153:
284:
303:(rather than axial chirality resulting from the twist); third, the substituents of the rings of the spiro compound may be such that the only reason they are chiral arises solely from the twist of their rings, e.g., in the simplest bicyclic case, where two structurally identical rings are attached via their spiro atom, resulting in a twisted presentation of the two rings. Hence, in the third case, the lack of planarity described above gives rise to what is termed
123:
236:
138:
17:
192:
204:
364:
is to follow with square brackets containing the number of atoms in the smaller ring then the number of atoms in the larger ring, separated by a period, in each case excluding the spiroatom (the atom by which the two rings are bonded) itself. Position-numbering starts with an atom of the smaller ring
211:
The spirocyclic core is usually prepared by dialkylation of an activated carbon center. The dialkylating group is often a 1,3-, 1,4-, etc. dihalide. In some cases the dialkylating group is a dilithio reagent, such as 1,5-dilithiopentane. For generating spirocycles containing a cyclopropane ring,
559:
De
Meijere, Armin; von Seebach, Malte; Zöllner, Stefan; Kozhushkov, Sergei I.; Belov, Vladimir N.; Boese, Roland; Haumann, Thomas; Benet-Buchholz, Jordi; Yufit, Dmitrii S.; Howard, Judith A. K. (2001). "Spirocyclopropanated Bicyclopropylidenes: Straightforward Preparation, Physical Properties, and
243:
Spiro compounds are considered heterocyclic if the spiro atom or any atom in either ring are not carbon atoms. Cases with a spiro heteroatom such as boron, silicon, and nitrogen (but also other Group IVA are often trivial to prepare. Many borate esters derived from glycols illustrate this case.
175:
that have two fully carbocyclic (all carbon) rings connected through a carbon atom are the usual focus of the topic of spirocycles. Simple parent spirocycles include spiropentane, spirohexane, etc. up to spiroundecane. Several exist as isomers. Lower members of the class are strained. The
692:
Die Übersetzung basiert auf der "Extension and
Revision of the Nomenclature for Spiro Compounds" der Commission on Nomenclature of Organic Chemistry (III.1) der Organic Chemistry Division der International Union of Pure and Applied Chemistry, veröffentlicht in Pure Appl. Chem. 1999, 71,
1389:
Eliel, et al., op. cit., introduces the synonym spirane and the Latin and translation as twist or whorl; Lewis' dictionary, op. cit., speaking to basic definitions in ancient use, and provides the vowel marking and definitions of coil, fold, twist, or
223:
953:
Nakamura, Masaharu; Wang, Xiao Qun; Isaka, Masahiko; Yamago, Shigeru; Nakamura, Eiichi (2003). "Synthesis and [3+2] Cycloaddition of a 2,2-Dialkoxy-1-methylenecyclopropane: 6,6-Dimethyl-1-methylene-4,8-Dioxaspiro[2.5]octane and
641:
Note, the article co-authors, the
Working Party of the IUPAC (1992-1998), were P. M. Giles, Jr., E. W. Godly, K.-H. Hellwich, A. K. Ikizler, M. V. Kisakürek, A. D. McNaught, G. P. Moss, J. Nyitrai, W. H. Powell, O. Weissbach, and A. Yerin.
844:
474:
1008:
Bartolo, Nicole D.; Robson, Ryan N.; Witt, Collin H.; Woerpel, K. A. (2024). "Preparation of a
Radical Clocks Bearing Carbonyl Groups: Synthesis of N-Methoxy-N-methylspiro[cyclopropane-1,9'-fluorene]-2-carboxamide".
222:
1191:"Stereocontrolled Preparation of 3-Acyltetrahydrofurans from Acid-Promoted Rearrangements of Allylic Ketals: (2S,3S)-3-Acetyl-8-Carboethoxy-2,3-Dimethyl-1-Oxa-8-Azaspiro[4.5]Decane".
