681:
treatment for patients positive for genetic risk factors, and elderly patients positive for indicators of AD are underway. This includes anti-AB treatment in
Asymptomatic Alzheimer's Disease (A4), the Alzheimer's Prevention Initiative (API), and DIAN-TU. The A4 study on older individuals who are positive for indicators of AD but are negative for genetic risk factors will test Solanezumab in Phase III Clinical Trials, as a follow-up of previous Solanezumab studies. DIAN-TU, launched in December 2012, focuses on young patients positive for genetic mutations that are risks for AD. This study uses Solanezumab and Gautenerumab. Gautenerumab, the first fully human MAB that preferentially interacts with oligomerized Aβ plaques in the brain, caused significant reduction in Aβ concentration in Phase I clinical trials, preventing plaque formation and concentration without altering plasma concentration of the brain. Phase II and III clinical trials are currently being conducted.
177:. The advantage of active monoclonal antibody therapy is the fact that the immune system will produce antibodies long-term, with only a short-term drug administration to induce this response. However, the immune response to certain antigens may be inadequate, especially in the elderly. Additionally, adverse reactions from these antibodies may occur because of long-lasting response to antigens. Passive monoclonal antibody therapy can ensure consistent antibody concentration, and can control for adverse reactions by stopping administration. However, the repeated administration and consequent higher cost for this therapy are major disadvantages.
842:
215:
536:(Aβ) into plaques via oligomerization leads to hallmark symptomatic conditions of AD through synaptic dysfunction and neurodegeneration. Immunotherapy via exogenous monoclonal antibody (mAb) administration has been known to treat various central nervous disorders. In the case of AD, immunotherapy is believed to inhibit Aβ-oligomerization or clearing of Aβ from the brain and thereby prevent
31:
720:(ADEPT) involves the application of cancer-associated monoclonal antibodies that are linked to a drug-activating enzyme. Systemic administration of a non-toxic agent results in the antibody's conversion to a toxic drug, resulting in a cytotoxic effect that can be targeted at malignant cells. The clinical success of ADEPT treatments is limited.
4785:(oncology and AIID) accounted for 80% of revenues in 2006. In 2007, eight of the 20 best-selling biotechnology drugs in the U.S. are therapeutic monoclonal antibodies. This rapid growth in demand for monoclonal antibody production has been well accommodated by the industrialization of mAb manufacturing.
374:
Humanised antibodies are produced by grafting murine hypervariable regions on amino acid domains into human antibodies. This results in a molecule of approximately 95% human origin. Humanised antibodies bind antigen much more weakly than the parent murine monoclonal antibody, with reported decreases
566:. In this case the antibodies is produced externally in cultured cells and are delivered to the patient in the form of a drug. In mice expressing APP, both active and passive immunization of anti-Aβ antibodies has been shown to be effective in clearing plaques, and can improve cognitive function.
680:
Failure of several drugs in Phase III clinical trials has led to AD prevention and early intervention for onset AD treatment endeavours. Passive anti-Aβ mAb treatment can be used for preventive attempts to modify AD progression before it causes extensive brain damage and symptoms. Trials using mAb
315:
Initially, murine antibodies were obtained by hybridoma technology, for which Jerne, Köhler and
Milstein received a Nobel prize. However the dissimilarity between murine and human immune systems led to the clinical failure of these antibodies, except in some specific circumstances. Major problems
652:
elevation of Aβ, thereby showing a reduced concentration of Aβ plaques. Additionally, there are no associated adverse side effects. Phase III clinical trials of
Solanezumab brought about significant reduction in cognitive impairment in patients with mild AD, but not in patients with severe AD.
447:
Monoclonal antibodies used to boost an anticancer immune response is another strategy to fight cancer where cancer cells are not targeted directly. Strategies include antibodies engineered to block mechanisms which downregulate anticancer immune responses, checkpoints such as PD-1 and CTLA-4
585:
while
Lecanemab has received full approval. Several clinical trials using passive and active immunization have been performed and some are on the way with expected results in a couple of years. The implementation of these drugs is often during the early onset of AD. Other research and drug
196:
that are proliferating at high rates, or body cells that are dying which subsequently cause physiological problems are generally not specifically targeted by the immune system, since tumor cells are the patient's own cells. Tumor cells, however are highly abnormal, and many display unusual
668:(BAN2401), is a humanized mAb that selectively targets toxic soluble Aβ protofibrils, In phase 3 clinical trials, Lecanemab showed a 27% slower cognitive decline after 18 months of treatment in comparison to placebo. The phase 3 clinical trials also reported infusion related reactions,
364:). Chimeric antibodies are composed of murine variable regions fused onto human constant regions. Taking human gene sequences from the kappa light chain and the IgG1 heavy chain results in antibodies that are approximately 65% human. This reduces immunogenicity, and thus increases
760:
Checkpoint therapy uses antibodies and other techniques to circumvent the defenses that tumors use to suppress the immune system. Each defense is known as a checkpoint. Compound therapies combine antibodies to suppress multiple defensive layers. Known checkpoints include
419:
genes into the murine genome and vaccinating the transgenic mouse against the desired antigen, leading to the production of appropriate monoclonal antibodies. Murine antibodies in vitro are thereby transformed into fully human antibodies.
731:(ADCs) are antibodies linked to one or more drug molecules. Typically when the ADC meets the target cell (e.g. a cancerous cell) the drug is released to kill it. Many ADCs are in clinical development. As of 2016 a few have been approved.
747:
and when conjugated with monoclonal antibodies, may be directed against malignant cells. Immunoliposomes have been successfully used in vivo to convey tumour-suppressing genes into tumours, using an antibody fragment against the human
636:
In Phase III clinical trials, Bapineuzumab showed promising positive effect on biomarkers of AD but failed to show effect on cognitive decline. Therefore, Bapineuzumab was discontinued after failing in the Phase III clinical trial.
625:, a humanized anti-Aβ mAb, is directed against the N-terminus of Aβ. Phase II clinical trials of Bapineuzumab in mild to moderate AD patients resulted in reduced Aβ concentration in the brain. However, in patients with increased
356:(attacks by the immune system against the antibody), murine molecules were engineered to remove immunogenic content and to increase immunologic efficiency. This was initially achieved by the production of chimeric (suffix
192:. Monoclonal antibody therapy can aid the immune system because the innate immune system responds to the environmental factors it encounters by discriminating against foreign cells from cells of the body. Therefore, tumor
307:
formation), limited penetration into tumour sites and inadequately recruit host effector functions. Chimeric and humanized antibodies have generally replaced them in therapeutic antibody applications. Understanding of
633:, a cytotoxic condition where the blood brain barrier has been disrupted thereby affecting white matter from excess accumulation of fluid from capillaries in intracellular and extracellular spaces of the brain.
271:
found mAb therapy of limited and generally short-lived success with blood malignancies. Treatment also had to be tailored to each individual patient, which was impracticable in routine clinical settings.
205:
are inappropriate for the cell type or its environment. Monoclonal antibodies can target tumor cells or abnormal cells in the body that are recognized as body cells, but are debilitating to one's health.
5104:
Nadler LM, Stashenko P, Hardy R, Kaplan WD, Button LN, Kufe DW, et al. (September 1980). "Serotherapy of a patient with a monoclonal antibody directed against a human lymphoma-associated antigen".
444:
is a recombinant human monoclonal antibody and is used in the treatment of advanced malignancies. In childhood lymphoma, phase I and II studies have found a positive effect of using antibody therapy.
672:
and headaches as the most common side effects of
Lecanemab. In July 2023 the FDA gave Lecanemab full approval for the treatment of Alzheimer's Disease and it was given the commercial name Leqembi.
6743:
6058:
Panza F, Frisardi V, Imbimbo BP, D'Onofrio G, Pietrarossa G, Seripa D, et al. (November 2010). "Bapineuzumab: anti-β-amyloid monoclonal antibodies for the treatment of
Alzheimer's disease".
554:
However, anti-Aβ vaccines can promote antibody-mediated clearance of Aβ plaques in transgenic mice models with amyloid precursor proteins (APP), and can reduce cognitive impairments.
383:(CDR), using techniques such as chain-shuffling, randomization of complementarity-determining regions and antibodies with mutations within the variable regions induced by error-prone
551:
proposes a mechanism where mAbs may not need to cross the blood–brain barrier. Therefore, many research studies are being conducted from failed attempts to treat AD in the past.
705:, as these are highly radio-sensitive malignancies. To limit radiation exposure, murine antibodies were chosen, as their high immunogenicity promotes rapid tumor clearance.
5938:
Panza F, Solfrizzi V, Imbimbo BP, Logroscino G (October 2014). "Amyloid-directed monoclonal antibodies for the treatment of
Alzheimer's disease: the point of no return?".
6736:
57:. They can be used to render their target ineffective (e.g. by preventing receptor binding), to induce a specific cell signal (by activating receptors), to cause the
586:
development for early intervention and AD prevention is ongoing. Examples of important mAb drugs that have been or are under evaluation for treatment of AD include
3184:
1753:
226:
6712:
558:
can stimulate the immune system to produce its own antibodies, in the case of
Alzheimer's disease by administration of the antigen Aβ. This is also known as
5567:
Vennepureddy A, Singh P, Rastogi R, Atallah JP, Terjanian T (October 2017). "Evolution of ramucirumab in the treatment of cancer - A review of literature".
6729:
6295:
528:
Alzheimer's disease (AD) is a multi-faceted, age-dependent, progressive neurodegenerative disorder, and is a major cause of dementia. According to the
8167:
873:
784:. Tumor vasculature helps tumors preferentially recruit other immune cells over T cells, in part through endothelial cell (EC)–specific expression of
4761:
Since 2000, the therapeutic market for monoclonal antibodies has grown exponentially. In 2006, the "big 5" therapeutic antibodies on the market were
5713:
Dempke WC, Fenchel K, Uciechowski P, Dale SP (March 2017). "Second- and third-generation drugs for immuno-oncology treatment-The more the better?".
657:, and hippocampal volume. Phase III clinical trials of Solanezumab failed as it did not show effect on cognitive decline in comparison to placebo.
150:
6321:"A phase I trial of antibody directed enzyme prodrug therapy (ADEPT) in patients with advanced colorectal carcinoma or other CEA producing tumours"
125:
chain, called the heavy (~50kDa) and the light chain (~25kDa). The two types of light chains are kappa (κ) and lambda (λ). By cleavage with enzyme
7735:
7378:
5369:
Chothia C, Lesk AM, Tramontano A, Levitt M, Smith-Gill SJ, Air G, et al. (1989). "Conformations of immunoglobulin hypervariable regions".
1928:
6460:
Hooks MA, Wade CS, Millikan WJ (1991). "Muromonab CD-3: a review of its pharmacology, pharmacokinetics, and clinical use in transplantation".
5760:
4903:
4872:
669:
6871:
4778:
3995:
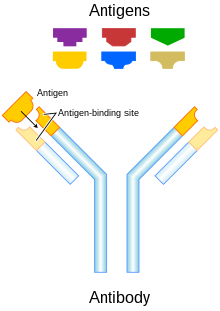
161:, playing a central role in both in the recognition of foreign antigens and the stimulation of an immune response to them. The advent of
2410:
1248:
380:
7849:
980:
230:
6235:
van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, et al. (January 2023). "Lecanemab in Early
Alzheimer's Disease".
547:, uptake of mAb into the brain is extremely limited, only approximately 1 of 1000 mAb molecules is estimated to pass. However, the
780:(TME) features prevents the recruitment of T cells to the tumor. Ways include chemokine CCL nitration, which traps T cells in the
7269:
6795:
3816:
3391:
2054:
4862:
1711:
149:
domains responsible for the antibody specificity embedded into constant regions. The four known IgG subclasses are involved in
648:, an anti-Aβ mAb, targets the N-terminus of Aβ. In Phase I and Phase II of clinical trials, Solanezumab treatment resulted in
8172:
4061:
1802:
375:
in affinity of up to several hundredfold. Increases in antibody-antigen binding strength have been achieved by introducing
4660:
4449:
4444:
4174:
3440:
3397:
2590:
1965:
1705:
1655:
1554:
1291:
1140:
1071:
1810:
7615:
4178:
3651:
3567:
1517:
910:
570:
5053:
Köhler G, Milstein C (August 1975). "Continuous cultures of fused cells secreting antibody of predefined specificity".
6144:"Safety and tolerability of BAN2401--a clinical study in Alzheimer's disease with a protofibril selective Aβ antibody"
3779:
3774:
3017:
2199:
1304:
809:
423:
The heavy and light chains of human IgG proteins are expressed in structural polymorphic (allotypic) forms. Human IgG
7796:
6702:
7921:
7770:
7519:
7021:
6368:
Krauss WC, Park JW, Kirpotin DB, Hong K, Benz CC (2000). "Emerging antibody-based HER2 (ErbB-2/neu) therapeutics".
3319:
1385:
1346:
752:
receptor. Tissue-specific gene delivery using immunoliposomes has been achieved in brain and breast cancer tissue.
582:
333:
165:
technology has made it possible to raise antibodies against specific antigens presented on the surfaces of tumors.
4132:
988:
7246:
7112:
6825:
4124:
2859:
2740:
2303:
1886:
1175:
392:
384:
4500:
3787:
3744:
2952:
2867:
2115:
1983:
1030:
548:
8177:
7256:
6782:
3830:
3234:
2839:
2245:
1525:
1483:
1256:
5178:
Stern M, Herrmann R (April 2005). "Overview of monoclonal antibodies in cancer therapy: present and promise".
4692:
4543:
4457:
4361:
4319:
4276:
4228:
4186:
4046:
3575:
3448:
3405:
3276:
3192:
3147:
3082:
2782:
2698:
2603:
2555:
2511:
2460:
2376:
2288:
2157:
2025:
1936:
1894:
1719:
1676:
1569:
1436:
1393:
544:
6752:
6707:
5829:
3174:
2630:
1788:
1195:
728:
654:
7624:
7107:
6836:
4645:
4589:
4403:
4311:
4268:
4089:
4003:
3957:
3915:
3872:
3701:
3659:
3617:
3533:
3490:
3362:
3131:
3066:
3001:
2910:
2824:
2690:
2648:
2640:
2418:
2405:
2334:
2068:
1852:
1761:
1612:
1214:
1168:
1148:
1099:
1079:
777:
189:
158:
7182:
7122:
7092:
6995:
6831:
6721:
4746:
4718:
4018:
3154:
2610:
2103:
1498:
1471:
1428:
789:
563:
424:
253:
166:
145:) part of the molecule. The Fab fragments contain the variable domains, which consist of three antibody
101:
42:
38:
2797:
7037:
6932:
6769:
6416:
5671:
5378:
5274:
5062:
4439:
4395:
4220:
4038:
3525:
3463:
3420:
3207:
3127:
3062:
3032:
2997:
2882:
2831:
2732:
2682:
2595:
2433:
2368:
2060:
1664:
1380:
1338:
1296:
1136:
1067:
1045:
1022:
886:
821:
701:-conjugated murine antibodies against cellular antigens. Most research involves their application to
559:
473:
449:
146:
5520:"Preclinical development of monoclonal antibodies: considerations for the use of non-human primates"
816: cells. CD8 cells can be suppressed by B cells regulation of TAM phenotypes. Cancer-associated
8162:
7890:
7157:
7147:
6967:
4736:
4633:
4170:
3864:
3693:
3505:
3482:
3226:
3135:
3070:
3005:
2686:
2260:
1768:
1144:
1075:
880:
649:
185:
162:
77:
6319:
Francis RJ, Sharma SK, Springer C, Green AJ, Hope-Stone LD, Sena L, et al. (September 2002).
