338:
1494:
1518:
1530:
1506:
98:
235:, and the temperature plotted against time to give a graph from which fundamental quantities can be calculated. Modern calorimeters are frequently supplied with automatic devices to provide a quick read-out of information, one example being the
247:
Several thermodynamic definitions are very useful in thermochemistry. A system is the specific portion of the universe that is being studied. Everything outside the system is considered the surroundings or environment. A system may be:
70:
release heat. Thermochemistry coalesces the concepts of thermodynamics with the concept of energy in the form of chemical bonds. The subject commonly includes calculations of such quantities as
201:
had already introduced the concept of latent heat in 1761, based on the observation that heating ice at its melting point did not raise the temperature but instead caused some ice to melt.
589:
381:
690:
56:
in the form of heat. Thermochemistry is useful in predicting reactant and product quantities throughout the course of a given reaction. In combination with
695:
559:
506:
396:
376:
52:. A reaction may release or absorb energy, and a phase change may do the same. Thermochemistry focuses on the energy exchange between a system and its
145:, as well the exchange of matter. When all forms of energy are considered, the concepts of exothermic and endothermic reactions are generalized to
673:
178:
of constant heat summation (1840): The energy change accompanying any transformation is the same whether the process occurs in one step or many.
717:
668:
729:
215:. Integration of this equation permits the evaluation of the heat of reaction at one temperature from measurements at another temperature.
141:, which deals with the exchange of all forms of energy between system and surroundings, including not only heat but also various forms of
1566:
582:
227:, usually an enclosed chamber within which the change to be examined occurs. The temperature of the chamber is monitored either using a
172:
law (1780): The energy change accompanying any transformation is equal and opposite to energy change accompanying the reverse process.
1534:
479:
439:
366:
236:
1413:
575:
60:
determinations, it is also used to predict whether a reaction is spontaneous or non-spontaneous, favorable or unfavorable.
856:
610:
277:
1561:
1133:
620:
406:
314:
A system undergoes a process when one or more of its properties changes. A process relates to the change of state. An
183:
1059:
1030:
1010:
963:
648:
386:
263:
91:
1556:
1403:
1319:
958:
1341:
1252:
1215:
1099:
1025:
846:
829:
772:
138:
31:
1259:
1247:
1138:
1003:
777:
643:
548:
1408:
1305:
1290:
1220:
1143:
975:
925:
834:
759:
658:
1510:
1398:
1353:
1128:
948:
878:
635:
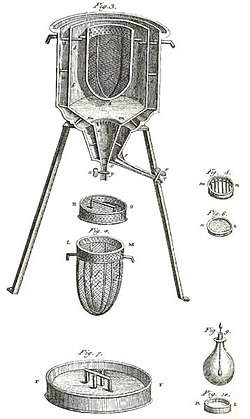
615:
391:
302:
169:
110:
1421:
1375:
1300:
1273:
1171:
1153:
1106:
1044:
940:
920:
789:
784:
685:
150:
63:
1517:
305:
which it can exchange both matter and energy with the surroundings, such as a pot of boiling water
1498:
1464:
1326:
1295:
1176:
1118:
816:
799:
794:
749:
712:
702:
663:
371:
315:
146:
142:
75:
67:
553:
1479:
1444:
1427:
1365:
1283:
1278:
1206:
1191:
1161:
1082:
1049:
1020:
1015:
990:
980:
900:
888:
767:
680:
475:
456:
435:
356:
323:
322:(same-pressure) process occurs when the pressure of the system remains constant. A process is
165:
106:
79:
41:
500:
1522:
1439:
1094:
953:
930:
883:
824:
520:
319:
285:
257:
204:
194:
161:
Thermochemistry rests on two generalizations. Stated in modern terms, they are as follows:
27:
Study of the heat energy associated with chemical reactions and/or physical transformations
1380:
1336:
1331:
1225:
1201:
1035:
998:
851:
724:
253:
118:
256:
which can exchange neither energy nor matter with the surroundings, such as an insulated
207:
showed in 1858 that the variation of the heat of reaction is given by the difference in
1264:
1242:
1237:
1232:
1187:
1183:
1166:
1123:
1054:
915:
910:
895:
707:
625:
411:
401:
343:
266:
which can exchange mechanical work but not heat or matter, such as an insulated closed
318:(same-temperature) process occurs when temperature of the system remains constant. An
1550:
1469:
1358:
1314:
1039:
873:
868:
861:
739:
495:
361:
291:
208:
71:
1346:
1196:
1111:
1087:
1077:
1069:
970:
905:
804:
653:
532:
337:
232:
198:
122:
53:
175:
744:
535:
and de Paula J., "Atkins' Physical
Chemistry" (8th edn, W.H. Freeman 2006), p.56
351:
228:
224:
190:
126:
1370:
333:
97:
17:
1432:
734:
599:
1454:
83:
567:
1474:
271:
87:
57:
49:
45:
552:
470:
Petrucci, Ralph H.; Harwood, William S.; Herring, F. Geoffrey (2002).
280:
which can exchange heat but not mechanical work or matter, such as an
267:
523:
and Meiser J.H., "Physical
Chemistry" (Benjamin/Cummings 1982), p.62
499:
96:
1449:
114:
571:
1459:
510:. Vol. 4 (11th ed.). Cambridge University Press.
40:
is the study of the heat energy which is associated with
294:
which can exchange energy but not matter, such as an
189:
Thermochemistry also involves the measurement of the
137:
Thermochemistry is one part of the broader field of
1391:
1152:
1068:
989:
939:
815:
758:
634:
223:The measurement of heat changes is performed using
563:. Vol. 26 (11th ed.). pp. 804–808.
