20:
138:
23:
27:
26:
22:
21:
28:
121:
25:
94:
59:, and solutions (both solid solutions and liquid solutions) involving mercury as a major component. Sodium amalgams are often used in reactions as strong reducing agents with better handling properties compared to solid sodium. They are less dangerously reactive toward water and in fact are often used as an aqueous suspension.
243:
solution, and mercury to be recycled through the process. In principle, all the mercury should be completely recycled, but inevitably a small portion goes missing. Because of concerns about this mercury escaping into the environment, the mercury cell process is generally being replaced by plants
24:
157:, i.e. with the release of heat, therefore, formation of sodium amalgam is famously dangerous for generating sparks. The process causes localised boiling of the mercury and for this reason the formation is usually conducted in a fume hood and often performed using
235:. Chlorine is formed at the anode, while sodium formed at the cathode dissolves into the mercury, making sodium amalgam. Normally this sodium amalgam is drawn off and reacted with water in a "decomposer cell" to produce
161:, such as synthesis under anhydrous liquid paraffin. Sodium amalgam may be prepared in the laboratory by dissolving sodium metal in mercury or the reverse. Sodium amalgams can be purchased from chemical supply houses.
512:
386:
605:
534:
Bachmann, W. E. (February 1933). "The
Mechanism of Reduction by Sodium Amalgam and Alcohol. I. The Reduction of Aromatic Ketones to Hydrols".
426:
334:
574:
932:
598:
1055:
1071:
62:
Sodium amalgam was used as a reagent as early as 1862. A synthesis method was described by J. Alfred
Wanklyn in 1866.
916:
591:
205:
188:
providing sodium to produce the proper color, and mercury to tailor the electrical characteristics of the lamp.
90:
of sodium. Amalgams with 2% Na are solids at room temperature, whereas some more dilute amalgams remain liquid.
1416:
888:
1421:
1039:
775:
56:
1388:
1023:
1003:
896:
847:
506:
380:
326:
349:
Keith R. Buszek "Sodium
Amalgam" in Encyclopedia of Reagents for Organic Synthesis, 2001, John Wiley.
1262:
1105:
839:
823:
718:
1360:
1352:
1344:
1323:
1313:
1308:
1303:
1298:
1280:
880:
863:
831:
815:
799:
767:
669:
657:
52:
855:
742:
730:
693:
645:
633:
628:
436:
158:
82:
Hg are well defined compounds. In sodium amalgams, the Hg-Hg distances are expanded to around 5
1426:
1336:
1331:
1293:
875:
794:
681:
614:
551:
498:
469:
422:
372:
330:
185:
170:
44:
784:
709:
543:
414:
350:
318:
300:
269:
240:
178:
571:
987:
959:
904:
789:
578:
448:
213:
971:
174:
87:
1410:
1368:
949:
1376:
217:
93:
1203:
1088:
493:
464:
367:
120:
262:
The London, Edinburgh, and Dublin
Philosophical Magazine and Journal of Science
1223:
418:
273:
197:
154:
555:
354:
1171:
141:
1138:
304:
236:
229:
225:
201:
86:
vs. about 3 Å for mercury itself. Usually amalgams are classified on the
83:
547:
409:
Babcock, S. H.; Lankelma, H. P.; Vopicka, E. (2007). "Sodium
Amalgam".
221:
145:
128:
48:
583:
288:
260:
Atkinson, E. (1862). "XLI. Chemical notices from foreign journals".
232:
209:
92:
40:
18:
114:
Demonstration and commentary on the preparation of sodium amalgam
587:
177:, which is safer to handle than sodium itself. It is used in
119:
492:
Tony C. T. Chang, Myron
Rosenblum, and Nancy Simms (1993).
181:, and also for reduction of aromatic ketones to hydrols.
70:
No particular formula is assigned to "sodium amalgam". Na
289:"XII.—On a new method of forming organo-metallic bodies"
494:"Vinylation of Enolates with a Vinyl Cation Equivalent"
490:
Procedure for making Na(Hg) by addition of Na to Hg:
1322:
1279:
1255:
948:
760:
621:
463:Richard N. McDonald and Charles E. Reineke (1988).
108:
465:"trans-3,5-Cyclohexadiene-1,2-dicarboxylic acid"
184:A sodium amalgam is used in the design of the
599:
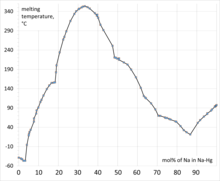
97:Phase diagram of sodium-mercury alloy system.
8:
511:: CS1 maint: multiple names: authors list (
366:W.4r B. Renfrow Jr and C. R. Hauser (1993).