6194:"A Study to Confirm Safety and Efficacy of BAN2401 in Participants With Early Alzheimer's Disease"
653:
However, Aβ concentration did not significantly change, along with other AD biomarkers, including
7545:
7177:
7162:
7082:
6485:
6442:
6217:
6093:
Sperling RA, Donohue MC, Raman R, Rafii MS, Johnson K, Masters CL, et al. (September 2023).
5963:
5804:
5695:
5592:
5451:
5402:
5351:
5243:
5086:
5035:
4989:
4680:
4537:
HER2-overexpressing breast cancer, metaststic gastric or gastroesophageal junction adenocarcinoma
4326:
4033:
3609:
3354:
3123:
3058:
2993:
2149:
1990:
1943:
1156:
1087:
694:
529:
481:
461:
440:
Anti-cancer monoclonal antibodies can be targeted against malignant cells by several mechanisms.
325:
296:
284:
62:
17:
4684:
4637:
3119:
3054:
2989:
2944:
2489:
2017:
1152:
1083:
477:
3632:
7222:
6681:
6632:
6583:
6534:
6477:
6434:
6385:
6350:
6252:
6209:
6175:
6124:
6075:
6012:
5955:
5913:
5863:
5796:
5756:
5730:
5687:
5641:
5584:
5549:
5500:
5443:
5394:
5343:
5320:
Presta LG, Lahr SJ, Shields RL, Porter JP, Gorman CM, Fendly BM, Jardieu PM (September 1993).
5302:
5235:
5195:
5155:
5114:
5078:
5027:
4981:
4945:
4899:
4868:
4840:
3822:
2546:
2322:
2083:
1650:
1604:
321:
85:
6031:
1951:
7062:
6904:
6899:
6671:
6663:
6652:"Industrialization of mAb production technology: the bioprocessing industry at a crossroads"
6622:
6614:
6573:
6565:
6524:
6516:
6469:
6424:
6377:
6340:
6332:
6244:
6201:
6165:
6155:
6114:
6106:
6067:
6002:
5994:
5947:
5903:
5853:
5845:
5788:
5722:
5679:
5631:
5623:
5576:
5539:
5531:
5490:
5482:
5433:
5386:
5333:
5292:
5282:
5227:
5187:
5145:
5070:
5019:
4973:
4935:
4830:
4822:
4629:
4581:
4507:
4353:
4264:
3268:
3139:
3074:
3009:
2774:
2452:
2191:
2107:
1844:
1798:
1776:
1619:
1475:
1160:
1091:
894:
717:
513:
388:
222:
170:
110:
6716:
5007:
4891:
4858:
4749:
have yielded promising results in clinical trials. In April 2009, the bispecific antibody
4604:
4291:
3291:
3089:
3024:
2967:
2959:
2789:
2755:
2713:
2467:
2172:
2040:
1971:
1668:
1106:
1037:
902:
630:
505:
174:
6142:
Logovinsky V, Satlin A, Lai R, Swanson C, Kaplow J, Osswald G, et al. (April 2016).
5779:
Pul R, Dodel R, Stangel M (March 2011). "Antibody-based therapy in
Alzheimer's disease".
6420:
6119:
6094:
5675:
5382:
5278:
5066:
855:
Please help update this article to reflect recent events or newly available information.
7999:
7897:
7845:
7443:
6756:
6676:
6651:
6627:
6602:
6578:
6553:
6529:
6504:
6473:
6345:
6320:
6170:
6143:
6007:
5982:
5858:
5833:
5636:
5611:
5544:
5519:
5518:
Chapman K, Pullen N, Coney L, Dempster M, Andrews L, Bajramovic J, et al. (2009).
5495:
5470:
4964:
Carter P (November 2001). "Improving the efficacy of antibody-based cancer therapies".
4835:
4810:
4624:
4620:
4576:
4572:
4348:
4201:
3949:
3845:
2769:
2233:
1859:
1691:
1271:
898:
885:
The first FDA-approved therapeutic monoclonal antibody was a murine IgG2a CD3 specific
797:
626:
416:
353:
304:
280:
193:
106:
5261:
Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL, et al. (May 1992).
5023:
804:, partly driven by hypoxic conditions and cytokine production, such as IFNβ. Aberrant
214:
8156:
8056:
7946:
7908:
7885:
7817:
7812:
7802:
7578:
7574:
7562:
7369:
7152:
7117:
6937:
6917:
6221:
5297:
5262:
5191:
4709:
4492:
4305:
4143:
4075:
3907:
3837:
3520:
3497:
3305:
2518:
2280:
1998:
1975:
1206:
770:
537:
497:
412:
341:
320:
and the formation of complexes after repeated administration, which resulted in mild
241:
202:
58:
46:
6489:
6446:
5983:"Anti-Amyloid-β Monoclonal Antibodies for Alzheimer's Disease: Pitfalls and Promise"
5967:
5834:"Anti-Amyloid-β Monoclonal Antibodies for Alzheimer's Disease: Pitfalls and Promise"
5808:
5596:
5247:
5039:
4993:
188:, and neurological disorders that result in the degeneration of body cells, such as
8096:
8081:
8051:
8031:
7913:
7861:
7822:
7710:
7689:
7596:
7558:
7459:
7434:
7430:
7314:
7279:
7232:
7217:
7172:
7047:
7017:
6942:
6760:
5998:
5849:
5699:
5627:
5455:
5406:
5090:
4806:
3540:
2874:
2498:
2237:
1726:
1683:
1659:
1532:
1353:
1311:
781:
622:
595:
587:
533:
61:
to attack specific cells, or to bring a drug to a specific cell type (such as with
50:
5355:
5951:
5792:
8126:
8106:
8101:
8091:
8071:
8046:
8041:
8024:
7972:
7962:
7957:
7952:
7854:
7781:
7751:
7746:
7741:
7725:
7715:
7695:
7570:
7566:
7554:
7550:
7479:
7383:
7329:
7324:
7289:
7227:
7212:
7202:
7197:
7192:
7187:
7167:
7132:
7087:
7067:
7057:
7012:
6977:
6957:
6913:
6876:
6864:
6859:
6821:
6805:
6270:
5338:
5321:
5134:"Utilization of monoclonal antibodies in the treatment of leukemia and lymphoma"
4766:
4762:
4750:
4700:
4652:
4596:
4550:
4464:
4410:
4368:
4334:
4235:
4010:
3964:
3922:
3794:
3751:
3582:
3369:
3334:
3326:
3283:
2502:
2349:
2252:
2164:
1901:
1576:
1540:
1443:
1221:
817:
749:
706:
698:
645:
591:
489:
441:
365:
287:
and human. Antibodies of each type are distinguished by suffixes on their name.
122:
114:
89:
6505:"Bispecific antibodies for cancer therapy: the light at the end of the tunnel?"
5726:
5267:
Proceedings of the National Academy of Sciences of the United States of America
4811:"Advances in targeting cell surface signalling molecules for immune modulation"
41:(mAbs) have varied therapeutic uses. It is possible to create a mAb that binds
8141:
8136:
8111:
8076:
8061:
8036:
8019:
8009:
7967:
7938:
7903:
7869:
7786:
7776:
7756:
7720:
7705:
7700:
7670:
7604:
7600:
7582:
7530:
7509:
7469:
7464:
7403:
7334:
7309:
7284:
7207:
7142:
7102:
7097:
7077:
7072:
7052:
7042:
7032:
6947:
6927:
6909:
6817:
6193:
6160:
5908:
5891:
5662:
Sharma P, Allison JP (April 2015). "The future of immune checkpoint therapy".
4774:
4770:
4727:
4527:
4283:
4053:
3879:
3708:
3666:
3624:
3241:
3199:
3109:
3044:
2979:
2917:
2902:
2896:
2747:
2562:
2425:
2383:
2341:
2122:
2075:
1867:
1817:
1639:
1408:
1263:
1126:
1057:
995:
975:
805:
744:
603:
599:
574:
509:
493:
469:
465:
408:
337:
309:
245:
6213:
6205:
5580:
8131:
8121:
8116:
8086:
8066:
8014:
8004:
7992:
7987:
7933:
7928:
7874:
7807:
7680:
7675:
7660:
7655:
7650:
7640:
7635:
7630:
7535:
7525:
7504:
7499:
7494:
7489:
7484:
7474:
7454:
7418:
7413:
7408:
7398:
7393:
7360:
7355:
7350:
7319:
7299:
7294:
7137:
7127:
6952:
6800:
6429:
6404:
5683:
5287:
5150:
5133:
4940:
4923:
4782:
4472:
4418:
4376:
4193:
4151:
4139:
4104:
4096:
3972:
3930:
3887:
3759:
3716:
3674:
3590:
3548:
3455:
3412:
3377:
3249:
2939:
2705:
2655:
2475:
2295:
2206:
2032:
1584:
1490:
1361:
1319:
1183:
953:
740:
702:
665:
611:
607:
578:
376:
368:
329:
317:
265:
249:
138:
66:
54:
6685:
6636:
6587:
6569:
6538:
6520:
6438:
6389:
6354:
6336:
6256:
6179:
6128:
6079:
6016:
5959:
5917:
5867:
5800:
5734:
5691:
5645:
5588:
5553:
5504:
5447:
5239:
5199:
5031:
4985:
4949:
4844:
6667:
6618:
6481:
6381:
6296:"FDA Converts Novel Alzheimer's Disease Treatment to Traditional Approval"
6248:
6110:
5535:
5486:
5471:"Human immunoglobulin allotypes: possible implications for immunogenicity"
5398:
5347:
5306:
5159:
5118:
5082:
7881:
7645:
7449:
7388:
7344:
7304:
7274:
7027:
6891:
6887:
6812:
4674:
4558:
4243:
3349:
2925:
2663:
2493:
1909:
1451:
1400:
961:
812:, can affect T cell functions directly and indirectly via cells such as T
555:
485:
268:
257:
154:
130:
6985:
5438:
5421:
5231:
2391:
1825:
1229:
501:
261:
198:
6071:
4924:"Active and passive immunity, vaccine types, excipients and licensing"
5390:
5074:
4977:
4081:
3990:
3986:
3944:
3736:
3688:
3311:
3221:
3097:
2447:
2326:
2130:
1839:
1560:
1003:
825:
762:
517:
276:
181:
126:
81:
73:
6405:"T cell exclusion, immune privilege, and the tumor microenvironment"
5263:"Humanization of an anti-p185HER2 antibody for human cancer therapy"
4826:
2858:
Relapsed or refractory low-grade, follicular, or transformed B-cell
452:), and antibodies modified to stimulate activation of immune cells.
30:
6703:
Cancer Management Handbook: Principles of Oncologic Pharmacotherapy
256:. These advances allowed for the specific targeting of tumors both
244:
developed in the 1970s following the discovery of the structure of
7865:
7591:
7425:
7374:
6854:
4740:
4515:
4259:
3802:
3263:
3162:
2618:
2584:
2570:
2526:
2363:
2317:
2228:
2214:
2097:
1881:
1734:
1627:
1465:
1422:
1375:
1333:
1285:
1114:
801:
629:(APOE) e4 carriers, Bapineuzumab treatment is also accompanied by
427:
is one of the many factors that can contribute to immunogenicity.
316:
associated with murine antibodies included reduced stimulation of
29:
905:. Most are concerned with immunological and oncological targets.
7980:
7766:
7264:
6990:
6972:
6962:
6922:
6790:
4731:
4722:
4713:
4704:
4532:
4486:
4434:
4390:
4215:
4165:
4118:
3901:
3859:
3646:
3604:
3562:
3477:
3434:
3179:
2853:
2635:
2540:
2186:
2144:
1923:
1793:
1748:
1644:
1598:
1512:
1243:
1200:
890:
785:
766:
234:
6725:
893:(also called muromonab), in 1986. This drug found use in solid
5218:
Hudson PJ, Souriau C (January 2003). "Engineered antibodies".
3730:
3114:
3049:
2984:
2816:
2811:
2727:
2677:
2274:
2012:
1131:
1062:
1017:
835:
118:
303:). These antibodies have: a short half-life in vivo (due to
180:
Monoclonal antibody therapy may prove to be beneficial for
312:
has proven essential in identifying novel tumour targets.
6554:"Catumaxomab: clinical development and future directions"
6095:"Trial of Solanezumab in Preclinical Alzheimer's Disease"
808:
production in the TME, such as the pathway regulation by
252:
technology, which provided the first reliable source of
275:
Four major antibody types that have been developed are
5610:
de Zwart V, Gouw SC, Meyer-Wentrup FA (January 2016).
5010:(February 2000). "Therapeutic monoclonal antibodies".
4861:; Paul Travers; Mark Walport; Mark Shlomchik (2001).
573:
approved antibody therapies for Alzheimer's disease,
820:(CAFs) have multiple TME functions, in part through
7837:
7614:
7255:
7245:
7005:
6886:
6846:
6781:
6768:
516:(IgE) and is useful in moderate-to-severe allergic
5322:"Humanization of an antibody directed against IgE"
5755:. Edinburgh: Churchill Livingstone. p. 241.
543:However, mAbs are large molecules and due to the
5824:
5822:
5820:
5818:
901:resistant. Hundreds of therapies are undergoing
5892:"Passive immunotherapy for Alzheimer's disease"
5890:Guo X, Yan L, Zhang D, Zhao Y (February 2024).
709:is an example used for non-Hodgkin's lymphoma.
800:and tumor cells can up-regulate expression of
34:Each antibody binds only one specific antigen.
6737:
6053:
6051:
5933:
5931:
5929:
5927:
5612:"Antibody therapies for lymphoma in children"
4917:
4915:
3185:Precursor B-cell acute lymphoblastic leukemia
1754:Precursor B-cell acute lymphoblastic leukemia
227:antibody-dependent cell-mediated cytotoxicity
8:
1970:Diagnostic imaging agent in newly diagnosed
5885:
5883:
5881:
5879:
5877:
5774:
5772:
5616:The Cochrane Database of Systematic Reviews
5469:Jefferis R, Lefranc MP (July–August 2009).
881:Monoclonal antibody § Therapeutic uses
743:. Liposomes can carry drugs or therapeutic
295:Initial therapeutic antibodies were murine
7252:
6778:
6744:
6730:
6722:
6294:Commissioner, Office of the (2023-07-07).
4886:
4884:
4800:
4798:
913:approved therapeutic monoclonal antibodies
907:
6675:
6626:
6577:
6528:
6428:
6344:
6169:
6159:
6118:
6006:
5907:
5857:
5635:
5543:
5494:
5437:
5422:"Immunotherapy: past, present and future"
5337:
5296:
5286:
5213:
5211:
5209:
5149:
4939:
4834:
874:List of therapeutic monoclonal antibodies
169:can be acquired in the immune system via
5173:
5171:
5169:
4867:. New York and London: Garland Science.
2505:deficiency) with Factor VIII inhibitors.