105:
1089:"Synthesis of Spiroborate Esters from 1,2-Aminoalcohols, Ethylene Glycol and Triisopropyl Borate: Preparation of (S)-1-(1,3,2-Dioxaborolan-2-Yloxy)-3-Methyl-1,1-Diphenylbutan-2-Amine"
330:
even when they lack the required four different substituents normally observed in chirality. When two rings are identical the priority is determined by a slight modification of the
91:
was first applied (though it is now used general of all spiro compounds). The two rings sharing the spiro atom are most often different, although they can be identical undecane and
319:, and alkylidenecycloalkanes as well). Assignment of absolute configuration of spiro compounds has been challenging, but a number of each type have been unequivocally assigned.
152:
1401:
52:(having just two rings). The presence of only one common atom connecting the two rings distinguishes spiro compounds from other bicyclics. Spiro compounds may be fully
1128:
334:
assigning a higher priority to one ring extension and a lower priority to an extension in the other ring. When rings are dissimilar the regular rules apply.
1768:
1484:
299:, and second, while again appearing twisted, the specific location of substituents, as with alkylidenecycloalkanes, may make a spiro compound display
365:
adjacent to the spiroatom around the atoms of that ring, then the spiroatom itself, then around the atoms of the larger ring. For example, compound
981:
Wender, Paul A.; White, Alan W.; McDonald, Frank E. (1992). "Spiroannelation Via
Organobis(Cuprates): 9,9-Dimethylspiro[4.5]Decan-7-One".
516:
Saragi, Tobat P. I.; Spehr, Till; Siebert, Achim; Fuhrmann-Lieker, Thomas; Salbeck, Josef (2007). "Spiro
Compounds for Organic Optoelectronics".
1418:
Quoting: 'spīra ae, f, σπεῖρα, a coil, fold, twist, spiral: in spirain se conligit anguis, V., O.: longo iactetur spira galero, i. e. tie, Iu.'
829:
725:
459:
425:
295:, in various ways. First, while nevertheless appearing to be twisted, they yet may have a chiral center making them analogous to any simple
404:, that presents a twisted structure of two or more rings (a ring system), in which 2 or 3 rings are linked together by one common atom,
789:, respectively, same access date. For the description featuring adjacent atoms for all but the isolated category, see Clayden, op. cit.
60:(having one or more non-carbon atom). One common type of spiro compound encountered in educational settings is a heterocyclic one— the
122:
1421:
The Greek transcription, σπεῖρα, reflects the use of this cognate as one ancient Greek term to refer to a coil or related fold, see
687:
1062:
573:
104:
1424:
1477:
137:
664:
Hellwich, Karl-Heinz; et al. (18 October 2002). "Erweiterung und
Revision der Nomenklatur der Spiroverbindungen".
1586:
1322:
606:
420:(2nd ed.). Oxford, UK: Oxford University Press. pp. 319f, 432, 604np, 653, 746int, 803ketals, 839, 846f.
1357:
The full author (Working Party) list and a link to a German translation are provided in a corresponding footnote.
1886:
1867:
1470:
1247:
1533:
787:
361:
1827:
1808:
1788:
1759:
1683:
1664:
1524:
843:
For a further but less stable source of the same text that provides access to the relevant material, see
473:
For a further but less stable source of the same text that provides access to the relevant material, see
1706:
1646:
1122:
720:(2nd ed.). Oxford, UK: Oxford University Press. pp. 319f, 432, 604, 653, 746, 803, 839, 846f.
327:
216:
900:
784:
750:
1850:
1710:
675:
283:
820:(1st ed.). New York, NY, USA: Wiley & Sons. pp. 1119–1190, esp. 1119ff, 1138ff, and
450:(1st ed.). New York, NY, USA: Wiley & Sons. pp. 1119–1190, esp. 1119ff, 1138ff, and
1621:
1348:
632:
342:
307:
in otherwise identical isomeric pair of spiro compounds, because they differ only in the right-
1037:
773:(Jan. 2016 ed.). East Lansing, MI, USA: Michigan State University, Department of Chemistry
1340:
1264:
1173:
882:
825:
751:"Saturated Hydrocarbons, Alkanes and Cycloalkanes: Cycloalkanes (Table: Examples of Isomeric C
721:
666:
624:
577:
533:
504:
455:
421:
397:
172:
80:
33:
1218:"Dichlorovinylation of an Enolate: 8-Ethynyl-8-Methyl-1,4-Dioxaspiro[4.5]Dec-6-Ene".
