complicate determining the mechanism of nucleophilic substitution reactions at secondary carbons:
1735:
1612:
1569:
1440:
J. Mikosch, S. Trippel, C. Eichhorn, R. Otto, U. Lourderaj, J. X. Zhang, W. L. Hase, M. Weidemüller, and R. Wester
Science 11 January
892:
1876:
1516:
150:, is that the displacement of the leaving group, which is the rate-determining step, is separate from the nucleophilic attack in S
882:
416:
anion, for example, is both a strong base and nucleophile because it is a methyl nucleophile, and is thus very much unhindered.
1372:
497:), are good examples because of their positive charge when bonded to the carbon center prior to nucleophilic attack. Halides (
1784:
1779:
1589:
1249:
Vermeeren, Pascal; Hansen, Thomas; Jansen, Paul; Swart, Marcel; Hamlin, Trevor A.; Bickelhaupt, F. Matthias (December 2020).
887:
728:
1 mechanism. In the case of a bromide leaving group in alcoholic solvent
Cowdrey et al. have shown that bromide can have an S
1944:
1484:
226:. Throughout the course of the reaction, a p orbital forms at the reaction center as the result of the transition from the
1949:
1400:
775:: the incoming anion can act as a base rather than as a nucleophile, abstracting a proton and leading to formation of the
703:
It has been shown that except in uncommon (but predictable cases) primary and secondary substrates go exclusively by the S
219:
719:
In reactions where the leaving group is also a good nucleophile (bromide for instance) the leaving group can perform an S
1975:
1509:
354:
2 pathway, as the greater steric hindrance between the nucleophile and nearby groups of the substrate will leave the S
1088:"Nucleophilic Substitution (SN2): Dependence on Nucleophile, Leaving Group, Central Atom, Substituents, and Solvent"
1914:
1604:
448:
447:
that comes from breaking its bond with the carbon center. This leaving group ability trend corresponds well to the
400:
264:
233:
1641:
1541:
127:
1871:
162:
30:"SN2" redirects here. For slush nitrogen, the mixture of solid and liquid nitrogen sometimes abbreviated as SN
1919:
1720:
1424:
872:
417:
393:
2 reaction. Electron-donating groups favor leaving-group displacement and are more likely to react via the S
291:
1674:
1061:
1904:
1836:
1694:
1684:
915:
662:
658:
181:
135:
100:
68:
yielding dimethylsulfonium. Note that the attacking group attacks from the backside of the leaving group
1251:"A Unified Framework for Understanding Nucleophilicity and Protophilicity in the S N 2/E2 Competition"
1145:"Nucleophilic Substitution in Solution: Activation Strain Analysis of Weak and Strong Solvent Effects"
1899:
1627:
877:
772:
590:
241:
119:
45:
361:
1909:
1841:
1826:
1769:
326:
The four factors that affect the rate of the reaction, in the order of decreasing importance, are:
197:
166:
1200:
Hansen, Thomas; Roozee, Jasper C.; Bickelhaupt, F. Matthias; Hamlin, Trevor A. (4 February 2022).
692:
2 the nucleophile forces off the leaving group in the limiting step. In other words, the rate of S
1934:
1704:
1533:
1036:
227:
108:
84:
41:
601:
1929:
1924:
1886:
1831:
1750:
1730:
1666:
1280:
1231:
1182:
1164:
1125:
1107:
1067:
1024:
1014:
947:
792:
634:
630:
424:
385:
88:
380:
1, allylic and benzylic carbocations are stabilized by delocalizing the positive charge. In S
1861:
1810:
1764:
1481:
Surprise From SN2 Snapshots Ion velocity measurements unveil additional unforeseen mechanism
1465:
1445:
1408:
1317:
1270:
1262:
1221:
1213:
1172:
1156:
1115:
1099:
982:
815:
444:
303:
299:
193:
1939:
1851:
1800:
973:
827:
614:
334:
The substrate plays the most important part in determining the rate of the reaction. For S
295:
185:
317:
2 mechanism of 1-bromo-1-fluoroethane with one of the carbon atoms being a chiral centre.