739:Immunoliposomes are antibody-conjugated
718:Antibody-directed enzyme prodrug therapy
713:Antibody-directed enzyme prodrug therapy
484:by their ability to bind to and inhibit
223:antibody directed enzyme prodrug therapy
213:
151:antibody-dependent cellular cytotoxicity
5746:
5744:
5657:
5655:
5180:Critical Reviews in Oncology/Hematology
4794:
5132:Ritz J, Schlossman SF (January 1982).
1929:Cryopyrin-associated periodic syndrome
5569:Journal of Oncology Pharmacy Practice
4779:autoimmune and inflammatory disorders
670:amyloid-related imaging abnormalities
7:
6552:Linke R, Klein A, Seimetz D (2010).
5940:Expert Opinion on Biological Therapy
5781:Expert Opinion on Biological Therapy
4753:was approved in the European Union.
3996:Wet age-related macular degeneration
2901:Emergency reversal of anticoagulant
1555:interleukin-5 receptor alpha subunit
532:, the accumulation of extracellular
403:Human monoclonal antibodies (suffix
92:prevention, and certain infections.
6237:The New England Journal of Medicine
6099:The New England Journal of Medicine
2411:Paroxysmal nocturnal hemoglobinuria
1249:B-cell chronic lymphocytic leukemia
872:For a more comprehensive list, see
832:FDA-approved therapeutic antibodies
824:(ECM)–mediated T cell trapping and
381:complementarity determining regions
6474:10.1002/j.1875-9114.1991.tb03595.x
6403:Joyce JA, Fearon DT (April 2015).
6148:Alzheimer's Research & Therapy
981:Percutaneous coronary intervention
504:and thereby help preventing acute
25:
4898:(6th ed.). Garland Science.
360:and humanized antibodies (suffix
231:complement-dependent cytotoxicity
219:Monoclonal antibodies for cancer.
137:) part can be separated from the
121:and are composed of two kinds of
18:Therapeutic monoclonal antibodies
8168:Monoclonal antibodies for tumors
5192:10.1016/j.critrevonc.2004.10.011
840:
773:and the tumor microenvironment.
562:. Another strategy is so called
415:libraries by transferring human
332:technology has been replaced by
1712:Clostridium difficile infection
460:Monoclonal antibodies used for
96:Antibody structure and function
6603:"mAbs: a business perspective"
6030:Goel, Ayush (20 August 2013).
5999:10.1016/j.biopsych.2017.08.010
5850:10.1016/j.biopsych.2017.08.010
5628:10.1002/14651858.cd011181.pub2
4815:Nature Reviews. Drug Discovery
4721:– Lumoxiti – September 2018 –
3735:Moderate to severe persistent
1803:Anaplastic large-cell lymphoma
143:fragment crystallizable region
1:
5981:van Dyck CH (February 2018).
5024:10.1016/S0140-6736(00)01034-5
4730:– Libtayo – September 2018 –
4450:juvenile idiopathic arthritis
4445:juvenile idiopathic arthritis
4175:Diffuse large B-cell lymphoma
3441:non-small cell lung carcinoma
3398:non-small cell lung carcinoma
2591:familial hypercholesterolemia
1706:Clostridium difficile toxin B
1656:Non-small-cell lung carcinoma
1292:familial hypercholesterolemia
1141:Juvenile idiopathic arthritis
1072:Juvenile idiopathic arthritis
828:-regulated T cell exclusion.
117:molecules, approximately 150
5952:10.1517/14712598.2014.935332
5793:10.1517/14712598.2011.552884
4864:Immunobiology; Fifth Edition
4712:– Poteligeo – August 2018 –
4179:Chronic lymphocytic leukemia
3652:Chronic lymphocytic leukemia
3568:Chronic lymphocytic leukemia
1518:Systemic lupus erythematosus
233:; MAb, monoclonal antibody;
6032:"Vasogenic cerebral oedema"
5339:10.4049/jimmunol.151.5.2623
3780:Respiratory syncytial virus
399:Human monoclonal antibodies
237:, single-chain Fv fragment.
157:are a key component of the
72:Major applications include
8194:
7247:Tyrosine kinase inhibitors
5727:10.1016/j.ejca.2017.01.001
5715:European Journal of Cancer
5420:Waldmann TA (March 2003).
4922:Baxter D (December 2007).
3519:Protective antigen of the
2850:murine, radioimmunotherapy
1386:non-small cell lung cancer
878:
871:
769:targeted by nivolumab and
697:(RIT) involves the use of
581:. Aducanemab has received
549:Peripheral Sink hypothesis
352:To reduce murine antibody
334:recombinant DNA technology
99:
7113:Mirvetuximab soravtansine
6503:Chames P, Baty D (2009).
6325:British Journal of Cancer
6161:10.1186/s13195-016-0181-2
5909:10.1016/j.arr.2024.102192
2304:Regeneron Pharmaceuticals
1887:X-linked hypophosphatemia
1176:ado-trastuzumab emtansine
849:This section needs to be
569:Currently, there are two
472:, which are effective in
393:site-specific mutagenesis
7257:Receptor tyrosine kinase
6783:Receptor tyrosine kinase
6206:10.31525/ct1-nct03887455
5581:10.1177/1078155216655474
2840:Spectrum Pharmaceuticals
1563:, eosinophilic phenotype
765:targeted by ipilimumab,
729:Antibody-drug conjugates
724:Antibody-drug conjugates
159:adaptive immune response
147:hypervariable amino acid
135:fragment-antigen binding
6847:Others for solid tumors
6753:Targeted cancer therapy
6430:10.1126/science.aaa6204
5896:Ageing Research Reviews
5684:10.1126/science.aaa8172
5288:10.1073/pnas.89.10.4285
5151:10.1182/blood.V59.1.1.1
4739:– Polivy – June 2019 –
4162:chimeric, co-formulated
3175:antibody-drug conjugate
2631:antibody-drug conjugate
1789:antibody-drug conjugate
1196:antibody-drug conjugate
523:
508:of kidney transplants.
248:and the development of
113:) antibodies are large
7108:Loncastuximab tesirine
6837:Trastuzumab deruxtecan
6570:10.4161/mabs.2.2.11221
6521:10.4161/mabs.1.6.10015
6337:10.1038/sj.bjc.6600517
4966:Nature Reviews. Cancer
4894:; et al. (2005).
4269:Ankylosing spondylitis
4125:non-Hodgkin's lymphoma
4032:Protective antigen of
3980:intravitreal injection
3132:Ankylosing spondylitis
3067:Ankylosing spondylitis
3002:Ankylosing spondylitis
2860:non-Hodgkin's lymphoma
2691:Ankylosing spondylitis
2641:Acute myeloid leukemia
2406:Complement component 5
1710:Prevent recurrence of
1149:Ankylosing spondylitis
1080:Ankylosing spondylitis
897:recipients who became
778:tumor microenvironment
735:Immunoliposome therapy
655:phospho-tau expression
348:Chimeric and humanized
264:. Initial research on
238:
35:
8173:Monoclonal antibodies
7183:Sacituzumab govitecan
7123:Moxetumomab pasudotox
7093:Inotuzumab ozogamicin
6996:Gemtuzumab ozogamicin
6832:Trastuzumab emtansine
6773:monoclonal antibodies
6757:antineoplastic agents
6708:registration required
6668:10.4161/mabs.1.5.9448
6619:10.4161/mabs.1.2.7736
6382:10.3233/bd-1999-11110
6249:10.1056/NEJMoa2212948
6198:Case Medical Research
6111:10.1056/NEJMoa2305032
5987:Biological Psychiatry
5838:Biological Psychiatry
5536:10.4161/mabs.1.5.9676
5487:10.4161/mabs.1.4.9122
5326:Journal of Immunology
4941:10.1093/occmed/kqm110
4928:Occupational Medicine
4747:bispecific antibodies
4719:Moxetumomab pasudotox
4019:Human Genome Sciences
3155:inotuzumab ozogamicin
2611:gemtuzumab ozogamicin
2486:humanized, bispecific
2104:acute organ rejection
1499:Human Genome Sciences
1472:acute organ rejection
1429:Merkel cell carcinoma
564:passive immunotherapy
534:amyloid beta peptides
407:) are produced using
254:monoclonal antibodies
217:
167:Monoclonal antibodies
102:Monoclonal antibodies
100:Further information:
39:Monoclonal antibodies
33:
27:Form of immunotherapy
7038:Belantamab mafodotin
6271:"Leqembi | ALZFORUM"
5751:Rang, H. P. (2003).
4440:Rheumatoid arthritis
4396:Rheumatoid arthritis
4251:subcutaneous (2015)
4221:Rheumatoid arthritis
4039:Inhalational anthrax
3526:Inhalational anthrax
3464:Bristol-Myers Squibb
3439:Metastatic squamous
3421:Bristol-Myers Squibb
3396:Metastatic squamous
3208:Bristol-Myers Squibb
3128:Rheumatoid arthritis
3063:Rheumatoid arthritis
3033:Celltrion Healthcare
2998:Rheumatoid arthritis
2883:Boehringer Ingelheim
2832:ibritumomab tiuxetan
2733:Rheumatoid arthritis
2683:Rheumatoid arthritis
2596:hypercholesterolemia
2434:Bristol-Myers Squibb
2369:Urothelial carcinoma
2279:Pediatric high-risk
2061:colorectal carcinoma
1962:murine, radiolabeled
1665:renal cell carcinoma
1381:Urothelial carcinoma
1339:Urothelial carcinoma
1297:hypercholesterolemia
1137:Rheumatoid arthritis
1068:Rheumatoid arthritis
1046:Boehringer Ingelheim
1023:Rheumatoid arthritis
887:transplant rejection
822:extracellular matrix
583:accelerated approval
560:active immunotherapy
474:rheumatoid arthritis
391:mutator strains and
7891:Denileukin diftitox
7553:(ALK, ROS1, NTRK),
7158:Polatuzumab vedotin
7148:Oportuzumab monatox
6601:Scolnik PA (2009).
6421:2015Sci...348...74J
5676:2015Sci...348...56S
5383:1989Natur.342..877C
5279:1992PNAS...89.4285C
5067:1975Natur.256..495K
4737:Polatuzumab vedotin
4634:Psoriatic arthritis
4312:Castleman's disease
4171:Follicular lymphoma
3865:Metastatic melanoma
3694:Soft tissue sarcoma
3506:Elusys Therapeutics
3483:Metastatic melanoma
3227:Metastatic melanoma
3136:Psoriatic arthritis
3071:Psoriatic arthritis
3006:Psoriatic arthritis
2687:Psoriatic arthritis
2261:United Therapeutics
1769:brentuximab vedotin
1145:Psoriatic arthritis
1076:Psoriatic arthritis
915:
650:cerebrospinal fluid
545:blood–brain barrier
524:Alzheimer's disease
462:autoimmune diseases
456:Autoimmune diseases
431:Targeted conditions
190:Alzheimer's disease
186:autoimmune diseases
163:monoclonal antibody
78:autoimmune diseases
7577:(ROS1, TRK, ALK),
7083:Enfortumab vedotin
6715:2009-05-15 at the
5439:10.1038/nm0303-269
5232:10.1038/nm0103-129
4703:– Bexxar – 2003 –
4681:Ulcerative colitis
4327:tildrakizumab-asmn
4253:intravenous (2023)
4034:Bacillus anthracis
3610:Multiple sclerosis
3355:Multiple sclerosis
3124:Ulcerative colitis
3059:Ulcerative colitis
2994:Ulcerative colitis
2150:Multiple sclerosis
1991:certolizumab pegol
1944:capromab pendetide
1157:Ulcerative colitis
1088:Ulcerative colitis
942:(Targeted disease)
908:
756:Checkpoint therapy
695:Radioimmunotherapy
690:Radioimmunotherapy
530:Amyloid hypothesis
482:ulcerative colitis
450:checkpoint therapy
326:anaphylactic shock
322:allergic reactions
239:
63:radioimmunotherapy
36:
8150:
8149:
7833:
7832:
7241:
7240:
7223:Tisotumab vedotin
6650:Kelley B (2009).
6105:(12): 1096–1107.
6072:10.2217/imt.10.80
5946:(10): 1465–1476.
5832:(February 2018).
5762:978-0-443-07145-4
5377:(6252): 877–883.
5273:(10): 4285–4289.
5061:(5517): 495–497.
5018:(9205): 735–740.
4905:978-0-443-07310-6
4874:978-0-8153-4101-7
4809:(February 2013).
4769:(both oncology),
4698:
4697:
3823:colorectal cancer
2547:Migraine headache
2323:Atopic dermatitis
1745:mouse, bispecific
1651:colorectal cancer
1605:colorectal cancer
1226:Campath, Lemtrada
870:
869:
676:Preventive trials
86:organ transplants
16:(Redirected from
8185:
7864:peptide against
7437:(AXL, ALK, LTK))
7253:
7063:Dinutuximab beta
6779:
6746:
6739:
6732:
6723:
6711:
6690:
6689:
6679:
6647:
6641:
6640:
6630:
6598:
6592:
6591:
6581:
6549:
6543:
6542:
6532:
6500:
6494:
6493:
6457:
6451:
6450:
6432:
6400:
6394:
6393:
6365:
6359:
6358:
6348:
6316:
6310:
6309:
6307:
6306:
6291:
6285:
6284:
6282:
6281:
6275:www.alzforum.org
6267:
6261:
6260:
6232:
6226:
6225:
6190:
6184:
6183:
6173:
6163:
6139:
6133:
6132:
6122:
6090:
6084:
6083:
6055:
6046:
6045:
6043:
6042:
6027:
6021:
6020:
6010:
5978:
5972:
5971:
5935:
5922:
5921:
5911:
5887:
5872:
5871:
5861:
5826:
5813:
5812:
5776:
5767:
5766:
5748:
5739:
5738:
5710:
5704:
5703:
5659:
5650:
5649:
5639:
5607:
5601:
5600:
5564:
5558:
5557:
5547:
5515:
5509:
5508:
5498:
5466:
5460:
5459:
5441:
5417:
5411:
5410:
5391:10.1038/342877a0
5366:
5360:
5359:
5341:
5332:(5): 2623–2632.
5317:
5311:
5310:
5300:
5290:
5258:
5252:
5251:
5215:
5204:
5203:
5175:
5164:
5163:
5153:
5129:
5123:
5122:
5113:(9): 3147–3154.
5101:
5095:
5094:
5075:10.1038/256495a0
5050:
5044:
5043:
5004:
4998:
4997:
4978:10.1038/35101072
4960:
4954:
4953:
4943:
4919:
4910:
4909:
4888:
4879:
4878:
4859:Janeway, Charles
4855:
4849:
4848:
4838:
4802:
4630:Plaque psoriasis
4582:Plaque psoriasis
4508:trastuzumab-dkst
4354:Plaque psoriasis
4265:Plaque psoriasis
3269:Plaque psoriasis
3140:Plaque psoriasis
3075:Plaque psoriasis
3010:Plaque psoriasis
2798:TaiMed Biologics
2775:Plaque psoriasis
2453:Multiple myeloma
2192:Multiple myeloma
2108:renal transplant
1845:Plaque psoriasis
1799:Hodgkin lymphoma
1777:Seattle Genetics
1620:bevacizumab-awwb
1476:renal transplant
1161:Plaque psoriasis
1092:Plaque psoriasis
916:
895:organ transplant
865:
862:
856:
844:
843:
836:
514:immunoglobulin E
171:passive immunity
49:target, such as
21:
8193:
8192:
8188:
8187:
8186:
8184:
8183:
8182:
8178:Antiviral drugs
8153:
8152:
8151:
8146:
8000:Pi3K inhibitors
7898:mTOR inhibitors
7829:
7610:
7581:(VEGFR, FGFR),
7237:
7001:
6882:
6842:
6764:
6750:
6717:Wayback Machine
6705:
6699:
6694:
6693:
6649:
6648:
6644:
6600:
6599:
6595:
6551:
6550:
6546:
6502:
6501:
6497:
6462:Pharmacotherapy
6459:
6458:
6454:
6415:(6230): 74–80.