385:: CS1 maint: numeric names: authors list (
945:
606:
592:
584:
536:Journal of the American Chemical Society
39:, with the common formula Na(Hg), is an
252:
504:
444:
434:
378:
105:
153:Metallic sodium dissolves in mercury
7:
14:
244:which use a less toxic cathode.
169:Sodium amalgam has been used in
136:
413:. Vol. 1. pp. 10–11.
1:
144:(31 December 2018), by
287:Wanklyn, J. Alfred (1866).
32:Synthesis of sodium amalgam
16:Alloy of mercury and sodium
1443:
522:, vol. 8, p. 479
479:, vol. 6, p. 461
396:, vol. 2, p. 607
142:Mixing sodium with mercury
66:Structure and compositions
419:10.1002/9780470132326.ch4
323:Chemistry of the Elements
321:; Earnshaw, Alan (1997).
274:10.1080/14786446208643359
220:between a liquid mercury
206:mercury cell electrolysis
192:Mercury cell electrolysis
186:high pressure sodium lamp
135:
118:
113:
368:"Sodium triphenylmethyl"
355:10.1002/047084289X.rs040
57:intermetallic compounds
124:
98:
33:
327:Butterworth-Heinemann
319:Greenwood, Norman N.
123:
96:
31:
305:10.1039/JS8661900128
196:Sodium amalgam is a
55:is used for alloys,
548:10.1021/ja01329a051
411:Inorganic Syntheses
159:air-free techniques
577:2011-12-17 at the
239:gas, concentrated
125:
99:
34:
1404:
1403:
1251:
1250:
615:Mercury compounds
520:Collected Volumes
499:Organic Syntheses
477:Collected Volumes
470:Organic Syntheses
461:see the notes in
428:978-0-470-13232-6
394:Collected Volumes
373:Organic Syntheses
336:978-0-08-037941-8
171:organic chemistry
151:
150:
29:
1434:
1134:
1133:
1132:
1124:
1123:
1115:
1114:
946:
608:
601:
594:
585:
560:
559:
531:
525:
523:
516:
510:
502:
488:
482:
480:
473:
459:
453:
452:
446:
442:
440:
432:
405:
399:
397:
390:
384:
376:
363:
357:
347:
341:
340:
325:(2nd ed.).
315:
309:
308:
284:
278:
277:
268:(161): 305–311.
257:
241:sodium hydroxide
208:. In this cell,
179:Emde degradation
140:
139:
106:
30:
1442:
1441:
1437:
1436:
1435:
1433:
1432:
1431:
1417:Reducing agents
1407:
1406:
1405:
1400:
1396:
1392:
1384:
1380:
1372:
1364:
1356:
1348:
1340:
1324:Mercury cations
1318:
1275:
1266:
1247:
1243:
1239:
1235:
1231:
1227:
1219:
1215:
1211:
1207:
1199:
1195:
1191:
1187:
1183:
1179:
1175:
1166:
1162:
1158:
1154:
1150:
1146:
1142:
1131:
1128:
1127:
1126:
1122:
1119:
1118:
1117:
1113:
1110:
1109:
1108:
1106:
1100:
1096:
1092:
1083:
1079:
1075:
1067:
1063:
1059:
1051:
1047:
1043:
1035:
1031:
1027:
1019:
1015:
1011:
1007:
999:
995:
991:
983:
979:
975:
967:
963:
952:
944:
940:
936:
928:
924:
920:
912:
908:
900:
892:
884:
871:
867:
859:
851:
843:
835:
827:
819:
811:
807:
803:
779:
771:
756:
746:
738:
734:
726:
722:
713:
705:
701:
697:
689:
685:
677:
673:
665:
661:
653:
649:
641:
637:
617:
612:
579:Wayback Machine
568:
563:
533:
532:
528:
518:
503:
491:
489:
485:
475:
462:
460:
456:
443:
433:
429:
408:
406:
402:
392:
377:
365:
364:
360:
348:
344:
337:
317:
316:
312:
286:
285:
281:
259:
258:
254:
250:
214:sodium chloride
194:
167:
137:
131:
109:External videos
104:
81:
77:
73:
68:
19:
17:
12:
11:
5:
1440:
1438:
1430:
1429:
1424:
1419:
1409:
1408:
1402:
1401:
1399:
1398:
1394:
1390:
1386:
1382:
1378:
1374:
1370:
1366:
1362:
1358:
1354:
1350:
1346:
1342:
1338:
1334:
1328:
1326:
1320:
1319:
1317:
1316:
1311:
1306:
1301:
1296:
1291:
1285:
1283:
1277:
1276:
1274:
1273:
1264:
1259:
1257:
1253:
1252:
1249:
1248:
1246:
1245:
1241:
1237:
1233:
1229:
1225:
1221:
1217:
1213:
1209:
1205:
1201:
1197:
1193:
1189:
1185:
1181:
1177:
1173:
1169:
1164:
1160:
1156:
1152:
1148:
1144:
1140:
1136:
1129:
1120:
1111:
1103:
1098:
1094:
1090:
1086:
1081:
1077:
1073:
1069:
1065:
1061:
1057:
1053:
1049:
1045:
1041:
1037:
1033:
1029:
1025:
1021:
1017:
1013:
1009:
1005:
1001:
997:
993:
989:
985:
981:
977:
973:
969:
965:
961:
956:
954:
943:
942:
938:
934:
930:
926:
922:
918:
914:
910:
906:
902:
898:
894:
890:
886:
882:
878:
873:
869:
865:
861:
857:
853:
849:
845:
841:
837:
833:
829:
825:
821:
817:
813:
809:
805:
801:
797:
792:
787:
782:
777:
773:
769:
764:
762:
758:
757:
755:
754:
744:
740:
736:
732:
728:
724:
720:
716:
711:
707:
703:
699:
695:
691:
687:
683:
679:
675:
671:
667:
663:
659:
655:
651:
647:
643:
639:
635:
631:
625:
623:
619:
618:
613:
611:
610:
603:
596:
588:
582:
581:
567:
566:External links
564:
562:
561:
542:(2): 770–774.
526:
483:
454:
445:|journal=
427:
400:
358:
342:
335:
310:
279:
251:
249:
246:
212:(concentrated
193:
190:
175:reducing agent
173:as a powerful
166:
163:
155:exothermically
149:
148:
133:
132:
126:
116:
115:
111:
110:
103:
100:
88:weight percent
79:
75:
71:
67:
64:
37:Sodium amalgam
15:
13:
10:
9:
6:
4:
3:
2:
1439:
1428:
1425:
1423:
1422:Sodium alloys
1420:
1418:
1415:
1414:
1412:
1397:
1387:
1385:
1375:
1373:
1367:
1365:
1359:
1357:
1351:
1349:
1343:
1341:
1335:
1333:
1330:
1329:
1327:
1325:
1321:
1315:
1312:
1310:
1307:
1305:
1302:
1300:
1297:
1295:
1292:
1290:
1287:
1286:
1284:
1282:
1278:
1271:
1267:
1261:
1260:
1258:
1254:
1244:
1222:
1220:
1202:
1200:
1170:
1168:
1137:
1135:
1104:
1102:
1087:
1085:
1070:
1068:
1054:
1052:
1038:
1036:
1022:
1020:
1002:
1000:
986:
984:
970:
968:
958:
957:
955:
951:
950:Organomercury
947:
941:
931:
929:
915:
913:
903:
901:
895:
893:
887:
885:
879:
877:
874:
872:
862:
860:
854:
852:
846:
844:
838:
836:
830:
828:
822:
820:
814:
812:
798:
796:
793:
791:
788:
786:
783:
781:
774:
772:
766:
765:
763:
759:
752:
748:
741:
739:
729:
727:
717:
715:
708:
706:
692:
690:
680:
678:
668:
666:
656:
654:
644:
642:
632:
630:
627:
626:
624:
620:
616:
609:
604:
602:
597:
595:
590:
589:
586:
580:
576:
573:
570:
569:
565:
557:
553:
549:
545:
541:
537:
530:
527:
521:
514:
508:
501:
500:
495:
487:
484:
478:
472:
471:
466:
458:
455:
450:
438:
430:
424:
420:
416:
412:
407:3% Na in Hg:
404:
401:
395:
388:
382:
375:
374:
369:
362:
359:
356:
352:
346:
343:
338:
332:
328:
324:
320:
314:
311:
306:
302:
298:
294:
290:
283:
280:
275:
271:
267:
263:
256:
253:
247:
245:
242:
238:
234:
231:
227:
223:
219:
216:solution) is
215:
211:
207:
203:
199:
191:
189:
187:
182:
180:
176:
172:
164:
162:
160:
156:
147:
143:
134:
130:
122:
117:
112:
107:
101:
95:
91:
89:
85:
65:
63:
60:
58:
54:
50:
46:
42:
38:
1288:
1270:hypothetical
1269:
751:hypothetical
750:
539:
535:
529:
519:
507:cite journal
497:
486:
476:
468:
457:
410:
403:
393:
381:cite journal
371:
361:
345:
322:
313:
296:
293:J. Chem. Soc
292:
282:
265:
261:
255:
218:electrolysed
195:
183:
168:
152:
69:
61:
36:
35:
1256:Mercury(IV)
761:Mercury(II)
572:Oxford MSDS
299:: 128–130.