1492:
665:
depends on the nucleophile concentration, as well as the concentration of substrate, .
1646:
1635:
1275:
1250:
1226:
1201:
1177:
1144:
1120:
1087:
642:
618:
346:
35:
1143:
Hamlin, Trevor A.; van Beek, Bas; Wolters, Lando P.; Bickelhaupt, F. Matthias (2018).
1964:
1894:
1866:
1774:
1725:
1699:
867:
847:
811:
764:
688:
1 reaction the nucleophile attacks after the rate-limiting step is over, whereas in S
669:
622:
223:
215:
104:
700:
2 reaction rate depends on the concentration of both the substrate and nucleophile.
1846:
1652:
1549:
1397:
Gas Phase
Studies of the Competition between Substitution and Elimination Reactions
897:
788:
582:
287:
143:
53:
716:
the reaction. This type of reaction is often called a pseudo first order reaction.
1805:
1740:
1057:
986:
529:
263:. Reactions such as this, with an alkoxide as the nucleophile, are known as the
131:
96:
61:
1028:
968:
525:
248:
1168:
1111:
412:
Like the substrate, steric hindrance affects the nucleophile's strength. The
1856:
1501:
1469:
1449:
1217:
784:
561:
553:
413:
256:
178:
1284:
1266:
1235:
1186:
1160:
1129:
1103:
1008:
203:
1321:
823:
819:
696:
1 reactions depend only on the concentration of the substrate while the S
545:
537:
516:
498:
479:
780:
638:
569:
504:
259:
group as the nucleophile and a halide as the leaving group, forming an
189:
1412:
1373:"Elimination Reactions Are Favored By Heat — Master Organic Chemistry"
783:. This effect can be demonstrated in the gas-phase reaction between a
814:, the reaction product is predominantly the substitution product. As
776:
510:
487:
161:
2 reaction can be considered as an organic-chemistry analogue of the
134:
mechanism, which means both the reacting species are involved in the
1086:
Hamlin, Trevor A.; Swart, Marcel; Bickelhaupt, F. Matthias (2018).
17:
1759:
475:
260:
40:
1063:
Advanced Organic Chemistry: Reactions, Mechanisms, and Structure
736:
The examples in textbooks of secondary substrates going by the S
443:
2 reactions. A good leaving group must be able to stabilize the
1505:
969:"Synthesis of the Bioherbicidal Fungus Metabolite Macrocidin A"
1579:
946:(2nd ed.). Oxford: Oxford University Press. p. 330.
906:
142:
2 from the other major type of nucleophilic substitution, the
188:, stable leaving group attached to it, which is frequently a
103:-hybridised carbon atom via a backside attack, all while the
799:
600:
581:
399:
360:
308:
269:
232:
202:
1464:
John I. Brauman (11 January 2008) Science 319 (5860), 168.
846:
observed in a gas-phase reaction between chloride ions and
524:
Leaving group reactivity of alcohols can be increased with
1202:"How Solvation Influences the S N 2 versus E2 Competition"
629:
2 manner of nucleophilic substitution reaction. Examples:
942:
Clayden, Jonathan; Greeves, Nick; Warren, Stuart (2012).
368:
Substrates with adjacent pi C=C systems can favor both S
838:
A development attracting attention in 2008 concerns a S
251:, involves an intramolecular ring closing step via an S
1007:
CURTIS, CLIFF. MURGATROYD, JASON. SCOTT, DAVE (2019).
1493:
http://pubsapp.acs.org/cen/news/86/i02/8602notw1.html
967:
Hasse, Robert; Schobert, Rainer (November 28, 2016).
439:
Good leaving groups on the substrate lead to faster S
247:
For example, the synthesis of macrocidin A, a fungal
1010:
Edexcel international a level chemistry student book
345:
2 occurs more quickly with substrates that are more
1885:
1819:
1793:
1749:
1713:
1665:
1626:
1603:
1540:
818:around the electrophilic center increases, as with
617:, are better solvents for this reaction than polar
707:2 mechanism while tertiary substrates go via the S
471:value, the faster the leaving group is displaced.
1066:(6th ed.), New York: Wiley-Interscience,
937:
935:
933:
931:
1517:
244:of tetrahedral geometry at the central atom.