6402:
6401:
6397:
6367:
6366:
6362:
6318:
6317:
6313:
6304:
6302:
6293:
6292:
6288:
6279:
6277:
6269:
6268:
6264:
6234:
6233:
6229:
6192:
6191:
6187:
6141:
6140:
6136:
6092:
6091:
6087:
6057:
6056:
6049:
6040:
6038:
6036:radiopaedia.org
6029:
6028:
6024:
5980:
5979:
5975:
5937:
5936:
5925:
5889:
5888:
5875:
5828:
5827:
5816:
5778:
5777:
5770:
5763:
5750:
5749:
5742:
5712:
5711:
5707:
5670:(6230): 56–61.
5661:
5660:
5653:
5622:(1): CD011181.
5609:
5608:
5604:
5566:
5565:
5561:
5517:
5516:
5512:
5468:
5467:
5463:
5426:Nature Medicine
5419:
5418:
5414:
5368:
5367:
5363:
5319:
5318:
5314:
5260:
5259:
5255:
5220:Nature Medicine
5217:
5216:
5207:
5177:
5176:
5167:
5131:
5130:
5126:
5107:Cancer Research
5103:
5102:
5098:
5052:
5051:
5047:
5006:
5005:
5001:
4963:
4961:
4957:
4921:
4920:
4913:
4906:
4890:
4889:
4882:
4875:
4857:
4856:
4852:
4827:10.1038/nrd3877
4804:
4803:
4796:
4791:
4759:
4685:Crohn's disease
4683:
4638:Crohn's disease
4636:
4632:
4623:
4613:
4605:Janssen Biotech
4575:
4447:
4442:
4427:
4292:Janssen Biotech
4267:
4252:
4177:
4173:
3989:
3292:GlaxoSmithKline
3138:
3134:
3130:
3126:
3122:
3120:Crohn's disease
3090:infliximab-qbtx
3073:
3069:
3065:
3061:
3057:
3055:Crohn's disease
3025:infliximab-dyyb
3008:
3004:
3000:
2996:
2992:
2990:Crohn's disease
2968:Samsung Bioepis
2960:infliximab-abda
2945:Crohn's disease
2790:ibalizumab-uiyk
2756:Janssen Biotech
2714:Janssen Biotech
2689:
2685:
2593:
2468:emicizumab-kxwh
2173:Janssen Biotech
2102:Prophylaxis of
2041:ImClone Systems
2018:Crohn's disease
1972:prostate cancer
1801:
1669:Cervical cancer
1667:
1662:
1658:
1653:
1470:Prophylaxis of
1383:
1294:
1159:
1155:
1153:Crohn's disease
1151:
1147:
1143:
1139:
1107:adalimumab-atto
1090:
1086:
1084:Crohn's disease
1082:
1078:
1074:
1070:
1038:adalimumab-adbm
941:
903:clinical trials
883:
877:
866:
860:
857:
854:
845:
841:
834:
815:
793:
758:
737:
726:
715:
692:
687:
678:
663:
643:
631:vasogenic edema
620:
526:
512:inhibits human
478:Crohn's disease
458:
438:
433:
401:
350:
293:
212:
175:active immunity
104:
98:
65:which delivers
28:
23:
22:
15:
12:
11:
5:
8191:
8189:
8181:
8180:
8175:
8170:
8165:
8155:
8154:
8148:
8147:
8145:
8144:
8139:
8134:
8129:
8124:
8119:
8114:
8109:
8104:
8099:
8094:
8089:
8084:
8079:
8074:
8069:
8064:
8059:
8054:
8049:
8044:
8039:
8034:
8029:
8028:
8027:
8022:
8017:
8012:
8007:
7997:
7996:
7995:
7990:
7977:
7976:
7975:
7970:
7965:
7960:
7955:
7947:CDK inhibitors
7943:
7942:
7941:
7936:
7931:
7918:
7917:
7916:
7911:
7906:
7894:
7878:
7858:
7846:fusion protein
7841:
7839:
7835:
7834:
7831:
7830:
7828:
7827:
7826:
7825:
7820:
7815:
7810:
7805:
7792:
7791:
7790:
7789:
7784:
7779:
7762:
7761:
7760:
7759:
7754:
7749:
7744:
7731:
7730:
7729:
7728:
7723:
7718:
7713:
7708:
7703:
7698:
7685:
7684:
7678:
7666:
7665:
7664:
7663:
7658:
7653:
7648:
7643:
7638:
7633:
7620:
7618:
7612:
7611:
7609:
7608:
7587:
7586:
7585:(VEGFR, EGFR).
7541:
7540:
7539:
7538:
7533:
7528:
7515:
7514:
7513:
7512:
7507:
7502:
7497:
7492:
7487:
7482:
7477:
7472:
7467:
7462:
7457:
7452:
7439:
7438:
7422:
7416:
7411:
7406:
7401:
7396:
7391:
7386:
7366:
7365:
7364:
7363:
7358:
7353:
7343:HER1/EGFR and
7339:
7338:
7332:
7327:
7322:
7317:
7312:
7307:
7302:
7297:
7292:
7287:
7282:
7277:
7261:
7259:
7250:
7243:
7242:
7239:
7238:
7236:
7235:
7230:
7225:
7220:
7215:
7210:
7205:
7200:
7195:
7190:
7185:
7180:
7175:
7170:
7165:
7160:
7155:
7150:
7145:
7140:
7135:
7130:
7125:
7120:
7115:
7110:
7105:
7100:
7095:
7090:
7085:
7080:
7075:
7070:
7065:
7060:
7055:
7050:
7045:
7040:
7035:
7030:
7025:
7022:+hyaluronidase
7015:
7009:
7007:
7003:
7002:
7000:
6999:
6982:
6981:
6955:
6950:
6945:
6940:
6935:
6930:
6896:
6894:
6884:
6883:
6881:
6880:
6868:
6862:
6850:
6848:
6844:
6843:
6841:
6840:
6834:
6829:
6826:+hyaluronidase
6809:
6803:
6787:
6785:
6776:
6766:
6765:
6751:
6749:
6748:
6741:
6734:
6726:
6720:
6719:
6698:
6697:External links
6695:
6692:
6691:
6662:(5): 443–452.
6642:
6613:(2): 179–184.
6593:
6564:(2): 129–136.
6544:
6515:(6): 539–547.
6495:
6452:
6395:
6370:Breast Disease
6360:
6331:(6): 600–607.
6311:
6286:
6262:
6227:
6200:. 2019-03-25.
6185:
6134:
6085:
6066:(6): 767–782.
6047:
6022:
5993:(4): 311–319.
5973:
5923:
5873:
5844:(4): 311–319.
5814:
5787:(3): 343–357.
5768:
5761:
5740:
5705:
5651:
5602:
5575:(7): 525–539.
5559:
5530:(5): 505–516.
5510:
5481:(4): 332–338.
5461:
5432:(3): 269–277.
5412:
5361:
5312:
5253:
5226:(1): 129–134.
5205:
5165:
5124:
5096:
5045:
4999:
4972:(2): 118–129.
4962:Modified from
4955:
4934:(8): 552–556.
4911:
4904:
4892:Janeway CA Jr.
4880:
4873:
4850:
4821:(2): 130–146.
4805:Yao S, Zhu Y,
4793:
4792:
4790:
4787:
4781:, 'AIID') and
4758:
4755:
4696:
4695:
4690:
4687:
4678:
4672:
4669:
4666:
4663:
4658:
4655:
4649:
4648:
4643:
4640:
4627:
4618:
4615:
4610:
4607:
4602:
4599:
4593:
4592:
4587:
4584:
4579:
4570:
4567:
4564:
4561:
4556:
4553:
4547:
4546:
4541:
4538:
4535:
4530:
4524:
4521:
4518:
4513:
4510:
4504:
4503:
4498:
4495:
4489:
4484:
4481:
4478:
4475:
4470:
4467:
4461:
4460:
4455:
4452:
4443:Polyarticular
4437:
4432:
4429:
4424:
4421:
4416:
4413:
4407:
4406:
4401:
4398:
4393:
4388:
4385:
4382:
4379:
4374:
4371:
4365:
4364:
4359:
4356:
4351:
4346:
4343:
4340:
4337:
4332:
4329:
4323:
4322:
4317:
4314:
4308:
4303:
4300:
4297:
4294:
4289:
4286:
4280:
4279:
4274:
4271:
4262:
4257:
4254:
4249:
4246:
4241:
4238:
4232:
4231:
4226:
4223:
4218:
4213:
4210:
4207:
4204:
4202:Sanofi Aventis
4199:
4196:
4190:
4189:
4184:
4181:
4168:
4163:
4160:
4157:
4154:
4149:
4148:Rituxan Hycela
4146:
4136:
4135:
4130:
4127:
4121:
4116:
4113:
4110:
4107:
4102:
4099:
4093:
4092:
4087:
4084:
4078:
4073:
4070:
4067:
4064:
4059:
4056:
4050:
4049:
4044:
4041:
4036:
4030:
4027:
4024:
4021:
4016:
4013:
4007:
4006:
4001:
3998:
3993:
3984:
3981:
3978:
3975:
3970:
3967:
3961:
3960:
3955:
3952:
3950:Gastric cancer
3947:
3942:
3939:
3936:
3933:
3928:
3925:
3919:
3918:
3913:
3910:
3904:
3899:
3896:
3893:
3890:
3885:
3882:
3876:
3875:
3870:
3867:
3862:
3857:
3854:
3851:
3848:
3843:
3840:
3834:
3833:
3828:
3825:
3819:
3814:
3811:
3808:
3805:
3800:
3797:
3791:
3790:
3785:
3782:
3777:
3771:
3768:
3765:
3762:
3757:
3754:
3748:
3747:
3742:
3739:
3733:
3728:
3725:
3722:
3719:
3714:
3711:
3705:
3704:
3699:
3696:
3691:
3686:
3683:
3680:
3677:
3672:
3669:
3663:
3662:
3657:
3654:
3649:
3644:
3641:
3638:
3635:
3630:
3627:
3621:
3620:
3615:
3612:
3607:
3602:
3599:
3596:
3593:
3588:
3585:
3579:
3578:
3573:
3570:
3565:
3560:
3557:
3554:
3551:
3546:
3543:
3537:
3536:
3531:
3528:
3523:
3517:
3514:
3511:
3508:
3503:
3500:
3494:
3493:
3488:
3485:
3480:
3475:
3472:
3469:
3466:
3461:
3458:
3452:
3451:
3446:
3443:
3437:
3432:
3429:
3426:
3423:
3418:
3415:
3409:
3408:
3403:
3400:
3394:
3389:
3386:
3383:
3380:
3375:
3372:
3366:
3365:
3360:
3357:
3352:
3346:
3343:
3340:
3337:
3332:
3329:
3323:
3322:
3317:
3314:
3308:
3303:
3300:
3297:
3294:
3289:
3286:
3280:
3279:
3274:
3271:
3266:
3261:
3258:
3255:
3252:
3247:
3244:
3238:
3237:
3232:
3229:
3224:
3219:
3216:
3213:
3210:
3205:
3202:
3196:
3195:
3190:
3187:
3182:
3177:
3171:
3168:
3165:
3160:
3157:
3151:
3150:
3145:
3142:
3117:
3112:
3106:
3103:
3100:
3095:
3092:
3086:
3085:
3080:
3077:
3052:
3047:
3041:
3038:
3035:
3030:
3027:
3021:
3020:
3015:
3012:
2987:
2982:
2976:
2973:
2970:
2965:
2962:
2956:
2955:
2950:
2947:
2942:
2937:
2934:
2931:
2928:
2923:
2920:
2914:
2913:
2908:
2905:
2899:
2894:
2891:
2888:
2885:
2880:
2877:
2871:
2870:
2865:
2862:
2856:
2851:
2848:
2845:
2842:
2837:
2834:
2828:
2827:
2822:
2819:
2814:
2809:
2806:
2803:
2800:
2795:
2792:
2786:
2785:
2780:
2777:
2772:
2767:
2764:
2761:
2758:
2753:
2750:
2744:
2743:
2738:
2735:
2730:
2725:
2722:
2719:
2716:
2711:
2708:
2702:
2701:
2696:
2693:
2680:
2675:
2672:
2669:
2666:
2661:
2658:
2652:
2651:
2646:
2643:
2638:
2633:
2627:
2624:
2621:
2616:
2613:
2607:
2606:
2601:
2598:
2587:
2582:
2579:
2576:
2573:
2568:
2565:
2559:
2558:
2553:
2550:
2544:
2538:
2535:
2532:
2529:
2524:
2521:
2515:
2514:
2509:
2506:
2496:
2487:
2484:
2481:
2478:
2473:
2470:
2464:
2463:
2458:
2455:
2450:
2445:
2442:
2439:
2436:
2431:
2428:
2422:
2421:
2416:
2413:
2408:
2403:
2400:
2397:
2394:
2389:
2386:
2380:
2379:
2374:
2371:
2366:
2361:
2358:
2355:
2352:
2347:
2344:
2338:
2337:
2332:
2329:
2320:
2315:
2312:
2309:
2306:
2301:
2298:
2292:
2291:
2286:
2283:
2277:
2272:
2269:
2266:
2263:
2258:
2255:
2249:
2248:
2243:
2240:
2234:Postmenopausal
2231:
2226:
2223:
2220:
2217:
2212:
2209:
2203:
2202:
2197:
2194:
2189:
2184:
2181:
2178:
2175:
2170:
2167:
2161:
2160:
2155:
2152:
2147:
2142:
2139:
2136:
2133:
2128:
2125:
2119:
2118:
2113:
2110:
2100:
2095:
2092:
2089:
2086:
2081:
2078:
2072:
2071:
2066:
2063:
2057:
2052:
2049:
2046:
2043:
2038:
2035:
2029:
2028:
2023:
2020:
2015:
2010:
2007:
2004:
2001:
1996:
1993:
1987:
1986:
1981:
1978:
1968:
1963:
1960:
1957:
1954:
1949:
1946:
1940:
1939:
1934:
1931:
1926:
1921:
1918:
1915:
1912:
1907:
1904:
1898:
1897:
1892:
1889:
1884:
1879:
1876:
1873:
1870:
1865:
1862:
1860:burosumab-twza
1856:
1855:
1850:
1847:
1842:
1837:
1834:
1831:
1828:
1823:
1820:
1814:
1813:
1808:
1805:
1796:
1791:
1785:
1782:
1779:
1774:
1771:
1765:
1764:
1759:
1756:
1751:
1746:
1743:
1740:
1737:
1732:
1729:
1723:
1722:
1717:
1714:
1708:
1703:
1700:
1697:
1694:
1689:
1686:
1680:
1679:
1674:
1671:
1647:
1642:
1636:
1633:
1630:
1625:
1622:
1616:
1615:
1610:
1607:
1601:
1596:
1593:
1590:
1587:
1582:
1579:
1573:
1572:
1567:
1564:
1557:
1552:
1549:
1546:
1543:
1538:
1535:
1529:
1528:
1523:
1520:
1515:
1510:
1507:
1504:
1501:
1496:
1493:
1487:
1486:
1481:
1478:
1468:
1463:
1460:
1457:
1454:
1449:
1446:
1440:
1439:
1434:
1431:
1425:
1420:
1417:
1414:
1411:
1406:
1403:
1397:
1396:
1391:
1388:
1378:
1373:
1370:
1367:
1364:
1359:
1356:
1350:
1349:
1344:
1341:
1336:
1331:
1328:
1325:
1322:
1317:
1314:
1308:
1307:
1302:
1299:
1288:
1283:
1280:
1277:
1274:
1272:Sanofi Aventis
1269:
1266:
1260:
1259:
1254:
1251:
1246:
1241:
1238:
1235:
1232:
1227:
1224:
1218:
1217:
1212:
1209:
1203:
1198:
1192:
1189:
1186:
1181:
1178:
1172:
1171:
1166:
1163:
1134:
1129:
1123:
1120:
1117:
1112:
1109:
1103:
1102:
1097:
1094:
1065:
1060:
1054:
1051:
1048:
1043:
1040:
1034:
1033:
1028:
1025:
1020:
1015:
1012:
1009:
1006:
1001:
998:
992:
991:
986:
983:
978:
973:
970:
967:
964:
959:
956:
950:
949:
946:
943:
938:
935:
932:
929:
926:
923:
920:
868:
867:
848:
846:
839:
833:
830:
813:
798:Myelomonocytic
791:
757:
754:
736:
733:
725:
722:
714:
711:
691:
688:
686:
683:
677:
674:
662:
659:
642:
639:
627:apolipoprotein
619:
616:
525:
522:
457:
454:
437:
434:
432:
429:
417:immunoglobulin
400:
397:
354:immunogenicity
349:
346:
324:and sometimes
305:immune complex
292:
289:
211:
208:
203:tumor antigens
107:Immunoglobulin
97:
94:
45:to almost any
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
8190:
8179:
8176:
8174:
8171:
8169:
8166:
8164:
8161:
8160:
8158:
8143:
8140:
8138:
8135:
8133:
8130:
8128:
8125:
8123:
8120:
8118:
8115:
8113:
8110:
8108:
8105:
8103:
8100:
8098:
8095:
8093:
8090:
8088:
8085:
8083:
8080:
8078:
8075:
8073:
8070:
8068:
8065:
8063:
8060:
8058:
8057:Larotrectinib
8055:
8053:
8050:
8048:
8045:
8043:
8040:
8038:
8035:
8033:
8030:
8026:
8023:
8021:
8018:
8016:
8013:
8011:
8008:
8006:
8003:
8002:
8001:
7998:
7994:
7991:
7989:
7986:
7985:
7984:
7982:
7978:
7974:
7971:
7969:
7966:
7964:
7961:
7959:
7956:
7954:
7951:
7950:
7949:
7948:
7944:
7940:
7937:
7935:
7932:
7930:
7927:
7926:
7925:
7923:
7919:
7915:
7912:
7910:
7909:Ridaforolimus
7907:
7905:
7902:
7901:
7900:
7899:
7895:
7892:
7888:
7887:
7883:
7879:
7876:
7872:
7871:
7867:
7863:
7859:
7856:
7852:
7851:
7847:
7843:
7842:
7840:
7836:
7824:
7821:
7819:
7818:Pirtobrutinib
7816:
7814:
7813:Orelabrutinib
7811:
7809:
7806:
7804:
7803:Acalabrutinib
7801:
7800:
7799:
7798:
7794:
7793:
7788:
7785:
7783:
7780:
7778:
7775:
7774:
7773:
7772:
7768:
7764:
7763:
7758:
7755:
7753:
7750:
7748:
7745:
7743:
7740:
7739:
7738:
7737:
7733:
7732:
7727:
7724:
7722:
7719:
7717:
7714:
7712:
7709:
7707:
7704:
7702:
7699:
7697:
7694:
7693:
7692:
7691:
7687:
7686:
7682:
7679:
7677:
7673:
7672:
7668:
7667:
7662:
7659:
7657:
7654:
7652:
7649:
7647:
7644:
7642:
7639:
7637:
7634:
7632:
7629:
7628:
7627:
7626:
7622:
7621:
7619:
7617:
7613:
7606:
7602:
7598:
7595:
7593:
7589:
7588:
7584:
7580:
7579:Selpercatinib
7576:
7575:Repotrectinib
7572:
7568:
7564:
7563:Larotrectinib
7560:
7556:
7552:
7549:
7547:
7543:
7542:
7537:
7534:
7532:
7529:
7527:
7524:
7523:
7522:
7521:
7517:
7516:
7511:
7508:
7506:
7503:
7501:
7498:
7496:
7493:
7491:
7488:
7486:
7483:
7481:
7478:
7476:
7473:
7471:
7468:
7466:
7463:
7461:
7458:
7456:
7453:
7451:
7448:
7447:
7446:
7445:
7441:
7440:
7436:
7432:
7428:
7427:
7423:
7420:
7417:
7415:
7412:
7410:
7407:
7405:
7402:
7400:
7397:
7395:
7392:
7390:
7387:
7385:
7381:
7380:
7376:
7371:
7370:RTK class III
7368:
7367:
7362:
7359:
7357:
7354:
7352:
7349:
7348:
7347:
7346:
7341:
7340:
7336:
7333:
7331:
7328:
7326:
7323:
7321:
7318:
7316:
7313:
7311:
7308:
7306:
7303:
7301:
7298:
7296:
7293:
7291:
7288:
7286:
7283:
7281:
7278:
7276:
7272:
7271:
7266:
7263:
7262:
7260:
7258:
7254:
7251:
7248:
7244:
7234:
7231:
7229:
7226:
7224:
7221:
7219:
7216:
7214:
7211:
7209:
7206:
7204:
7201:
7199:
7196:
7194:
7191:
7189:
7186:
7184:
7181:
7179:
7176:
7174:
7171:
7169:
7166:
7164:
7161:
7159:
7156:
7154:
7153:Pembrolizumab
7151:
7149:
7146:
7144:
7141:
7139:
7136:
7134:
7131:
7129:
7126:
7124:
7121:
7119:
7118:Mogamulizumab
7116:
7114:
7111:
7109:
7106:
7104:
7101:
7099:
7096:
7094:
7091:
7089:
7086:
7084:
7081:
7079:
7076:
7074:
7071:
7069:
7066:
7064:
7061:
7059:
7056:
7054:
7051:
7049:
7046:
7044:
7041:
7039:
7036:
7034:
7031:
7029:
7026:
7023:
7019:
7016:
7014:
7011:
7010:
7008:
7004:
6997:
6993:
6992:
6987:
6984:
6983:
6979:
6975:
6974:
6969:
6965:
6964:
6959:
6956:
6954:
6951:
6949:
6946:
6944:
6941:
6939:
6938:Mosunetuzumab
6936:
6934:
6931:
6929:
6925:
6924:
6919:
6918:Mosunetuzumab
6915:
6911:
6907:
6906:
6901:
6898:
6897:
6895:
6893:
6889:
6885:
6878:
6874:
6873:
6869:
6866:
6863:
6861:
6857:
6856:
6852:
6851:
6849:
6845:
6838:
6835:
6833:
6830:
6827:
6823:
6819:
6815:
6814:
6810:
6807:
6804:
6802:
6798:
6797:
6792:
6789:
6788:
6786:
6784:
6780:
6777:
6774:
6771:
6767:
6762:
6758:
6754:
6747:
6742:
6740:
6735:
6733:
6728:
6727:
6724:
6718:
6714:
6709:
6704:
6701:
6700:
6696:
6687:
6683:
6678:
6673:
6669:
6665:
6661:
6657:
6653:
6646:
6643:
6638:
6634:
6629:
6624:
6620:
6616:
6612:
6608:
6604:
6597:
6594:
6589:
6585:
6580:
6575:
6571:
6567:
6563:
6559:
6555:
6548:
6545:
6540:
6536:
6531:
6526:
6522:
6518:
6514:
6510:
6506:
6499:
6496:
6491:
6487:
6483:
6479:
6475:
6471:
6467:
6463:
6456:
6453:
6448:
6444:
6440:
6436:
6431:
6426:
6422:
6418:
6414:
6410:
6406:
6399:
6396:
6391:
6387:
6383:
6379:
6375:
6371:
6364:
6361:
6356:
6352:
6347:
6342:
6338:
6334:
6330:
6326:
6322:
6315:
6312:
6301:
6297:
6290:
6287:
6276:
6272:
6266:
6263:
6258:
6254:
6250:
6246:
6242:
6238:
6231:
6228:
6223:
6219:
6215:
6211:
6207:
6203:
6199:
6195:
6189:
6186:
6181:
6177:
6172:
6167:
6162:
6157:
6153:
6149:
6145:
6138:
6135:
6130:
6126:
6121:
6116:
6112:
6108:
6104:
6100:
6096:
6089:
6086:
6081:
6077:
6073:
6069:
6065:
6061:
6060:Immunotherapy
6054:
6052:
6048:
6037:
6033:
6026:
6023:
6018:
6014:
6009:
6004:
6000:
5996:
5992:
5988:
5984:
5977:
5974:
5969:
5965:
5961:
5957:
5953:
5949:
5945:
5941:
5934:
5932:
5930:
5928:
5924:
5919:
5915:
5910:
5905:
5901:
5897:
5893:
5886:
5884:
5882:
5880:
5878:
5874:
5869:
5865:
5860:
5855:
5851:
5847:
5843:
5839:
5835:
5831:
5825:
5823:
5821:
5819:
5815:
5810:
5806:
5802:
5798:
5794:
5790:
5786:
5782:
5775:
5773:
5769:
5764:
5758:
5754:
5747:
5745:
5741:
5736:
5732:
5728:
5724:
5720:
5716:
5709:
5706:
5701:
5697:
5693:
5689:
5685:
5681:
5677:
5673:
5669:
5665:
5658:
5656:
5652:
5647:
5643:
5638:
5633:
5629:
5625:
5621:
5617:
5613:
5606:
5603:
5598:
5594:
5590:
5586:
5582:
5578:
5574:
5570:
5563:
5560:
5555:
5551:
5546:
5541:
5537:
5533:
5529:
5525:
5521:
5514:
5511:
5506:
5502:
5497:
5492:
5488:
5484:
5480:
5476:
5472:
5465:
5462:
5457:
5453:
5449:
5445:
5440:
5435:
5431:
5427:
5423:
5416:
5413:
5408:
5404:
5400:
5396:
5392:
5388:
5384:
5380:
5376:
5372:
5365:
5362:
5357:
5353:
5349:
5345:
5340:
5335:
5331:
5327:
5323:
5316:
5313:
5308:
5304:
5299:
5294:
5289:
5284:
5280:
5276:
5272:
5268:
5264:
5257:
5254:
5249:
5245:
5241:
5237:
5233:
5229:
5225:
5221:
5214:
5212:
5210:
5206:
5201:
5197:
5193:
5189:
5185:
5181:
5174:
5172:
5170:
5166:
5161:
5157:
5152:
5147:
5143:
5139:
5135:
5128:
5125:
5120:
5116:
5112:
5108:
5100:
5097:
5092:
5088:
5084:
5080:
5076:
5072:
5068:
5064:
5060:
5056:
5049:
5046:
5041:
5037:
5033:
5029:
5025:
5021:
5017:
5013:
5009:
5003:
5000:
4995:
4991:
4987:
4983:
4979:
4975:
4971:
4967:
4959:
4956:
4951:
4947:
4942:
4937:
4933:
4929:
4925:
4918:
4916:
4912:
4907:
4901:
4897:
4896:Immunobiology
4893:
4887:
4885:
4881:
4876:
4870:
4866:
4865:
4860:
4854:
4851:
4846:
4842:
4837:
4832:
4828:
4824:
4820:
4816:
4812:
4808:
4801:
4799:
4795:
4788:
4786:
4784:
4780:
4776:
4772:
4768:
4764:
4756:
4754:
4752:
4748:
4743:
4742:
4738:
4734:
4733:
4729:
4725:
4724:
4720:
4716:
4715:
4711:
4710:Mogamulizumab
4707:
4706:
4702:
4694:
4691:
4688:
4686:
4682:
4679:
4676:
4673:
4670:
4667:
4664:
4662:
4659:
4656:
4654:
4651:
4650:
4647:
4644:
4641:
4639:
4635:
4631:
4628:
4626:
4622:
4619:
4616:
4611:
4608:
4606:
4603:
4600:
4598:
4595:
4594:
4591:
4588:
4585:
4583:
4580:
4578:
4574:
4571:
4568:
4565:
4562:
4560:
4557:
4554:
4552:
4549:
4548:
4545:
4542:
4539:
4536:
4534:
4531:
4529:
4525:
4522:
4519:
4517:
4514:
4511:
4509:
4506:
4505:
4502:
4499:
4496:
4494:
4493:breast cancer
4490:
4488:
4485:
4482:
4479:
4476:
4474:
4471:
4468:
4466:
4463:
4462:
4459:
4456:
4453:
4451:
4446:
4441:
4438:
4436:
4433:
4430:
4425:
4422:
4420:
4417:
4414:
4412:
4409:
4408:
4405:
4402:
4399:
4397:
4394:
4392:
4389:
4386:
4383:
4380:
4378:
4375:
4372:
4370:
4367:
4366:
4363:
4360:
4357:
4355:
4352:
4350:
4347:
4344:
4341:
4338:
4336:
4333:
4330:
4328:
4325:
4324:
4321:
4318:
4315:
4313:
4310:Multicentric
4309:
4307:
4304:
4301:
4298:
4295:
4293:
4290:
4287:
4285:
4282:
4281:
4278:
4275:
4272:
4270:
4266:
4263:
4261:
4258:
4255:
4250:
4247:
4245:
4242:
4239:
4237:
4234:
4233:
4230:
4227:
4224:
4222:
4219:
4217:
4214:
4211:
4208:
4205:
4203:
4200:
4197:
4195:
4192:
4191:
4188:
4185:
4182:
4180:
4176:
4172:
4169:
4167:
4164:
4161:
4158:
4155:
4153:
4150:
4147:
4145:
4144:hyaluronidase
4141:
4138:
4137:
4134:
4131:
4128:
4126:
4122:
4120:
4117:
4114:
4111:
4108:
4106:
4103:
4100:
4098:
4095:
4094:
4091:
4088:
4085:
4083:
4079:
4077:
4074:
4071:
4068:
4065:
4063:
4060:
4057:
4055:
4052:
4051:
4048:
4045:
4042:
4040:
4037:
4035:
4031:
4028:
4025:
4022:
4020:
4017:
4014:
4012:
4009:
4008:
4005:
4002:
3999:
3997:
3994:
3992:
3988:
3985:
3982:
3979:
3976:
3974:
3971:
3968:
3966:
3963:
3962:
3959:
3956:
3953:
3951:
3948:
3946:
3943:
3940:
3937:
3934:
3932:
3929:
3926:
3924:
3921:
3920:
3917:
3914:
3911:
3909:
3908:breast cancer