1376:
1330:
1298:
1282:
1256:
1227:
1200:
1163:
1155:
1108:
1100:
1018:
990:
963:
935:
872:
683:
614:
569:
525:
494:
401:
346:
180:
41:
1245:
Rios, Ramon (2012). "Enantioselective
Methodologies for the Synthesis of Spiro Compounds".
1513:
1493:
323:
304:
296:
143:
92:
45:
679:
215:
Spiro compounds are often prepared by diverse rearrangement reactions. For example, the
1168:
1143:
1113:
1088:
128:
1142:
Craig, Robert; Smith, R. C.; Pritchett, B. P.; Estipona, B. I.; Stoltz, B. M. (2016).
1880:
268:
1352:
636:
1559:
235:
196:
57:
813:
715:
443:
415:
926:
Dixon, Joseph A.; Naro, Paul A. (1960). "Syntheses of Four Spiro
Hydrocarbons".
53:
75:
The common atom that connects the two (or sometimes three) rings is called the
1302:
331:
1344:
1231:
1204:
1159:
1104:
1022:
994:
967:
628:
1508:
1335:
1317:
619:
601:
292:
112:
16:
1268:
1177:
886:
688:
10.1002/1521-3757(20021018)114:20<4073::AID-ANGE4073>3.0.CO;2-T
661:
Also available in German, with et al. indicating the same working party, at
581:
537:
508:
252:
cation) can be the spiro center. Many such compounds have been described.
1362:
954:
cis-5-(5,5-Dimethyl-1,3-dioxan-2-ylidene)hexahydro-1(2H)-pentalen-2-one".
647:
1741:
574:
10.1002/1521-3765(20010917)7:18<4021::AID-CHEM4021>3.0.CO;2-E
249:
245:
219:
is illustrated below. is employed in the preparation of aspirodecane.].
195:
Synthesis route to Fecht's ester, illustrating a dialkylation route to a
168:
79:. In carbocyclic spiro compounds like spiroundecane, the spiro-atom is a
49:
939:
1260:
877:
860:
499:
482:
264:
191:
158:
1-Bromo-3-chlorospirodecan-7-ol, and '1-bromo-3-chlorospirodecan-7-ol.
529:
1087:
Viatcheslav
Stepanenko, Kun Huang, Margarita Ortiz-Marciales (2010).
316:
312:
272:
256:
69:
61:
1286:
203:
87:
ending implies, these are the types of molecules to which the name
1462:
350:
282:
234:
202:
190:
15:
861:"Total Syntheses of Natural Products Containing Spirocarbocycles"
812:
Eliel, Ernest Ludwig; Wilen, Samuel H.; Mander, Lewis N. (1994).
483:"Total Syntheses of Natural Products Containing Spirocarbocycles"
442:
Eliel, Ernest Ludwig; Wilen, Samuel H.; Mander, Lewis N. (1994).
1363:"Extension and Revision of the Nomenclature for Spiro Compounds"
1318:"Extension and Revision of the Nomenclature for Spiro Compounds"
648:"Extension and Revision of the Nomenclature for Spiro Compounds"
602:"Extension and Revision of the Nomenclature for Spiro Compounds"
311:
left-handed "twist" of structurally identical rings (as seen in
260:
65:
1466:
212:
cyclopropanation with cyclic carbenoids has been demonstrated.
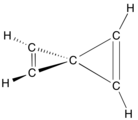
28:, which contains seven spiro atoms and eight cyclopropane rings
814:"Chirality in Molecules Devoid of Chiral Centers (Chapter 14)"
444:"Chirality in Molecules Devoid of Chiral Centers (Chapter 14)"
226:
The synthesis of a spiro-keto compound form a symmetrical diol
1429:
English-Greek Dictionary: A Vocabulary of the Attic Language
1287:"Systematik und Nomenclatur Bicyclischer Kohlenwasserstoffe"
360:
denotes two rings with a spiro junction. The main method of
221:
1144:"Preparation of 1,5-Dioxaspiro[5.5]undecan-3-one"
714:
Clayden, Jonathan; Greeves, Nick; Warren, Stuart (2012).
414:
Clayden, Jonathan; Greeves, Nick; Warren, Stuart (2012).