102:Preparation
51:. The term
1411:Categories
622:Mercury(I)
248:References
198:by-product
953:compounds
556:0002-7863
447:ignored (
437:cite book
1427:Amalgams
1281:Amalgams
917:Hg(Si(CH
575:Archived
237:hydrogen
230:graphite
226:titanium
204:made by
202:chlorine
1188:OBrOCHC
897:Hg(CNO)
889:Hg(SCN)
222:cathode
146:NileRed
129:YouTube
53:amalgam
45:mercury
1314:Sn(Hg)
1309:Tl(Hg)
1304:Au(Hg)
1294:Al(Hg)
1289:Na(Hg)
1139:HgOHCH
1101:(NHCO)
1089:HgOHCH
1032:OB(OH)
848:Hg(OH)
832:Hg(CN)
554:
425:
333:
224:and a
78:and Na
49:sodium
1299:K(Hg)
1224:NaHgC
1180:HOBrC
1176:HgOHC
1151:NHCOC
1143:CHOCH
1093:CHOCH
1072:HgClC
960:Hg(CH
864:Hg(NO
233:anode
210:brine
41:alloy
1369:HgCH
1204:HgOC
988:Hg(C
972:Hg(C
881:HgSO
824:HgCl
816:HgBr
800:Hg(O
795:HgTe
785:HgSe
776:HgNH
552:ISSN
513:link
449:help
423:ISBN
387:link
331:ISBN
165:Uses
127:via
47:and
1389:HgC
1377:HgC
1263:HgF
1159:OCH
1125:HgO
1064:CCl
1056:HgC
1040:HgC
1024:HgC
1004:HgC
937:HgI
876:HgO
856:HgI
840:HgF
804:CCH
790:HgS
768:HgH
698:(NO
629:HgH
544:doi
415:doi
351:doi
301:doi
270:doi
228:or
200:of
43:of
1413::
1361:Hg
1353:Hg
1345:Hg
1337:Hg
1332:Hg
1240:CO
1232:SC
1216:NO
1212:CH
1196:CO
1172:Na
1163:CO
1147:CH
1121:70
1112:36
1097:CH
1080:CO
1048:NO
1016:CO
1012:CH
905:Hg
780:Cl
743:Hg
735:SO
731:Hg
723:CO
719:Hg
710:Hg
694:Hg
682:Hg
670:Hg
662:Cl
658:Hg
650:Br
646:Hg
634:Hg
550:.
540:55
538:.
517:;
509:}}
505:{{
496:.
474:;
467:.
441::
439:}}
435:{{
421:.
391:;
383:}}
379:{{
370:.
329:.
297:19
295:.
291:.
266:24
264:.
74:Hg
1395:5
1393:H
1391:6
1383:5
1381:H
1379:2
1371:3
1363:3
1355:4
1347:3
1339:2
1272:)
1268:(
1265:4
1242:2
1238:4
1236:H
1234:6
1230:5
1228:H
1226:2
1218:2
1214:3
1210:2
1208:H
1206:6
1198:2
1194:4
1192:H
1190:6
1186:2
1184:H
1182:6
1178:6
1174:2
1167:H
1165:2
1161:2
1157:4
1155:H
1153:6
1149:2
1145:3
1141:2
1130:4
1116:H
1107:C
1099:2
1095:3
1091:2
1084:H
1082:2
1078:4
1076:H
1074:6
1066:3
1062:5
1060:H
1058:6
1050:3
1046:5
1044:H
1042:6
1034:2
1030:5
1028:H
1026:6
1018:2
1014:3
1010:5
1008:H
1006:6
998:2
996:)
994:5
992:H
990:6
982:2
980:)
978:5
976:H
974:2
966:2
964:)
962:3
939:4
935:2
933:K
927:2
925:)
923:3
921:)
919:3
911:2
909:N
907:3
899:2
891:2
883:4
870:2
868:)
866:3
858:2
850:2
842:2
834:2
826:2
818:2
810:2
808:)
806:3
802:2
778:2
770:2
753:)
749:(
747:S
745:2
737:4
733:2
725:3
721:2
714:O
712:2
704:2
702:)
700:3
696:2
688:2
686:I
684:2
676:2
674:F
672:2
664:2
660:2
652:2
648:2
640:2
638:H
636:2
607:e
600:t
593:v
558:.
546::
524:.
515:)
481:.
451:)
431:.
417::
398:.
389:)
353::
339:.
307:.
303::
276:.
272::
84:Å
80:3
76:8
72:5
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.