3905:
3903:
3900:
3897:
3894:
3891:
3889:
3886:
3883:
3881:
3878:
3877:
3874:
3871:
3868:
3866:
3863:
3861:
3858:
3855:
3852:
3849:
3847:
3844:
3841:
3839:
3838:pembrolizumab
3836:
3835:
3832:
3829:
3826:
3824:
3820:
3818:
3815:
3812:
3809:
3806:
3804:
3801:
3798:
3796:
3793:
3792:
3789:
3786:
3783:
3781:
3778:
3776:
3773:F protein of
3772:
3769:
3767:intramuscular
3766:
3763:
3761:
3758:
3755:
3753:
3750:
3749:
3746:
3743:
3740:
3738:
3734:
3732:
3729:
3726:
3723:
3720:
3718:
3715:
3712:
3710:
3707:
3706:
3703:
3700:
3697:
3695:
3692:
3690:
3687:
3684:
3681:
3678:
3676:
3673:
3670:
3668:
3665:
3664:
3661:
3658:
3655:
3653:
3650:
3648:
3645:
3642:
3639:
3636:
3634:
3631:
3628:
3626:
3623:
3622:
3619:
3616:
3613:
3611:
3608:
3606:
3603:
3600:
3597:
3594:
3592:
3589:
3586:
3584:
3581:
3580:
3577:
3574:
3571:
3569:
3566:
3564:
3561:
3558:
3555:
3552:
3550:
3547:
3544:
3542:
3539:
3538:
3535:
3532:
3529:
3527:
3524:
3522:
3521:Anthrax toxin
3518:
3515:
3512:
3509:
3507:
3504:
3501:
3499:
3498:obiltoxaximab
3496:
3495:
3492:
3489:
3486:
3484:
3481:
3479:
3476:
3473:
3470:
3467:
3465:
3462:
3459:
3457:
3454:
3453:
3450:
3447:
3444:
3442:
3438:
3436:
3433:
3430:
3427:
3424:
3422:
3419:
3416:
3414:
3411:
3410:
3407:
3404:
3401:
3399:
3395:
3393:
3390:
3387:
3384:
3381:
3379:
3376:
3373:
3371:
3368:
3367:
3364:
3361:
3358:
3356:
3353:
3351:
3347:
3344:
3341:
3338:
3336:
3333:
3330:
3328:
3325:
3324:
3321:
3318:
3315:
3313:
3309:
3307:
3304:
3301:
3298:
3295:
3293:
3290:
3287:
3285:
3282:
3281:
3278:
3275:
3272:
3270:
3267:
3265:
3262:
3259:
3256:
3253:
3251:
3248:
3245:
3243:
3240:
3239:
3236:
3233:
3230:
3228:
3225:
3223:
3220:
3217:
3214:
3211:
3209:
3206:
3203:
3201:
3198:
3197:
3194:
3191:
3188:
3186:
3183:
3181:
3178:
3176:
3172:
3169:
3166:
3164:
3161:
3158:
3156:
3153:
3152:
3149:
3146:
3143:
3141:
3137:
3133:
3129:
3125:
3121:
3118:
3116:
3113:
3111:
3107:
3104:
3101:
3099:
3096:
3093:
3091:
3088:
3087:
3084:
3081:
3078:
3076:
3072:
3068:
3064:
3060:
3056:
3053:
3051:
3048:
3046:
3042:
3039:
3036:
3034:
3031:
3028:
3026:
3023:
3022:
3019:
3016:
3013:
3011:
3007:
3003:
2999:
2995:
2991:
2988:
2986:
2983:
2981:
2977:
2974:
2971:
2969:
2966:
2963:
2961:
2958:
2957:
2954:
2951:
2948:
2946:
2943:
2941:
2938:
2935:
2932:
2929:
2927:
2924:
2921:
2919:
2916:
2915:
2912:
2909:
2906:
2904:
2900:
2898:
2895:
2893:humanized Fab
2892:
2889:
2886:
2884:
2881:
2878:
2876:
2873:
2872:
2869:
2866:
2863:
2861:
2857:
2855:
2852:
2849:
2846:
2843:
2841:
2838:
2835:
2833:
2830:
2829:
2826:
2823:
2820:
2818:
2815:
2813:
2810:
2807:
2804:
2801:
2799:
2796:
2793:
2791:
2788:
2787:
2784:
2781:
2778:
2776:
2773:
2771:
2768:
2765:
2762:
2759:
2757:
2754:
2751:
2749:
2746:
2745:
2742:
2739:
2736:
2734:
2731:
2729:
2726:
2723:
2720:
2717:
2715:
2712:
2709:
2707:
2704:
2703:
2700:
2697:
2694:
2692:
2688:
2684:
2681:
2679:
2676:
2673:
2670:
2667:
2665:
2662:
2659:
2657:
2654:
2653:
2650:
2647:
2644:
2642:
2639:
2637:
2634:
2632:
2628:
2625:
2622:
2620:
2617:
2614:
2612:
2609:
2608:
2605:
2602:
2599:
2597:
2592:
2589:Heterozygous
2588:
2586:
2583:
2580:
2577:
2574:
2572:
2569:
2566:
2564:
2561:
2560:
2557:
2554:
2551:
2548:
2545:
2542:
2539:
2536:
2533:
2530:
2528:
2525:
2522:
2520:
2519:erenumab-aooe
2517:
2516:
2513:
2510:
2507:
2504:
2500:
2497:
2495:
2491:
2488:
2485:
2482:
2479:
2477:
2474:
2471:
2469:
2466:
2465:
2462:
2459:
2456:
2454:
2451:
2449:
2446:
2443:
2440:
2437:
2435:
2432:
2429:
2427:
2424:
2423:
2420:
2417:
2414:
2412:
2409:
2407:
2404:
2401:
2398:
2395:
2393:
2390:
2387:
2385:
2382:
2381:
2378:
2375:
2372:
2370:
2367:
2365:
2362:
2359:
2356:
2353:
2351:
2348:
2345:
2343:
2340:
2339:
2336:
2333:
2330:
2328:
2324:
2321:
2319:
2316:
2313:
2310:
2307:
2305:
2302:
2299:
2297:
2294:
2293:
2290:
2287:
2284:
2282:
2281:neuroblastoma
2278:
2276:
2273:
2270:
2267:
2264:
2262:
2259:
2256:
2254:
2251:
2250:
2247:
2244:
2241:
2239:
2235:
2232:
2230:
2227:
2224:
2221:
2218:
2216:
2213:
2211:Prolia, Xgeva
2210:
2208:
2205:
2204:
2201:
2198:
2195:
2193:
2190:
2188:
2185:
2182:
2179:
2176:
2174:
2171:
2168:
2166:
2163:
2162:
2159:
2156:
2153:
2151:
2148:
2146:
2143:
2140:
2137:
2134:
2132:
2129:
2126:
2124:
2121:
2120:
2117:
2114:
2111:
2109:
2105:
2101:
2099:
2096:
2093:
2090:
2087:
2085:
2082:
2079:
2077:
2074:
2073:
2070:
2067:
2064:
2062:
2058:
2056:
2053:
2050:
2047:
2044:
2042:
2039:
2036:
2034:
2031:
2030:
2027:
2024:
2021:
2019:
2016:
2014:
2011:
2008:
2005:
2002:
2000:
1999:UCB (company)
1997:
1994:
1992:
1989:
1988:
1985:
1982:
1979:
1977:
1976:prostatectomy
1973:
1969:
1967:
1964:
1961:
1958:
1955:
1953:
1950:
1947:
1945:
1942:
1941:
1938:
1935:
1932:
1930:
1927:
1925:
1922:
1919:
1916:
1913:
1911:
1908:
1905:
1903:
1900:
1899:
1896:
1893:
1890:
1888:
1885:
1883:
1880:
1877:
1874:
1871:
1869:
1866:
1863:
1861:
1858:
1857:
1854:
1851:
1848:
1846:
1843:
1841:
1838:
1835:
1832:
1829:
1827:
1824:
1821:
1819:
1816:
1815:
1812:
1809:
1806:
1804:
1800:
1797:
1795:
1792:
1790:
1786:
1783:
1780:
1778:
1775:
1772:
1770:
1767:
1766:
1763:
1760:
1757:
1755:
1752:
1750:
1747:
1744:
1741:
1738:
1736:
1733:
1730:
1728:
1725:
1724:
1721:
1718:
1715:
1713:
1709:
1707:
1704:
1701:
1698:
1695:
1693:
1690:
1687:
1685:
1682:
1681:
1678:
1675:
1672:
1670:
1666:
1661:
1657:
1654:Non-squamous
1652:
1648:
1646:
1643:
1641:
1637:
1634:
1631:
1629:
1626:
1623:
1621:
1618:
1617:
1614:
1611:
1608:
1606:
1602:
1600:
1597:
1594:
1591:
1588:
1586:
1583:
1580:
1578:
1575:
1574:
1571:
1568:
1565:
1562:
1558:
1556:
1553:
1550:
1547:
1544:
1542:
1539:
1536:
1534:
1531:
1530:
1527:
1524:
1521:
1519:
1516:
1514:
1511:
1508:
1505:
1502:
1500:
1497:
1494:
1492:
1489:
1488:
1485:
1482:
1479:
1477:
1473:
1469:
1467:
1464:
1461:
1458:
1455:
1453:
1450:
1447:
1445:
1442:
1441:
1438:
1435:
1432:
1430:
1426:
1424:
1421:
1418:
1415:
1412:
1410:
1407:
1404:
1402:
1399:
1398:
1395:
1392:
1389:
1387:
1382:
1379:
1377:
1374:
1371:
1368:
1365:
1363:
1360:
1357:
1355:
1352:
1351:
1348:
1345:
1342:
1340:
1337:
1335:
1332:
1329:
1326:
1323:
1321:
1318:
1315:
1313:
1310:
1309:
1306:
1303:
1300:
1298:
1293:
1290:Heterozygous
1289:
1287:
1284:
1281:
1278:
1275:
1273:
1270:
1267:
1265:
1262:
1261:
1258:
1255:
1252:
1250:
1247:
1245:
1242:
1239:
1236:
1233:
1231:
1228:
1225:
1223:
1220:
1219:
1216:
1213:
1210:
1208:
1207:breast cancer
1204:
1202:
1199:
1197:
1193:
1190:
1187:
1185:
1182:
1179:
1177:
1174:
1173:
1170:
1167:
1164:
1162:
1158:
1154:
1150:
1146:
1142:
1138:
1135:
1133:
1130:
1128:
1125:fully human,
1124:
1121:
1118:
1116:
1113:
1110:
1108:
1105:
1104:
1101:
1098:
1095:
1093:
1089:
1085:
1081:
1077:
1073:
1069:
1066:
1064:
1061:
1059:
1056:fully human,
1055:
1052:
1049:
1047:
1044:
1041:
1039:
1036:
1035:
1032:
1029:
1026:
1024:
1021:
1019:
1016:
1013:
1010:
1007:
1005:
1002:
999:
997:
994:
993:
990:
987:
984:
982:
979:
977:
974:
971:
968:
965:
963:
960:
957:
955:
952:
951:
947:
944:
939:
936:
933:
930:
928:Approval date
927:
924:
921:
918:
917:
914:
912:
906:
904:
900:
896:
892:
888:
882:
875:
864:
852:
847:
838:
837:
831:
829:
827:
823:
819:
811:
807:
803:
799:
795:
787:
783:
779:
774:
772:
771:pembrolizumab
768:
764:
755:
753:
751:
746:
742:
734:
732:
730:
723:
721:
719:
712:
710:
708:
704:
700:
699:radioactively
696:
689:
685:Therapy types
684:
682:
675:
673:
671:
667:
660:
658:
656:
651:
647:
640:
638:
634:
632:
628:
624:
617:
615:
613:
609:
605:
601:
597:
593:
589:
584:
580:
576:
572:
567:
565:
561:
557:
552:
550:
546:
541:
539:
538:neurotoxicity
535:
531:
521:
519:
515:
511:
507:
503:
500:on activated
499:
495:
491:
487:
483:
479:
475:
471:
467:
463:
455:
453:
451:
445:
443:
435:
430:
428:
426:
421:
418:
414:
413:phage display
410:
406:
398:
396:
394:
390:
386:
382:
378:
372:
370:
367:
363:
359:
355:
347:
345:
343:
342:phage display
339:
335:
331:
327:
323:
319:
313:
311:
306:
302:
298:
290:
288:
286:
282:
278:
273:
270:
267:
263:
259:
255:
251:
247:
243:
242:Immunotherapy
236:
232:
228:
224:
220:
216:
209:
207:
204:
200:
195:
191:
187:
183:
178:
176:
172:
168:
164:
160:
156:
152:
148:
144:
140:
136:
132:
128:
124:
120:
116:
115:heterodimeric
112:
108:
103:
95:
93:
91:
87:
83:
79:
75:
70:
68:
64:
60:
59:immune system
56:
53:proteins and
52:
48:
47:extracellular
44:
40:
32:
19:
8097:Pexidartinib
8082:Odronextamab
8052:Gilteritinib
8032:Cabozantinib
7979:
7945:
7920:
7914:Temsirolimus
7896:
7880:
7862:proapoptotic
7860:
7844:
7823:Zanubrutinib
7795:
7765:
7734:
7711:Lestaurtinib
7690:Janus kinase
7688:
7669:
7623:
7616:Non-receptor
7597:Cabozantinib
7590:
7559:Infigratinib
7544:
7518:
7460:Fruquintinib
7442:
7435:Gilteritinib
7431:Lestaurtinib
7424:
7373:
7342:
7315:Mobocertinib
7280:Aumolertinib
7268:
7233:Tremelimumab
7218:Tislelizumab
7173:Retifanlimab
7048:Blinatumomab
7018:Atezolizumab
6989:
6971:
6961:
6943:Obinutuzumab
6921:
6903:
6870:
6853:
6811:
6794:
6772:
6659:
6655:
6645:
6610:
6606:
6596:
6561:
6557:
6547:
6512:
6508:
6498:
6468:(1): 26–37.
6465:
6461:
6455:
6412:
6408:
6398:
6373:
6369:
6363:
6328:
6324:
6314:
6303:. Retrieved
6299:
6289:
6278:. Retrieved
6274:
6265:
6240:
6236:
6230:
6197:
6188:
6151:
6147:
6137:
6102:
6098:
6088:
6063:
6059:
6039:. Retrieved
6035:
6025:
5990:
5986:
5976:
5943:
5939:
5899:
5895:
5841:
5837:
5784:
5780:
5753:Pharmacology
5752:
5718:
5714:
5708:
5667:
5663:
5619:
5615:
5605:
5572:
5568:
5562:
5527:
5523:
5513:
5478:
5474:
5464:
5429:
5425:
5415:
5374:
5370:
5364:
5329:
5325:
5315:
5270:
5266:
5256:
5223:
5219:
5186:(1): 11–29.