8:
1041:: CS1 maint: multiple names: authors list (
230:of the reactants to those of the products.
1524:
1510:
1502:
1438:Imaging Nucleophilic Substitution Dynamics
322:Factors affecting the rate of the reaction
1274:
1225:
1176:
1119:
804:Competition experiment between SN2 and E2
474:Leaving groups that are neutral, such as
575:
493:
457:of the leaving group's conjugate acid (p
431:2 manner of nucleophillic substitution.
927:
107:detaches from the reaction center in a
1550:Unimolecular nucleophilic substitution
1034:
858:2 displacement mechanism takes place.
676:This is a key difference between the S
1560:Bimolecular nucleophilic substitution
1462:PERSPECTIVES CHEMISTRY: Not So Simple
282:If the substrate that is undergoing S
177:The reaction most often occurs at an
73:Bimolecular nucleophilic substitution
7:
1971:Nucleophilic substitution reactions
1613:Electrophilic aromatic substitution
621:because polar protic solvents will
222:between the central carbon and the
126:" indicates that the reaction is a
1580:Nucleophilic internal substitution
1570:Nucleophilic aromatic substitution
893:Nucleophilic aromatic substitution
25:
1407:; 36(11) pp 848 - 857; (Article)
200:and approximately sp-hybridised.
130:, and "2" that it proceeds via a
1206:The Journal of Organic Chemistry
883:Neighbouring group participation
850:with a special technique called
302:) may occur; this is called the
196:in which the reaction center is
1736:Lindemann–Hinshelwood mechanism
1485:Chemical & Engineering News
552:). Poor leaving groups include
274:Synthesis of macrocidin A via S
1785:Outer sphere electron transfer
1780:Inner sphere electron transfer
1590:Nucleophilic acyl substitution
1377:www.masterorganicchemistry.com
1255:Chemistry – A European Journal
1149:Chemistry – A European Journal
888:Nucleophilic acyl substitution
852:crossed molecular beam imaging
214:2 reaction can be viewed as a
1:
1950:Diffusion-controlled reaction
1401:Accounts of Chemical Research
111:(i.e. simultaneous) fashion.
46:Ball-and-stick representation
1605:Electrophilic substitutions
987:10.1021/acs.orglett.6b03240
358:1 reaction to occur first.
1992:
1915:Energy profile (chemistry)
1877:More O'Ferrall–Jencks plot
1542:Nucleophilic substitutions
265:Williamson ether synthesis
29:
27:Organic chemistry reaction
1945:Michaelis–Menten kinetics
1491:Volume 86, Number 2 p. 9
128:nucleophilic substitution
1872:Potential energy surface
1751:Electron/Proton transfer
1636:Unimolecular elimination
515:, with the exception of
163:associative substitution
1920:Transition state theory
1721:Intramolecular reaction
1647:Bimolecular elimination
1470:10.1126/science.1152387
1450:10.1126/science.1150238
1425:electrospray ionization
1218:10.1021/acs.joc.1c02354
873:Christopher Kelk Ingold
99:forms a new bond to an
1714:Unimolecular reactions
1675:Electrophilic addition
1423:The technique used is
1267:10.1002/chem.202003831
1161:10.1002/chem.201706075
1104:10.1002/cphc.201701363
805:
791:taking place inside a
684:2 mechanisms. In the S
605:
586:
404:
365:
318:
279:
237:
220:σ* antibonding orbital
207:
184:carbon center with an
138:. What distinguishes S
69:
1905:Rate-determining step
1837:Reactive intermediate
1695:Free-radical addition
1685:Nucleophilic addition
1628:Elimination reactions
1013:. : EDEXCEL Limited.
916:Substitution reaction
803:
663:rate-determining step
604:
585:
403:
364:
347:sterically accessible
312:
273:
236:
216:HOMO–LUMO interaction
206:
136:rate-determining step
122:of the mechanism: "S
95:2 reaction, a strong
44:
1900:Equilibrium constant
1322:10.1039/JR9370001252
878:Finkelstein reaction
844:roundabout mechanism
834:Roundabout mechanism
591:Finkelstein reaction
290:, then inversion of
120:Hughes-Ingold symbol
1976:Reaction mechanisms
1910:Reaction coordinate
1842:Radical (chemistry)
1827:Elementary reaction
1770:Grotthuss mechanism
1534:reaction mechanisms
1335:10.1021/ja00478a031
1261:(67): 15538–15548.