479:
Examples of spiro natural products and their synthesis:
267:. A simple case is the acetal 1,4-dioxaspirodecane from
244:
Likewise, a tetravalent neutral silicon and quaternary
1377:"Spiro Hydrocarbons. Rule A-41. Compounds: Method 1"
275:. Cases of such ketals and dithioketals are common.
48:
sharing one common atom. Simple spiro compounds are
1849:
1826:
1807:
1787:
1767:
1758:
1740:
1728:
1705:
1682:
1663:
1645:
1620:
1585:
1558:
1532:
1523:
1500:
854:
852:
859:Smith, Laura K. & Baxendale, Ian R. (2015).
481:Smith, Laura K. & Baxendale, Ian R. (2015).
1365:. London, GBR: Queen Mary University of London.
1291:Berichte der Deutschen Chemischen Gesellschaft
650:. London, GBR: Queen Mary University of London
353:provides advice on naming of spiro compounds.
1478:
807:
805:
803:
801:
799:
797:
795:
709:
707:
705:
703:
701:
595:
593:
591:
259:formed by condensation of cyclic ketones and
8:
1431:. Ludgate Hill : George Routledge & Sons
1127:: CS1 maint: multiple names: authors list (
345:for spiro compounds was first discussed by
1764:
1529:
1485:
1471:
1463:
1408:. New York, NY, USA: American Book Company
176:symmetric isomer of spiroundecane is not.
1334:
1167:
1112:
876:
763:Nomenclature: Cycloalkanes (same Table),
618:
498:
1425:"Fold, subs. [dictionary entry]"
255:Particularly common spiro compounds are
1789:Pseudoasymmetric (pseudochiral) centers
1769:CIP (Cahn–Ingold–Prelog) priority rules
551:
97:
1120:
783:The specific chapters can be found at
7:
818:Stereochemistry of Organic Compounds
448:Stereochemistry of Organic Compounds
287:Two enantiomers of a spiro diketone.
179:Some spirocyclic compounds occur as
1063:"1,4-Dioxaspiro[4.5]decane"
1402:"spīra [dictionary entry]"
326:. Spiroatoms can be the origin of
131:, a commercial diuretic medication
14:
771:Virtual Text of Organic Chemistry
207:Synthesis route to spiroundecane.
928:The Journal of Organic Chemistry
396:, meaning a twist or coil, is a
217:pinacol-pinacolone rearrangement
151:
136:
121:
103:
379:1-bromo-3-chlorospirodecan-7-ol
371:1-bromo-3-chlorospirodecan-7-ol
1406:An Elementary Latin Dictionary
1038:"1,1'-Bicyclopentyl-1,1'-diol"
562:Chemistry - A European Journal
1:
747:For all four categories, see
322:Some spiro compounds exhibit
239:Preparation of a spiro ketal.
901:"Elusive bowtie pinned down"
231:Heterocyclic spiro compounds
1400:Lewis, Charlton T. (1890).
560:Chemical Transformations".
164:Carbocyclic spiro compounds
146:, which is highly strained.
1903:
1851:Octahedral propeller twist
1587:Arene substitution pattern
338:Nomenclature and etymology
1865:
1525:Configuration descriptors
1303:10.1002/cber.190003303187
1868:Category:Stereochemistry
1441:Quoting: 'Fold, subs. …
1423:Woodhouse, S.C. (1910).
1359:Also available online at
1248:Chemical Society Reviews
1232:10.15227/orgsyn.064.0073
1205:10.15227/orgsyn.071.0063
1160:10.15227/orgsyn.093.0210
1105:10.15227/orgsyn.087.0026
1023:10.15227/orgsyn.101.0061
995:10.15227/orgsyn.070.0204
968:10.15227/orgsyn.080.0144
749:Reusch, William (1999).
644:Also available online at
99:Selected spiro compounds
64:formed by reaction of a
1760:Absolute configurations
1665:Three identical ligands
1336:10.1351/pac199971030531
620:10.1351/pac199971030531
369:in the image is called
362:systematic nomenclature
44:that have at least two
1828:Relative configuration
315:, sterically hindered
288:
240:
227:
208:
200:
110:Elatol, isolated from
29:
1647:Syn and anti addition
286:
238:
225:
206:
194:
19:
1677:(facies, meridonal)
113:Laurencia dendroidea
1684:In carbon skeletons
1316:Moss, G.P. (1999).