5183:
5179:
5141:
5137:
5127:
5110:
5106:
5099:
5058:
5054:
5048:
5015:
5011:
5008:Breedveld FC
5002:
4969:
4965:
4958:
4931:
4927:
4895:
4863:
4853:
4818:
4814:
4760:
4744:
4735:
4726:
4717:
4708:
4699:
4612:subcutaneous
4566:subcutaneous
4428:subcutaneous
4342:subcutaneous
4209:subcutaneous
4159:subcutaneous
3724:subcutaneous
3541:obinutuzumab
3299:subcutaneous
3257:subcutaneous
2875:idarucizumab
2763:subcutaneous
2710:Simponi Aria
2671:subcutaneous
2578:subcutaneous
2534:subcutaneous
2501:(congenital
2499:Hemophilia A
2483:subcutaneous
2311:subcutaneous
2238:osteoporosis
2222:subcutaneous
2138:subcutaneous
2006:subcutaneous
1917:subcutaneous
1875:subcutaneous
1833:subcutaneous
1727:blinatumomab
1684:bezlotoxumab
1660:Glioblastoma
1548:subcutaneous
1533:benralizumab
1354:atezolizumab
1312:atezolizumab
1279:subcutaneous
1122:subcutaneous
1053:subcutaneous
1011:subcutaneous
972:chimeric Fab
909:
884:
858:
850:
796:, and B7H3.
775:
759:
738:
727:
716:
693:
679:
664:
644:
635:
623:Bapineuzumab
621:
618:Bapineuzumab
596:Gautenerumab
588:Bapineuzumab
568:
553:
542:
527:
459:
446:
439:
422:
404:
402:
373:
361:
357:
351:
318:cytotoxicity
314:
300:
294:
274:
240:
218:
201:. Some such
179:
142:
134:
105:
71:
69:radiation).
51:cell surface
43:specifically
37:
8127:Tebentafusp
8107:Regorafenib
8102:Quizartinib
8092:Pemigatinib
8072:Midostaurin
8047:Erdafitinib
8042:Entrectinib
8025:Parsaclisib
7973:Trilaciclib
7963:Palbociclib
7958:Dalpiciclib
7953:Abemaciclib
7855:Aflibercept
7782:Entrectinib
7752:Selumetinib
7747:Cobimetinib
7742:Binimetinib
7726:Ruxolitinib
7716:Momelotinib
7696:Baricitinib
7594:inhibitors:
7571:Pralsetinib
7567:Pemigatinib
7555:Futibatinib
7551:Entrectinib
7548:inhibitors:
7480:Regorafenib
7384:Avapritinib
7330:Rociletinib
7325:Osimertinib
7290:Dacomitinib
7228:Toripalimab
7213:Teclistamab
7203:Talquetamab
7198:Tafasitamab
7193:Sugemalimab
7188:Serplulimab
7178:Sabatolimab
7168:Ramucirumab
7163:Prolgolimab
7133:Necitumumab
7088:Epcoritamab
7068:Dostarlimab
7058:Daratumumab
7013:Amivantamab
6978:Alemtuzumab
6968:Brentuximab
6958:Tositumomab
6933:Ibritumomab
6914:Elranatamab
6877:Bevacizumab
6865:Edrecolomab
6860:Catumaxomab
6822:Trastuzumab
6806:Panitumumab
6376:: 113–124.
6243:(1): 9–21.
5830:van Dyck CH
5144:(1): 1–11.
4767:trastuzumab
4763:bevacizumab
4751:catumaxomab
4701:Tositumomab
4668:intravenous
4653:vedolizumab
4617:fully human
4614:intravenous
4597:ustekinumab
4569:fully human
4551:ustekinumab
4526:humanized,
4523:intravenous
4491:Metastatic
4480:intravenous
4465:trastuzumab
4426:intravenous
4411:tocilizumab
4384:intravenous
4369:tocilizumab
4299:intravenous
4256:fully human
4236:secukinumab
4212:fully human
4112:intravenous
4069:intravenous
4029:fully human
4026:intravenous
4015:Raxibacumab
4011:raxibacumab
3965:ranibizumab
3941:fully human
3938:intravenous
3923:ramucirumab
3906:Metastatic
3895:intravenous
3853:intravenous
3821:Metastatic
3813:fully human
3810:intravenous
3795:panitumumab
3752:palivizumab
3685:fully human
3682:intravenous
3643:fully human
3640:intravenous
3598:intravenous
3583:ocrelizumab
3556:intravenous
3513:intravenous
3474:fully human
3471:intravenous
3431:fully human
3428:intravenous
3388:fully human
3385:intravenous
3370:necitumumab
3342:intravenous
3335:Biogen Idec
3327:natalizumab
3284:mepolizumab
3218:fully human
3215:intravenous
3173:humanized,
3170:intravenous
3105:intravenous
3040:intravenous
2975:intravenous
2933:intravenous
2890:intravenous
2847:intravenous
2805:intravenous
2766:fully human
2724:fully human
2721:intravenous
2674:fully human
2629:humanized,
2626:intravenous
2594:Refractory
2581:fully human
2537:fully human
2503:Factor VIII
2441:intravenous
2399:intravenous
2360:fully human
2357:intravenous
2350:AstraZeneca
2314:fully human
2268:intravenous
2253:dinutuximab
2236:women with
2225:fully human
2183:fully human
2180:intravenous
2165:daratumumab
2091:intravenous
2059:Metastatic
2048:intravenous
1959:intravenous
1948:ProstaScint
1920:fully human
1902:canakinumab
1878:fully human
1784:intravenous
1742:intravenous
1702:fully human
1699:intravenous
1663:Metastatic
1649:Metastatic
1638:humanized,
1635:intravenous
1603:Metastatic
1592:intravenous
1577:bevacizumab
1541:AstraZeneca
1509:fully human
1506:intravenous
1459:intravenous
1444:basiliximab
1427:Metastatic
1419:fully human
1416:intravenous
1384:Metastatic
1369:intravenous
1327:intravenous
1295:Refractory
1282:fully human
1237:intravenous
1222:alemtuzumab
1205:Metastatic
1194:humanized,
1191:intravenous
1014:fully human
969:intravenous
948:Drug Label
818:fibroblasts
750:transferrin
745:nucleotides
707:Tositumomab
646:Solanezumab
641:Solanezumab
592:Solanezumab
490:Basiliximab
442:Ramucirumab
123:polypeptide
8163:Immunology
8157:Categories
8142:Venetoclax
8137:Vandetanib
8112:Ripretinib
8077:Nintedanib
8062:Lenvatinib
8037:Capmatinib
8020:Idelalisib
8010:Copanlisib
7983:inhibitors
7968:Ribociclib
7939:Vismodegib
7924:inhibitors
7904:Everolimus
7870:prohibitin
7787:Lorlatinib
7777:Crizotinib
7757:Trametinib
7721:Pacritinib
7706:Filgotinib
7701:Fedratinib
7605:Crizotinib
7601:Capmatinib
7583:Vandetanib
7531:Brigatinib
7510:Vandetanib
7470:Nintedanib
7465:Lenvatinib
7404:Ripretinib
7335:Vandetanib
7310:Lazertinib
7285:Brigatinib
7208:Tarlatamab
7143:Olaratumab
7103:Isatuximab
7098:Ipilimumab
7078:Elotuzumab
7073:Durvalumab
7053:Cemiplimab
7043:Bermekimab
7033:Axatilimab
6948:Ofatumumab
6928:Glofitamab
6910:Glofitamab
6818:Pertuzumab
6305:2024-02-14
6280:2024-02-14
6041:2017-11-01
5902:: 102192.
4789:References
4775:infliximab
4771:adalimumab
4728:Cemiplimab
4528:biosimilar
4423:10/21/2013
4284:siltuximab
4109:11/26/1997
4054:reslizumab
4023:12/24/2012
3880:pertuzumab
3709:omalizumab
3679:10/19/2016
3667:olaratumab
3637:10/26/2009
3625:ofatumumab
3468:12/22/2014
3382:11/24/2015
3339:11/23/2004
3242:ixekizumab
3200:ipilimumab
3110:biosimilar
3108:chimeric,
3045:biosimilar
3043:chimeric,
2980:biosimilar
2978:chimeric,
2918:infliximab
2903:dabigatran
2897:dabigatran
2887:10/16/2015
2748:guselkumab
2563:evolocumab
2549:prevention
2490:Factor IXa
2438:11/30/2015
2426:elotuzumab
2384:eculizumab
2342:durvalumab
2177:11/16/2015
2123:daclizumab
2088:12/10/1997
2076:daclizumab
1956:10/28/1996
1868:Ultragenyx
1818:brodalumab
1787:chimeric,
1696:10/21/2016
1640:biosimilar
1409:EMD Serono
1366:10/18/2016
1264:alirocumab
1127:biosimilar
1058:biosimilar
1008:12/31/2002
996:adalimumab
976:GPIIb/IIIa
966:12/22/1994
940:Indication
922:Brand name
879:See also:
806:metabolite
604:Aducanemab
600:Crenezumab
575:Aducanemab
510:Omalizumab
494:daclizumab
470:adalimumab
466:infliximab
409:transgenic
338:transgenic
310:proteomics
246:antibodies
155:Antibodies
90:blood clot
8132:Tepotinib
8122:Sunitinib
8117:Sorafenib
8087:Pazopanib
8067:Masitinib
8015:Duvelisib
8005:Alpelisib
7993:Sotorasib
7988:Adagrasib
7934:Sonidegib
7929:Glasdegib
7875:Adipotide
7808:Ibrutinib
7681:Dasatinib
7676:Bosutinib
7661:Radotinib
7656:Ponatinib
7651:Nilotinib
7641:Dasatinib
7636:Bosutinib
7631:Asciminib
7599:(VEGFR),
7557:(FGFR2),
7536:Ceritinib
7526:Alectinib
7505:Toceranib
7500:Tivozanib
7495:Sunitinib
7490:Sorafenib
7485:Semaxanib
7475:Pazopanib
7455:Cediranib
7419:Toceranib
7414:Sunitinib
7409:Sorafenib
7399:Pazopanib
7394:Masitinib
7361:Tucatinib
7356:Neratinib
7351:Lapatinib
7320:Olmutinib
7300:Gefitinib
7295:Erlotinib
7270:HER1/EGFR
7138:Nivolumab
7128:Naxitamab
6953:Rituximab
6801:Cetuximab
6796:HER1/EGFR
6222:242999976
6214:2643-4652
6154:(1): 14.
5721:: 55–72.
4783:rituximab
4757:Economics
4671:humanized
4665:5/20/2014
4609:9/23/2016
4563:9/25/2009
4483:humanized
4477:9/25/1998
4473:Genentech
4469:Herceptin
4448:Systemic
4431:humanized
4419:Genentech
4387:humanized
4377:Genentech
4345:humanized
4296:4/23/2014
4248:1/21/2015
4194:sarilumab
4152:Genentech
4140:rituximab
4105:Genentech
4097:rituximab
4072:humanized
4066:3/23/2016
3983:humanized
3977:6/30/2006
3973:Genentech
3935:4/21/2014
3931:Eli Lilly
3898:humanized
3888:Genentech
3856:humanized
3807:9/27/2006
3770:humanized
3764:6/19/1998
3760:MedImmune
3727:humanized
3721:6/20/2003
3717:Genentech
3675:Eli Lilly
3633:Glaxo Grp
3601:humanized
3595:3/28/2017
3591:Genentech
3559:humanized
3553:11/1/2013
3549:Genentech
3510:3/18/2016
3456:nivolumab
3413:nivolumab
3378:Eli Lilly
3374:Portrazza
3345:humanized
3302:humanized
3296:11/4/2015
3260:humanized
3254:3/22/2016
3250:Eli Lilly
3212:3/25/2011
3029:Inflectra
2972:4/21/2017
2964:Renflexis
2940:TNF alpha
2930:8/24/1998
2844:2/19/2002
2808:humanized
2718:7/18/2013
2706:golimumab
2668:4/24/2009
2656:golimumab
2575:8/27/2015
2476:Genentech
2444:humanized
2430:Empliciti
2402:humanized
2396:3/16/2007
2308:3/28/2017
2296:dupilumab
2265:3/10/2015
2207:denosumab
2141:humanized
2135:5/27/2016
2094:humanized
2045:2/12/2004
2033:cetuximab
2009:humanized
2003:4/22/2008
1914:6/17/2009
1830:2/15/2017
1781:9/19/2011
1739:12/3/2014
1595:humanized
1589:2/26/2004
1585:Genentech
1551:humanized
1491:belimumab
1456:5/12/1998
1413:3/23/2017
1372:humanized
1362:Genentech
1358:Tecentriq
1330:humanized
1324:5/18/2016
1320:Genentech
1316:Tecentriq
1276:7/24/2015
1240:humanized
1188:2/22/2013
1184:Genentech
1119:9/23/2016
954:abciximab
741:liposomes
703:lymphomas
666:Lecanemab
661:Lecanemab
612:Donanemab
608:Lecanemab
579:Lecanemab
506:rejection
379:into the
377:mutations
369:half-life
340:mice and
330:Hybridoma
297:analogues
285:humanised
269:neoplasms
266:malignant
250:hybridoma
67:cytotoxic
55:cytokines
7922:hedgehog
7884:against
7882:exotoxin
7848:against
7797:Bruton's
7646:Imatinib
7569:(FGFR),
7565:(NTRK),
7450:Axitinib
7389:Axitinib
7345:HER2/neu
7305:Icotinib
7275:Afatinib
7249:("-nib")
7028:Avelumab
6900:lymphoid
6892:lymphoma
6888:Leukemia
6813:HER2/neu
6775:("-mab")
6713:Archived
6686:20065641
6637:20061824
6588:20190561
6539:20073127
6490:25271222
6447:11603692
6439:25838376
6390:15687597
6355:12237768
6257:36449413
6180:27048170
6129:37458272
6120:10559996
6080:21091109
6017:28967385
5968:26323381
5960:24981190
5918:38219962
5868:28967385
5809:19375883
5801:21261567
5735:28335888
5692:25838373
5646:26784573
5597:21298489
5589:27306885
5554:20065651
5505:20073133
5448:12612576
5248:19243664
5240:12514726
5200:15780905
5040:43781004
5032:10703815