1056:Smith, Michael B.;
767:taking place with S
167:inorganic chemistry
1935:Arrhenius equation
1705:Oxidative addition
1667:Addition reactions
806:
606:
587:
464:); the lower its p
405:
366:
319:
280:
255:2 reaction with a
238:
228:molecular orbitals
208:
173:Reaction mechanism
165:from the field of
87:that is common in
85:reaction mechanism
70:
1958:
1957:
1930:Activated complex
1925:Activation energy
1887:Chemical kinetics
1832:Reaction dynamics
1731:Photodissociation
1413:10.1021/ar020042n
1155:(22): 5927–5938.
1098:(11): 1315–1330.
1073:978-0-471-72091-1
1020:978-1-292-24472-3
981:(24): 6352–6355.
953:978-0-19-927029-3
944:Organic chemistry
793:mass spectrometer
649:Reaction kinetics
635:dimethylformamide
631:dimethylsulfoxide
425:electronegativity
376:2 reactions. In S
286:2 reaction has a
278:2 etherification.
89:organic chemistry
16:(Redirected from
1983:
1862:Collision theory
1811:Matrix isolation
1765:Harpoon reaction
1642:E1cB-elimination
1526:
1519:
1512:
1503:
1496:
1495:, video included
1478:
1472:
1459:
1453:
1435:
1429:
1421:
1415:
1394:
1388:
1387:
1385:
1383:
1369:
1363:
1360:
1354:
1351:
1345:
1342:
1336:
1332:
1326:
1325:
1308:
1302:
1295:
1289:
1288:
1278:
1246:
1240:
1239:
1229:
1212:(3): 1805–1813.
1197:
1191:
1190:
1180:
1140:
1134:
1133:
1123:
1083:
1077:
1076:
1053:
1047:
1046:
1040:
1032:
1004:
998:
997:
995:
993:
964:
958:
957:
939:
830:are eliminated.
816:steric hindrance
653:The rate of an S
578:
567:
559:
551:
543:
535:
519:
513:
507:
501:
496:
485:
445:electron density
384:2, however, the
304:Walden inversion
300:optical activity
194:transition state
118:2 refers to the
21:
1991:
1990:
1986:
1985:
1984:
1982:
1981:
1980:
1961:
1960:
1959:
1954:
1940:Eyring equation
1881:
1852:Stereochemistry
1815:
1801:Solvent effects
1789:
1745:
1709:
1690:
1680:
1661:
1656:
1622:
1618:
1599:
1595:
1585:
1575:
1565:
1555:
1536:
1530:
1500:
1499:
1479:
1475:
1460:
1456:
1436:
1432:
1428:molecular ions.