940:10.1021/jo01082a006
846:, same access date.
680:2002AngCh.114.4073H
600:Moss, G.P. (1999).
476:, same access date.
171:ring structures in
1261:10.1039/C1CS15156H
878:10.1039/C5OB01524C
500:10.1039/C5OB01524C
289:
241:
228:
209:
201:
30:
1874:
1873:
1861:
1860:
1754:
1753:
1283:von Baeyer, Adolf
1220:Organic Syntheses
1193:Organic Syntheses
1148:Organic Syntheses
1093:Organic Syntheses
1011:Organic Syntheses
983:Organic Syntheses
956:Organic Syntheses
934:(12): 2094–2097.
871:(39): 9907–9933.
865:Org. Biomol. Chem
831:978-0-471-01670-0
727:978-0-19-927029-3
717:Organic Chemistry
674:(20): 4073–4089.
667:Angewandte Chemie
568:(18): 4021–4034.
530:10.1021/cr0501341
493:(39): 9907–9933.
487:Org. Biomol. Chem
461:978-0-471-01670-0
427:978-0-19-927029-3
417:Organic Chemistry
398:chemical compound
392:, from the Latin
301:central chirality
173:organic chemistry
81:quaternary carbon
34:organic chemistry
1894:
1843:
1842:
1837:
1836:
1809:Optical rotation
1765:
1530:
1487:
1480:
1473:
1464:
1452:
1451:
1438:
1436:
1420:
1415:
1413:
1397:
1391:
1387:
1381:
1380:
1373:
1367:
1366:
1356:
1338:
1323:Pure Appl. Chem.
1313:
1307:
1306:
1297:(3): 3771–3775.
1279:
1273:
1272:
1255:(3): 1060–1074.
1242:
1236:
1235:
1215:
1209:
1208:
1188:
1182:
1181:
1171:
1139:
1133:
1132:
1126:
1118:
1116:
1084:
1078:
1077:
1075:
1073:
1059:
1053:
1052:
1050:
1048:
1033:
1027:
1026:
1005:
999:
998:
978:
972:
971:
950:
944:
943:
923:
917:
916:
914:
912:
905:The Free Library
897:
891:
890:
880:
856:
847:
842:
840:
838:
809:
790:
782:
780:
778:
759:Bicycloalkanes)
745:
739:
738:
736:
734:
711:
696:
695:
659:
657:
655:
640:
622:
607:Pure Appl. Chem.
597:
586:
585:
556:
541:
524:(4): 1011–1065.
518:Chemical Reviews
512:
502:
472:
470:
468:
438:
436:
434:
402:organic compound
347:Adolf von Baeyer
291:Spiranes can be
181:natural products
155:
140:
125:
107:
56:(all carbon) or
1902:
1901:
1897:
1896:
1895:
1893:
1892:
1891:
1887:Spiro compounds
1877:
1876:
1875:
1870:
1857:
1845:
1840:
1839:
1834:
1833:
1822:
1803:
1783:
1750:
1736:
1724:
1701:
1678:
1659:
1641:
1616:
1581:
1554:
1519:
1518:
1514:Racemic mixture
1496:
1494:Stereochemistry
1491:
1461:
1456:
1455:
1445:V. σπεῖρα… see
1434:
1432:
1422:
1411:
1409:
1399:
1398:
1394:
1388:
1384:
1375:
1374:
1370:
1361:
1315:
1314:
1310:
1281:
1280:
1276:
1244:
1243:
1239:
1217:
1216:
1212:
1190:
1189:
1185:
1141:
1140:
1136:
1119:
1086:
1085:
1081:
1071:
1069:
1061:
1060:
1056:
1046:
1044:
1035:
1034:
1030:
1007:
1006:
1002:
980:
979:
975:
952:
951:
947:
925:
924:
920:
910:
908:
899:
898:
894:
858:
857:
850:
836:
834:
832:
811:
810:
793:
776:
774:
758:
754:
748:
746:
742:
732:
730:
728:
713:
712:
699:
663:
653:
651:
646:
599:
598:
589:
558:
557:
553:
548:
515:
480:
466:
464:
462:
441:
432:
430:
428:
413:
410:
408:Further reading
400:, typically an
373:, and compound
340:
324:axial chirality
305:axial chirality
297:chiral compound
281:
233:
189:
166:
159:
156:
147:
144:Spiropentadiene
141:
132:
126:
117:
108:
93:spiropentadiene
46:molecular rings
38:spiro compounds
27:
23:
12:
11:
5:
1900:
1898:
1890:
1889:
1879:
1878:
1872:
1871:
1866:
1863:
1862:
1859:
1858:
1855:
1853:
1847:
1846:
1832:
1830:
1824:
1823:
1814:(+)-, (−)- or
1813:
1811:
1805:
1804:
1793:
1791:
1785:
1784:
1773:
1771:
1762:
1756:
1755:
1752:
1751:
1746:
1744:
1738:
1737:
1734:
1732:
1730:Spiro compound
1726:
1725:
1715:
1713:
1703:
1702:
1688:
1686:
1680:
1679:
1669:
1667:
1661:
1660:
1651:
1649:
1643:
1642:
1633:
1631:
1618:
1617:
1591:
1589:
1583:
1582:
1571:
1569:
1556:
1555:
1545:
1543:
1527:
1521:
1520:
1517:
1516:
1511:
1505:
1504:
1502:
1498:
1497:
1492:
1490:
1489:
1482:
1475:
1467:
1460:
1459:External links
1457:
1454:
1453:
1392:
1382:
1368:
1329:(3): 531–558.
1308:
1274:
1237:
1210:
1183:
1134:
1079:
1067:chemspider.com
1054:
1028:
1000:
973:
945:
918:
907:. 13 July 1991
892:
848:
830:
791:
756:
752:
740:
726:
697:
613:(3): 531–558.
587:
550:
549:
547:
544:
543:
542:
513:
477:
460:
439:
426:
409:
406:
386:spiro compound
339:
336:
280:
277:
257:ketal (acetal)
232:
229:
188:
185:
165:
162:
161:
160:
157:
150:
148:
142:
135:
133:
129:Spironolactone
127:
120:
118:
109:
102:
100:
68:with a cyclic
25:
21:
20:Structure of C
13:
10:
9:
6:
4:
3:
2:
1899:
1888:
1885:
1884:
1882:
1869:
1864:
1854:
1852:
1848:
1831:
1829:
1825:
1821:
1817:
1812:
1810:
1806:
1801:
1797:
1792:
1790:
1786:
1781:
1777:
1772:
1770:
1766:
1763:
1761:
1757:
1749:
1745:
1743:
1739:
1733:
1731:
1727:
1722:
1718:
1714:
1712:
1708:
1704:
1699:
1695:
1691:
1687:
1685:
1681:
1676:
1672:
1668:
1666:
1662:
1658:
1654:
1650:
1648:
1644:
1640:
1636:
1632:
1630:
1628:
1624:
1619:
1614:
1610:
1606:
1602:
1598:
1594:
1590:
1588:
1584:
1579:
1575:
1570:
1568:
1566:
1562:
1557:
1552:
1548:
1544:
1542:
1540:
1536:
1531:
1528:
1526:
1522:
1515:
1512:
1510:
1507:
1506:
1503:
1499:
1495:
1488:
1483:
1481:
1476:
1474:
1469:
1468:
1465:
1458:
1450:
1448:
1444:
1430:
1426:
1419:
1407:
1403:
1396:
1393:
1386:
1383:
1378:
1372:
1369:
1364:
1360:
1354:
1350:
1346:
1342:
1337:
1332:
1328:
1325:
1324:
1319:
1312:
1309:
1304:
1300:
1296:
1292:
1288:
1284:
1278:
1275:
1270:
1266:
1262:
1258:
1254:
1250:
1249:
1241:
1238:
1233:
1229:
1225:
1221:
1214:
1211:
1206:
1202:
1198:
1194:
1187:
1184:
1179:
1175:
1170:
1165:
1161:
1157:
1153:
1149:
1145:
1138:
1135:
1130:
1124:
1115:
1110:
1106:
1102:
1098:
1094:
1090:
1083:
1080:
1068:
1064:
1058:
1055:
1043:
1039:
1032:
1029:
1024:
1020:
1016:
1012:
1004:
1001:
996:
992:
988:
984:
977:
974:
969:
965:
961:
957:
949:
946:
941:
937:
933:
929:
922:
919:
906:
902:
896:
893:
888:
884:
879:
874:
870:
866:
862:
855:
853:
849:
845:
833:
827:
823:
819:
815:
808:
806:
804:
802:
800:
798:
796:
792:
788:
785:
772:
768:
766:
762:
744:
741:
729:
723:
719:
718:
710:
708:
706:
704:
702:
698:
694:
689:
685:
681:
677:
673:
669:
668:
662:
649:
645:
638:
634:
630:
626:
621:
616:
612:
609:
608:
603:
596:
594:
592:
588:
583:
579:
575:
571:
567:
563:
555:
552:
545:
539:
535:
531:
527:
523:
519:
514:
510:
506:
501:
496:
492:
488:
484:
478:
475:
463:
457:
453:
449:
445:
440:
429:
423:
419:
418:
412:
411:
407:
405:
403:
399:
395:
391:
387:
382:
380:
376:
372:
368:
363:
359:
354:
352:
348:
344:
337:
335:
333:
329:
325:
320:
318:
314:
310:
306:
302:
298:
294:
285:
278:
276:
274:
270:
269:cyclohexanone
266:
262:
258:
253:
251:
247:
237:
230:
224:
220:
218:
213:
205:
198:
193:
186:
184:
182:
177:
174:
170:
163:
154:
149:
145:
139:
134:
130:
124:
119:
115:
114:
106:
101:
98:
96:
95:, at right].
94:
90:
86:
83:, and as the
82:
78:
73:
71:
67:
63:
59:
55:
51:
47:
43:
39:
35:
18:
1819:
1815:
1799:
1795:
1779:
1775:
1747:
1729:
1720:
1716:
1697:
1693:
1689:
1674:
1670:
1656:
1652:
1638:
1634:
1626:
1622:
1612:
1608:
1604:
1600:
1596:
1592:
1577:
1573:
1564:
1560:
1550:
1546:
1538:
1534:
1446:
1442:
1440:
1433:. Retrieved
1428:
1417:
1410:. Retrieved
1405:
1395:
1385:
1371:
1358:
1326:
1321:
1311:
1294:
1290:
1277:
1252:
1246:
1240:
1226:: 73. 1986.
1223:
1219:
1213:
1199:: 63. 1993.
1196:
1192:
1186:
1151:
1147:
1137:
1123:cite journal
1096:
1092:
1082:
1070:. Retrieved
1066:
1057:
1045:. Retrieved
1041:
1031:
1014:
1010:
1003:
986:
982:
976:
959:
955:
948:
931:
927:
921:
909:. Retrieved
904:
895:
868:
864:
835:. Retrieved
821:
817:
775:. Retrieved
770:
764:
760:
743:
731:. Retrieved
716:
691:
671:
665:
660:
652:. Retrieved
643:
610:
605:
565:
561:
554:
521:
517:
490:
486:
465:. Retrieved
451:
447:
431:. Retrieved
416:
393:
389:
385:
383:
378:
374:
370:
366:
357:
355:
343:Nomenclature
341:
321:
308:
300:
290:
254:
242:
214:
210:
197:spiroheptane
178:
167:
116:(red algae)
111:
88:
84:
76:
74:
58:heterocyclic
37:
31:
1443:Coil :
1154:: 210–227.
356:The prefix
187:Preparation
54:carbocyclic
1435:3 February
1412:3 February
1072:3 February
911:2 February
837:2 February
777:3 February
765:and passim
733:2 February
654:3 February
546:References
467:2 February
433:2 February
377:is called
349:in 1900.
332:CIP system
77:spiro atom
1707:Secondary
1629:isomerism
1541:isomerism
1509:Chirality
1345:1365-3075
1036:Pubchem.
1017:: 61–80.