4994:10169378
4986:11905803
4950:18045976
4845:23370250
4677:receptor
4675:integrin
4559:Centocor
4381:1/8/2010
4302:chimeric
4244:Novartis
4240:Cosentyx
4115:chimeric
3969:Lucentis
3892:6/8/2012
3850:9/4/2014
3842:Keytruda
3799:Vectibix
3671:Lartruvo
3516:chimeric
3425:3/4/2015
3350:integrin
3348:alpha-4
3159:Besponsa
3102:12/13/17
3037:4/5/2016
2936:chimeric
2926:Centocor
2922:Remicade
2879:Praxbind
2794:Trogarzo
2664:Centocor
2615:Mylotarg
2543:receptor
2494:Factor X
2480:11/16/17
2472:Hemlibra
2354:5/1/2017
2300:Dupixent
2271:chimeric
2257:Unituxin
2219:6/1/2010
2169:Darzalex
2127:Zinbryta
2051:chimeric
1974:or post-
1910:Novartis
1864:Crysvita
1836:chimeric
1773:Adcetris
1731:Blincyto
1688:Zinplava
1545:11/14/17
1503:3/9/2011
1495:Benlysta
1462:chimeric
1452:Novartis
1448:Simulect
1405:Bavencio
1401:avelumab
1268:Praluent
1234:5/7/2001
1111:Amjevita
962:Centocor
919:Antibody
861:May 2021
556:Vaccines
496:inhibit
464:include
425:allotype
411:mice or
299:(suffix
281:chimeric
258:in vitro
225:; ADCC:
199:antigens
7625:bcr-abl
6986:myeloid
6677:2759494
6628:2725420
6579:2840231
6530:2791310
6482:1902291
6417:Bibcode
6409:Science
6346:2364249
6171:4822297
6008:5767539
5859:5767539
5700:4608450
5672:Bibcode
5664:Science
5637:8719646
5545:2759500
5496:2726606
5456:9745527
5407:4241051
5399:2687698
5379:Bibcode
5348:8360482
5307:1350088
5275:Bibcode
5160:7032624
5119:7427932
5091:4161444
5083:1172191
5063:Bibcode
4836:3698571
4657:Entyvio
4601:Stelara
4555:Stelara
4520:12/1/17
4415:Actemra
4373:Actemra
4339:3/20/18
4288:Sylvant
4206:5/22/17
4198:Kevzara
4156:6/22/17
4123:B-cell
4101:Rituxan
4080:Severe
4058:Cinqair
3927:Cyramza
3884:Perjeta
3756:Synagis
3629:Arzerra
3587:Ocrevus
3331:Tysabri
3310:Severe
3167:8/17/17
2836:Zevalin
2760:7/13/17
2752:Tremfya
2660:Simponi
2567:Repatha
2531:5/17/18
2523:Aimovig
2392:Alexion
2388:Soliris
2346:Imfinzi
2080:Zenapax
2037:Erbitux
1952:Cytogen
1872:4/17/18
1826:Valeant
1632:9/14/17
1581:Avastin
1559:Severe
1537:Fasenra
1230:Genzyme
1180:Kadcyla
1050:8/25/17
1042:Cyltezo
945:BLA STN
925:Company
899:steroid
851:updated
502:T cells
389:E. coli
358:-ximab)
262:in vivo
229:; CDC:
221:ADEPT:
210:History
6872:VEGF-A
6684:
6674:
6635:
6625:
6586:
6576:
6537:
6527:
6488:
6480:
6445:
6437:
6388:
6353:
6343:
6255:
6220:
6212:
6178:
6168:
6127:
6117:
6078:
6015:
6005:
5966:
5958:
5916:
5866:
5856:
5807:
5799:
5759:
5733:
5698:
5690:
5644:
5634:
5595:
5587:
5552:
5542:
5503:
5493:
5454:
5446:
5405:
5397:
5371:Nature
5356:904440
5354:
5346:
5305:
5295:
5246:
5238:
5198:
5158:
5117:
5089:
5081:
5055:Nature
5038:
5030:
5012:Lancet
4992:
4984:
4948:
4902:
4871:
4843:
4833:
4807:Chen L
4777:(both
4689:125476
4661:Takeda
4642:761044
4586:125261
4540:761074
4512:Ogivri
4497:103792
4454:125472
4400:125276
4358:761067
4331:Ilumya
4316:125496
4273:125504
4225:761037
4183:761064
4129:103705
4086:761033
4082:asthma
4043:125349
4000:125156
3991:VEGFR2
3987:VEGFR1
3954:125477
3945:VEGFR2
3912:125409
3869:125514
3827:125147
3784:103770
3741:103976
3737:asthma
3713:Xolair
3698:761038
3689:PDGFRA
3656:125326
3614:761053
3572:125486
3545:Gazyva
3530:125509
3502:Anthem
3487:125554
3460:Opdivo
3445:125527
3417:Opdivo
3402:125547
3359:125104
3316:125526
3312:asthma
3288:Nucala
3273:125521
3231:125377
3222:CTLA-4
3204:Yervoy
3189:761040
3144:761072
3098:Pfizer
3079:125544
3014:761054
2949:103772
2907:761025
2864:125019
2821:761065
2802:3/6/18
2779:761061
2737:125433
2695:125289
2645:761060
2623:9/1/17
2600:125522
2552:761077
2508:761083
2457:761035
2448:SLAMF7
2415:125166
2373:761069
2331:761055
2327:asthma
2285:125516
2242:125320
2196:761036
2154:761029
2131:Biogen
2112:103749
2065:125084
2022:125160
1995:Cimzia
1980:103608
1933:125319
1906:Ilaris
1891:761068
1849:761032
1840:IL17RA
1807:125388
1758:125557
1716:761046
1673:761028
1609:125085
1566:761070
1561:asthma
1522:125370
1480:103764
1433:761049
1390:761041
1343:761034
1301:125559
1253:103948
1211:125427
1165:761024
1096:761058
1027:125057
1004:Abbvie
1000:Humira
985:103575
958:ReoPro
937:Target
889:drug,
826:CXCL12
782:stroma
763:CTLA-4
518:asthma
436:Cancer
362:-zumab
291:Murine
277:murine
182:cancer
129:, the
127:papain
82:asthma
74:cancer
7866:ANXA2
7838:Other
7736:MAP2K
7607:(ALK)
7592:c-MET
7444:VEGFR
7379:PDGFR
7375:C-kit
7006:Other
6855:EpCAM
6486:S2CID
6443:S2CID
6218:S2CID
5964:S2CID
5805:S2CID
5696:S2CID
5593:S2CID
5452:S2CID
5403:S2CID
5352:S2CID
5298:49066
5244:S2CID
5138:Blood
5087:S2CID
5036:S2CID
4990:S2CID
4741:CD79B
4516:Mylan
4335:Merck
4260:IL17A
3846:Merck
3803:Amgen
3264:IL17A
3246:Taltz
3163:Wyeth
3094:Ixifi
2619:Wyeth
2585:PCSK9
2571:Amgen
2527:Amgen
2364:PD-L1
2318:IL4RA
2229:RANKL
2215:Amgen
2098:IL2RA
2084:Roche
1882:FGF23
1822:Siliq
1735:Amgen
1692:Merck
1628:Amgen
1624:Mvasi
1466:IL2RA
1423:PD-L1
1376:PD-L1
1334:PD-L1
1286:PCSK9
1115:Amgen
931:Route
802:PD-L1
486:TNF-α
405:-umab
366:serum
301:-omab
194:cells
7981:KRAS
7886:IL-2
7868:and
7850:VEGF
7767:EML4
7426:FLT3
7377:and
7265:ErbB
6991:CD33
6973:CD52
6963:CD30
6923:CD20
6791:ErbB
6682:PMID
6656:mAbs
6633:PMID
6607:mAbs
6584:PMID
6558:mAbs
6535:PMID
6509:mAbs
6478:PMID
6435:PMID
6386:PMID
6351:PMID
6253:PMID
6210:ISSN
6176:PMID
6125:PMID
6076:PMID
6013:PMID
5956:PMID
5914:PMID
5864:PMID
5797:PMID
5757:ISBN
5731:PMID
5688:PMID
5642:PMID
5620:2016
5585:PMID
5550:PMID
5524:mAbs
5501:PMID
5475:mAbs
5444:PMID
5395:PMID
5344:PMID
5303:PMID
5236:PMID
5196:PMID
5156:PMID
5115:PMID
5079:PMID
5028:PMID
4982:PMID
4946:PMID
4900:ISBN
4869:ISBN
4841:PMID
4745:The
4732:PD-1
4723:CD22
4714:CCR4
4705:CD20
4693:Link
4646:Link
4625:IL23
4621:IL12
4590:Link
4577:IL23
4573:IL12
4544:Link
4533:HER2
4501:Link
4487:HER2
4458:Link
4435:IL6R
4404:Link
4391:IL6R
4362:Link
4349:IL23
4320:Link
4277:Link
4229:Link
4216:IL6R
4187:Link
4166:CD20
4142:and
4133:Link
4119:CD20
4090:Link
4062:Teva
4047:Link
4004:Link
3958:Link
3916:Link
3902:HER2
3873:Link
3860:PD-1
3831:Link
3817:EGFR
3788:Link
3745:Link
3702:Link
3660:Link
3647:CD20
3618:Link
3605:CD20
3576:Link
3563:CD20
3534:Link
3491:Link
3478:PD-1
3449:Link
3435:PD-1
3406:Link
3392:EGFR
3363:Link
3320:Link
3277:Link
3235:Link
3193:Link
3180:CD22
3148:Link
3083:Link
3018:Link
2953:Link
2911:Link
2868:Link
2854:CD20
2825:Link
2783:Link
2770:IL23
2741:Link
2699:Link
2649:Link
2636:CD33
2604:Link
2556:Link
2541:CGRP
2512:Link
2461:Link
2419:Link
2377:Link
2335:Link
2289:Link
2246:Link
2200:Link
2187:CD38
2158:Link
2145:IL2R
2116:Link
2069:Link
2055:EGFR
2026:Link
1984:Link
1966:PSMA
1937:Link
1924:IL1B
1895:Link
1853:Link
1811:Link
1794:CD30
1762:Link
1749:CD19
1720:Link
1677:Link
1645:VEGF
1613:Link
1599:VEGF
1570:Link
1526:Link
1513:BLyS
1484:Link
1437:Link
1394:Link
1347:Link
1305:Link
1257:Link
1244:CD52
1215:Link
1201:HER2
1169:Link
1100:Link
1031:Link
989:Link
934:Type
891:OKT3
786:FasL
776:The
767:PD-1
610:and
577:and
498:IL-2
492:and
480:and
468:and
260:and
235:scFv
7771:ALK
7671:Src
7546:RET
7520:ALK
6970:),
6960:),
6920:),
6905:CD3
6761:L01
6672:PMC
6664:doi
6623:PMC
6615:doi
6574:PMC
6566:doi
6525:PMC
6517:doi
6470:doi
6425:doi
6413:348
6378:doi
6341:PMC
6333:doi
6300:FDA
6245:doi
6241:388
6202:doi
6166:PMC
6156:doi
6115:PMC
6107:doi
6103:389
6068:doi
6003:PMC
5995:doi
5948:doi
5904:doi
5854:PMC
5846:doi
5789:doi
5723:doi
5680:doi
5668:348
5632:PMC
5624:doi
5577:doi
5540:PMC
5532:doi
5491:PMC
5483:doi
5434:doi
5387:doi
5375:342
5334:doi
5330:151
5293:PMC
5283:doi
5228:doi
5188:doi
5146:doi
5071:doi
5059:256
5020:doi
5016:355
4974:doi
4936:doi
4831:PMC
4823:doi
4306:IL6
4076:IL5
3775:RSV
3731:IgE
3306:IL5
3115:TNF
3050:TNF
2985:TNF
2817:HIV
2812:CD4
2728:TNF
2678:TNF
2275:GD2
2106:in
2013:TNF
1474:in
1132:TNF
1063:TNF
1018:TNF
911:FDA
814:reg
810:IDO
571:FDA
540:.
385:PCR
173:or
131:Fab
119:kDa
111:IgG
109:G (
8159::
7603:,
7573:,
7561:,
7433:,
7372::
7267::
6988::
6916:,
6912:,
6902::
6820:,
6793::
6770:CI
6755:/
6680:.
6670:.
6658:.
6654:.
6631:.
6621:.
6609:.
6605:.
6582:.
6572:.
6560:.
6556:.
6533:.
6523:.
6511:.
6507:.
6484:.
6476:.
6466:11
6464:.
6441:.
6433:.
6423:.
6411:.
6407:.
6384:.
6374:11
6372:.
6349:.
6339:.
6329:87
6327:.
6323:.
6298:.
6273:.
6251:.
6239:.
6216:.
6208:.
6196:.
6174:.
6164:.
6150:.
6146:.
6123:.
6113:.
6101:.
6097:.
6074:.
6062:.
6050:^
6034:.
6011:.
6001:.
5991:83
5989:.
5985:.
5962:.
5954:.
5944:14
5942:.
5926:^
5912:.
5900:94
5898:.
5894:.
5876:^
5862:.
5852:.
5842:83
5840:.
5836:.
5817:^
5803:.
5795:.
5785:11
5783:.
5771:^
5743:^
5729:.
5719:74
5717:.
5694:.
5686:.
5678:.
5666:.
5654:^
5640:.
5630:.
5618:.
5614:.
5591:.
5583:.
5573:23
5571:.
5548:.
5538:.
5526:.
5522:.
5499:.
5489:.
5477:.
5473:.
5450:.
5442:.
5428:.
5424:.
5401:.
5393:.
5385:.
5373:.
5350:.
5342:.
5328:.
5324:.
5301:.
5291:.
5281:.
5271:89
5269:.
5265:.
5242:.
5234:.
5222:.
5208:^
5194:.
5184:54
5182:.
5168:^
5154:.
5142:59
5140:.
5136:.
5111:40
5109:.
5085:.
5077:.
5069:.
5057:.
5034:.
5026:.
5014:.
4988:.
4980:.
4968:.
4944:.
4932:57
4930:.
4926:.
4914:^
4883:^
4839:.
4829:.
4819:12
4817:.
4813:.
4797:^
4773:,
4765:,
2492:,
2325:,
790:ET
788:,
614:.
606:,
602:,
598:,
594:,
590:,
520:.
488:.
476:,
395:.
387:,
371:.
344:.
336:,
328:.
283:,
279:,
184:,
153:.
139:Fc
88:,
84:,
80:,
76:,
7893:)
7889:(
7877:)
7873:(
7857:)
7853:(
7769:-
7683:)
7674:(
7429:(
7421:)
7382:(
7337:)
7273:(
7024:)
7020:(
6998:)
6994:(
6980:)
6976:(
6966:(
6926:(
6908:(
6890:/
6879:)
6875:(
6867:)
6858:(
6839:)
6828:)
6824:(
6816:(
6808:)
6799:(
6763:)
6759:(
6745:e
6738:t
6731:v
6710:)
6706:(
6688:.
6666::
6660:1
6639:.
6617::
6611:1
6590:.
6568::
6562:2
6541:.
6519::
6513:1
6492:.
6472::
6449:.
6427::
6419::
6392:.
6380::
6357:.
6335::
6308:.
6283:.
6259:.
6247::
6224:.
6204::
6182:.
6158::
6152:8
6131:.
6109::
6082:.
6070::
6064:2
6044:.
6019:.
5997::
5970:.
5950::
5920:.
5906::
5870:.
5848::
5811:.
5791::
5765:.
5737:.
5725::
5702:.
5682::
5674::
5648:.
5626::
5599:.
5579::
5556:.
5534::
5528:1
5507:.
5485::
5479:1
5458:.
5436::
5430:9
5409:.
5389::
5381::
5358:.
5336::
5309:.
5285::
5277::
5250:.
5230::
5224:9
5202:.
5190::
5162:.
5148::
5121:.
5093:.
5073::
5065::
5042:.
5022::
4996:.
4976::
4970:1
4952:.
4938::
4908:.
4877:.
4847:.
4825::
876:.
863:)
859:(
853:.
794:R
792:B
448:(
141:(
133:(
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.