1422:
1418:
1395:
1391:
1381:
1379:
1371:
1370:
1366:
1361:
1357:
1352:
1348:
1343:
1339:
1333:
1329:
1310:
1309:
1305:
1300:
1296:
1292:
1248:
1247:
1243:
1199:
1198:
1194:
1142:
1141:
1137:
1085:
1084:
1080:
1074:
1055:
1054:
1050:
1033:
1021:
1006:
1005:
1001:
991:
989:
974:Organic Letters
966:
965:
961:
954:
941:
940:
929:
924:
910:
901:
864:
857:
841:
836:
828:solvent effects
771:2 reactions is
770:
761:
754:
749:
744:
739:
731:
727:
722:
710:
706:
699:
695:
691:
687:
683:
679:
656:
651:
643:protic solvents
628:
619:protic solvents
615:tetrahydrofuran
611:
596:
577:
573:
565:
557:
549:
541:
533:
517:
511:
505:
499:
495:
491:
483:
470:
463:
455:
442:
437:
430:
410:
396:
392:
383:
379:
375:
371:
357:
353:
344:
337:
332:
324:
316:
296:stereochemistry
285:
277:
254:
213:
198:pentacoordinate
186:electronegative
175:
160:
153:
147:
141:
125:
117:
94:
83:) is a type of
80:
65:
57:
51:
39:
33:
28:
23:
22:
15:
12:
11:
5:
1989:
1987:
1979:
1978:
1973:
1963:
1962:
1956:
1955:
1953:
1952:
1947:
1942:
1937:
1932:
1927:
1922:
1917:
1912:
1907:
1902:
1897:
1891:
1889:
1883:
1882:
1880:
1879:
1874:
1869:
1864:
1859:
1854:
1849:
1844:
1839:
1834:
1829:
1823:
1821:
1820:Related topics
1817:
1816:
1814:
1813:
1808:
1803:
1797:
1795:
1794:Medium effects
1791:
1790:
1788:
1787:
1782:
1777:
1772:
1767:
1762:
1756:
1754:
1747:
1746:
1744:
1743:
1738:
1733:
1728:
1723:
1717:
1715:
1711:
1710:
1708:
1707:
1702:
1697:
1692:
1688:
1682:
1678:
1671:
1669:
1663:
1662:
1660:
1659:
1654:
1650:
1644:
1639:
1632:
1630:
1624:
1623:
1621:
1620:
1616:
1609:
1607:
1601:
1600:
1598:
1597:
1593:
1587:
1583:
1577:
1573:
1567:
1563:
1557:
1553:
1546:
1544:
1538:
1537:
1531:
1529:
1528:
1521:
1514:
1506:
1498:
1497:
1473:
1454:
1430:
1416:
1399:Scott Gronert
1389:
1364:
1355:
1346:
1337:
1327:
1303:
1298:
1290:
1241:
1192:
1135:
1078:
1072:
1048:
1019:
999:
959:
952:
926:
925:
923:
920:
919:
918:
913:
908:
904:
899:
895:
890:
885:
880:
875:
870:
863:
860:
855:
839:
835:
832:
808:
807:
773:E2 elimination
768:
760:
759:E2 competition
757:
752:
747:
742:
737:
734:
733:
729:
725:
720:
717:
708:
704:
697:
693:
689:
685:
681:
677:
674:
673:
657:2 reaction is
654:
650:
647:
626:
610:
607:
594:
468:
461:
453:
440:
436:
433:
428:
409:
406:
394:
390:
381:
377:
373:
369:
355:
351:
342:
335:
331:
328:
323:
320:
314:
283:
275:
252:
211:
174:
171:
158:
151:
145:
139:
123:
115:
92:
78:
63:
55:
52:2 reaction of
49:
36:slush nitrogen
31:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1988:
1977:
1974:
1972:
1969:
1968:
1966:
1951:
1948:
1946:
1943:
1941:
1938:
1936:
1933:
1931:
1928:
1926:
1923:
1921:
1918:
1916:
1913:
1911:
1908:
1906:
1903:
1901:
1898:
1896:
1895:Rate equation
1893:
1892:
1890:
1888:
1884:
1878:
1875:
1873:
1870:
1868:
1867:Arrow pushing
1865:
1863:
1860:
1858:
1855:
1853:
1850:
1848:
1845:
1843:
1840:
1838:
1835:
1833:
1830:
1828:
1825:
1824:
1822:
1818:
1812:
1809:
1807:
1804:
1802:
1799:
1798:
1796:
1792:
1786:
1783:
1781:
1778:
1776:
1775:Marcus theory
1773:
1771:
1768:
1766:
1763:
1761:
1758:
1757:
1755:
1752:
1748:
1742:
1739:
1737:
1734:
1732:
1729:
1727:
1726:Isomerization
1724:
1722:
1719:
1718:
1716:
1712:
1706:
1703:
1701:
1700:Cycloaddition
1698:
1696:
1693:
1686:
1683:
1676:
1673:
1672:
1670:
1668:
1664:
1658:
1651:
1648:
1645:
1643:
1640:
1637:
1634:
1633:
1631:
1629:
1625:
1614:
1611:
1610:
1608:
1606:
1602:
1591:
1588:
1581:
1578:
1571:
1568:
1561:
1558:
1551:
1548:
1547:
1545:
1543:
1539:
1535:
1527:
1522:
1520:
1515:
1513:
1508:
1507:
1504:
1494:
1490:
1486:
1483:Carmen Drahl
1482:
1477:
1474:
1471:
1467:
1463:
1458:
1455:
1451:
1447:
1444:319: 183-186
1443:
1439:
1434:
1431:
1426:
1420:
1417:
1414:
1410:
1406:
1402:
1398:
1393:
1390:
1378:
1374:
1368:
1365:
1359:
1356:
1350:
1347:
1341:
1338:
1331:
1328:
1323:
1319:
1316:: 1252–1271.