629:1365-3075
328:chirality
279:Chirality
42:compounds
1881:Category
1742:Catenane
1711:tertiary
1567:notation
1353:20131819
1285:(1900).
1269:21975423
1178:28729749
887:26356301
693:531–558.
637:20131819
582:11596945
538:17381160
509:26356301
265:dithiols
250:ammonium
246:nitrogen
169:Bicyclic
50:bicyclic
1700:, cyclo
1563:–
1390:spiral.
1169:5514842
1114:2915795
1047:7 March
1042:nih.gov
989:: 204.
962:: 144.
676:Bibcode
390:spirane
317:biaryls
313:allenes
89:spirane
1856:Λ-, Δ-
1748:catena
1501:Topics
1351:
1343:
1267:
1176:
1166:
1111:
1099:: 26.
885:
828:
822:passim
724:
635:
627:
580:
536:
507:
458:
452:passim
424:
309:versus
293:chiral
273:glycol
248:atom (
70:ketone
62:acetal
1735:spiro
1605:ortho
1576:)-, (
1551:trans
1539:trans
1349:S2CID
633:S2CID
394:spīra
388:, or
358:spiro
351:IUPAC
261:diols
1798:), (
1778:), (
1721:tert
1709:and
1657:anti
1635:endo
1623:Endo
1613:para
1609:meta
1447:coil
1437:2016
1414:2016
1341:ISSN
1265:PMID
1174:PMID
1129:link
1074:2016
1049:2016
913:2016
883:PMID
839:2016
826:ISBN
786:and
779:2016
735:2016
722:ISBN
656:2016
625:ISSN
578:PMID
534:PMID
505:PMID
469:2016
456:ISBN
435:2016
422:ISBN
271:and
263:and
85:-ane
66:diol
40:are
1838:-,
1719:-,
1717:sec
1698:neo
1694:iso
1675:mer
1671:fac
1653:syn
1639:exo
1637:,
1627:exo
1603:- (
1599:-,
1595:-,
1549:-,
1547:cis
1535:cis
1449:.'
1331:doi
1299:doi
1257:doi
1228:doi
1201:doi
1164:PMC
1156:doi
1109:PMC
1101:doi
1019:doi
1015:101
991:doi
964:doi
936:doi
873:doi
684:doi
672:114
615:doi
570:doi
526:doi
522:107
495:doi
183:.
72:.
32:In
1883::
1820:l-
1818:,
1816:d-
1696:,
1692:,
1673:,
1655:,
1611:,
1607:,
1580:)-
1439:.
1427:.
1416:.
1404:.
1347:.
1339:.
1327:71
1320:.
1295:33
1293:.
1289:.
1263:.
1253:41
1251:.
1224:64
1222:.
1197:71
1195:.
1172:.
1162:.
1152:93
1150:.
1146:.
1125:}}
1121:{{
1107:.
1097:87
1095:.
1091:.
1065:.
1040:.
1013:.
987:70
985:.
960:80
958:.
932:25
930:.
903:.
881:.
869:13
867:.
863:.
851:^
824:.
816:.
794:^
769:.
761:or
757:14
700:^
690:.
682:.
670:.
631:.
623:.
611:71
604:.
590:^
576:.
564:.
532:.
520:.
503:.
491:13
489:.
485:.
454:.
446:.
384:A
381:.
36:,
26:20
22:17
1844:-
1841:L
1835:D
1802:)
1800:s
1796:r
1794:(
1782:)
1780:S
1776:R
1774:(
1723:-
1690:n
1625:-
1615:)
1601:p
1597:m
1593:o
1578:Z
1574:E
1572:(
1565:Z
1561:E
1553:-
1537:–
1486:e
1479:t
1472:v
1379:.
1355:.
1333::
1305:.
1301::
1271:.
1259::
1234:.
1230::
1207:.
1203::
1180:.
1158::
1131:)
1117:.
1103::
1076:.
1051:.
1025:.
1021::
997:.
993::
970:.
966::
942:.
938::
915:.
889:.
875::
841:.
781:.
767:"
755:H
753:8
737:.
686::
678::
658:.
639:.
617::
584:.
572::
566:7
540:.
528::
511:.
497::
471:.
437:.
375:B
367:A
199:.
24:H
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.