1315:
1314:J. Chem. Soc.
1307:
1304:
1294:
1291:
1286:
1282:
1277:
1272:
1268:
1264:
1260:
1256:
1252:
1245:
1242:
1237:
1233:
1228:
1223:
1219:
1215:
1211:
1207:
1203:
1196:
1193:
1188:
1184:
1179:
1174:
1170:
1166:
1162:
1158:
1154:
1150:
1146:
1139:
1136:
1131:
1127:
1122:
1117:
1113:
1109:
1105:
1101:
1097:
1093:
1089:
1082:
1079:
1075:
1069:
1065:
1064:
1059:
1052:
1049:
1044:
1038:
1030:
1026:
1022:
1016:
1012:
1011:
1003:
1000:
988:
984:
980:
976:
975:
970:
963:
960:
955:
949:
945:
938:
936:
934:
932:
928:
921:
917:
914:
912:
905:
903:
896:
894:
891:
889:
886:
884:
881:
879:
876:
874:
871:
869:
868:Arrow pushing
866:
865:
861:
859:
853:
849:
848:methyl iodide
845:
833:
831:
829:
825:
821:
817:
813:
812:ethyl bromide
802:
798:
797:
796:
794:
790:
789:alkyl bromide
787:and a simple
786:
782:
778:
774:
766:
765:side reaction
758:
756:
755:2 mechanism.
718:
714:
713:
712:
701:
671:
668:
667:
666:
664:
660:
648:
646:
644:
640:
636:
632:
624:
623:hydrogen bond
620:
616:
608:
603:
599:
592:
584:
580:
571:
563:
555:
547:
539:
531:
527:
522:
520:
514:
508:
502:
489:
481:
477:
472:
467:
460:
456:
452:
446:
435:Leaving group
434:
432:
426:
422:
420:
415:
407:
402:
398:
387:
363:
359:
348:
339:
329:
327:
321:
311:
307:
305:
301:
297:
293:
292:configuration
289:
288:chiral centre
272:
268:
266:
262:
258:
250:
245:
243:
235:
231:
229:
225:
224:leaving group
221:
217:
205:
201:
199:
195:
191:
187:
183:
180:
172:
170:
168:
164:
155:
149:
137:
133:
129:
121:
112:
110:
106:
105:leaving group
102:
98:
90:
86:
82:
74:
67:
59:
47:
43:
37:
19:
1847:Molecularity
1559:
1488:
1487:January 14,
1480:
1476:
1461:
1457:
1452:(in Reports)
1441:
1437:
1433:
1419:
1404:
1396:
1392:
1380:. Retrieved
1376:
1367:
1358:
1349:
1340:
1330:
1313:
1306:
1297:Absence of S
1293:
1258:
1254:
1244:
1209:
1205:
1195:
1152:
1148:
1138:
1095:
1092:ChemPhysChem
1091:
1081:
1062:
1058:March, Jerry
1051:
1009:
1002:
992:December 30,
990:. Retrieved
978:
972:
962:
943:
851:
843:
837:
809:
762:
735:
702:
675:
659:second order
652:
612:
588:
523:
473:
465:
458:
450:
438:
418:
411:
367:
340:
333:
325:
281:
246:
239:
209:
176:
156:
113:
76:
72:
71:
1806:Cage effect
1741:RRKM theory
1657:elimination
408:Nucleophile
397:1 pathway.
386:conjugation
132:bimolecular
97:nucleophile
1965:Categories
1029:1084791738
922:References
902:1 reaction
528:, such as
526:sulfonates
249:metabolite
148:1 reaction
114:The name S
91:. In the S
1857:Catalysis
1753:reactions
1169:1521-3765
1112:1439-7641
1037:cite book
785:phenolate
763:A common
661:, as the
562:alkoxides
554:hydroxide
421:-Butoxide
414:methoxide
330:Substrate
257:phenoxide
242:inversion
179:aliphatic
109:concerted
1382:13 April
1285:32866336
1236:34932346
1187:29457865
1130:29542853
1060:(2007),
862:See also
824:benzoate
820:isobutyl
593:is one S
546:mesylate
538:triflate
530:tosylate
480:alcohols
48:of the S
1276:7756690
1227:8822482
1178:5947303
1121:6001448
781:entropy
746:of an S
680:1 and S
639:acetone
609:Solvent
568:), and
544:), and
486:), and
372:1 and S
190:halogen
1532:Basic
1283:
1273:
1234:
1224:
1185:
1175:
1167:
1128:
1118:
1110:
1070:
1027:
1017:
950:
777:alkene
570:amides
509:, and
488:amines
34:, see
1760:Redox
1596:Acyl)
810:With
476:water
261:ether
210:The S
157:The S
60:with
1649:(E2)
1638:(E1)
1489:2008
1442:2008
1405:2003
1384:2018
1281:PMID
1232:PMID
1183:PMID
1165:ISSN
1126:PMID
1108:ISSN
1068:ISBN
1043:link
1025:OCLC
1015:ISBN
994:2023
948:ISBN
589:The
492:R−NH
484:R−OH
419:tert
298:and
1619:Ar)
1576:Ar)
1466:doi
1446:doi
1409:doi
1318:doi
1271:PMC
1263:doi
1222:PMC
1214:doi
1173:PMC
1157:doi
1116:PMC
1100:doi
983:doi
672:= k
579:).
560:),
550:OMs
542:OTf
536:),
534:OTs
154:1.
18:SN2
1967::
1687:(A
1677:(A
1615:(S
1592:(S
1586:i)
1582:(S
1572:(S
1566:2)
1562:(S
1556:1)
1552:(S
1403:;
1375:.
1279:.
1269:.
1259:26
1257:.
1253:.
1230:.
1220:.
1210:87
1208:.
1204:.
1181:.
1171:.
1163:.
1153:24
1151:.
1147:.
1124:.
1114:.
1106:.
1096:19
1094:.
1090:.
1039:}}
1035:{{
1023:.
979:18
977:.
971:.
930:^
842:2
795::
741:(S
637:,
633:,
574:NR
566:OR
558:OH
506:Br
503:,
500:Cl
478:,
469:aH
462:aH
267:.
182:sp
169:.
101:sp
62:CH
58:SH
54:CH
1691:)
1689:N
1681:)
1679:E
1655:i
1653:E
1617:E
1594:N
1584:N
1574:N
1564:N
1554:N
1525:e
1518:t
1511:v
1468::
1448::
1411::
1386:.
1324:.
1320::
1299:N
1287:.
1265::
1238:.
1216::
1189:.
1159::
1132:.
1102::
1045:)
1031:.
996:.
985::
956:.
911:i
909:N
907:S
900:N
898:S
856:N
840:N
769:N
753:N
751:S
748:N
743:N
738:N
730:N
726:N
724:S
721:N
709:N
705:N
698:N
694:N
690:N
686:N
682:N
678:N
670:r
655:N
627:N
595:N
576:2
572:(
564:(
556:(
548:(
540:(
532:(
518:F
512:I
494:2
490:(
482:(
466:K
459:K
454:a
451:K
449:p
441:N
429:N
395:N
391:N
389:S
382:N
378:N
374:N
370:N
356:N
352:N
350:S
343:N
341:S
336:N
315:N
313:S
294:(
284:N
276:N
253:N
212:N
159:N
152:N
146:N
144:S
140:N
124:N
116:N
93:N
81:2
79:N
77:S
75:(
66:I
64:3
56:3
50:N
38:.
32:2
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.