474:(8th ed.). Prentice Hall. pp. 241–3.
382:Photoelectron photoion coincidence spectroscopy
583:
211:between products and reactants: dΔH / dT = ΔC
8:
129:. These experiments mark the foundation of
397:Thermodynamic databases for pure substances
590:
576:
568:
377:Important publications in thermochemistry
422:
186:(1845) and helped in its formulation.
7:
1505:
105:, used in the winter of 1782–83, by
1529:
121:; calculations which were based on
298:insulated closed piston or balloon
25:
460:by Frederick Hutton Getman (1918)
457:Outlines of Theoretical Chemistry
367:Differential scanning calorimetry
237:differential scanning calorimeter
1528:
1516:
1504:
1493:
1492:
336:
326:when no heat exchange occurs.
182:These statements preceded the
1:
857:Interface and colloid science
611:Glossary of chemical formulae
44:and/or phase changes such as
278:mechanically isolated system
1134:Bioorganometallic chemistry
621:List of inorganic compounds
434:. Oxford University Press.
407:Thomsen-Berthelot principle
184:first law of thermodynamics
1583:
1567:Branches of thermodynamics
1060:Dynamic covalent chemistry
1031:Enantioselective synthesis
1011:Physical organic chemistry
964:Organolanthanide chemistry
29:
1488:
649:Electroanalytical methods
606:
387:Principle of maximum work
264:thermally isolated system
1404:Nobel Prize in Chemistry
1320:Supramolecular chemistry
959:Organometallic chemistry
432:A to Z of Thermodynamics
1342:Combinatorial chemistry
1253:Food physical chemistry
1216:Environmental chemistry
1100:Bioorthogonal chemistry
1026:Retrosynthetic analysis
847:Chemical thermodynamics
830:Spectroelectrochemistry
773:Computational chemistry
560:Encyclopædia Britannica
554:"Thermochemistry"
507:Encyclopædia Britannica
430:Perrot, Pierre (1998).
139:chemical thermodynamics
32:chemical thermodynamics
1414:of element discoveries
1260:Agricultural chemistry
1248:Carbohydrate chemistry
1139:Bioinorganic chemistry
1004:Alkane stereochemistry
949:Coordination chemistry
778:Mathematical chemistry
644:Instrumental chemistry
134:
125:'s prior discovery of
1409:Timeline of chemistry
1306:Post-mortem chemistry
1291:Clandestine chemistry
1221:Atmospheric chemistry
1144:Biophysical chemistry
976:Solid-state chemistry
926:Equilibrium chemistry
835:Photoelectrochemistry
501:"Black, Joseph"
100:
64:Endothermic reactions
1399:History of chemistry
1354:Chemical engineering
1129:Bioorganic chemistry
879:Structural chemistry
616:List of biomolecules
392:Reaction Calorimeter
151:endergonic reactions
111:Pierre-Simon Laplace
68:exothermic reactions
30:For other uses, see
1422:The central science
1376:Ceramic engineering
1301:Forensic toxicology
1274:Chemistry education
1172:Radiation chemistry
1154:Interdisciplinarity
1107:Medicinal chemistry
1045:Fullerene chemistry
921:Microwave chemistry
790:Molecular mechanics
785:Molecular modelling
147:exergonic reactions
117:evolved in various
113:, to determine the
66:absorb heat, while
1562:Physical chemistry
1465:Chemical substance
1327:Chemical synthesis
1296:Forensic chemistry
1177:Actinide chemistry
1119:Clinical chemistry
800:Molecular geometry
795:Molecular dynamics
750:Elemental analysis
703:Separation process
372:Isodesmic reaction
135:
101:The world's first
76:heat of combustion
42:chemical reactions
1544:
1543:
1480:Quantum mechanics
1445:Chemical compound
1428:Chemical reaction
1366:Materials science
1284:General chemistry
1279:Amateur chemistry
1207:Photogeochemistry
1192:Stellar chemistry
1162:Nuclear chemistry
1083:Molecular biology
1050:Polymer chemistry
1021:Organic synthesis
1016:Organic reactions
981:Ceramic chemistry
971:Cluster chemistry
901:Chemical kinetics
889:Molecular physics
768:Quantum chemistry
681:Mass spectrometry
472:General Chemistry
357:Chemical kinetics
195:phase transitions
107:Antoine Lavoisier
80:heat of formation
16:(Redirected from
1574:
1532:
1531:
1520:
1508:
1507:
1496:
1495:
1440:Chemical element
1095:Chemical biology
954:Magnetochemistry
931:Mechanochemistry
884:Chemical physics
825:Electrochemistry
730:Characterization
592:
585:
578:
569:
564:
556:
536:
530:
524:
518:
512:
511:
503:
492:
486:
485:
467:
461:
454:See page 290 of
452:
446:
445:
427:
346:
341:
340:
286:bomb calorimeter
258:bomb calorimeter
205:Gustav Kirchhoff
119:chemical changes
21:
1582:
1581:
1577:
1576:
1575:
1573:
1572:
1571:
1557:Thermochemistry
1547:
1546:
1545:
1540:
1484:
1387:
1381:Polymer science
1337:Click chemistry
1332:Green chemistry
1226:Ocean chemistry
1202:Biogeochemistry
1148:
1064:
1036:Total synthesis
999:Stereochemistry
985:
935:
852:Surface science
842:Thermochemistry
811:
754:
725:Crystallography
630:
602:
596:
547:
544:
539:
531:
527:
519:
515:
494:
493:
489:
482:
469:
468:
464:
453:
449:
442:
429:
428:
424:
420:
342:
335:
332:
312:
254:isolated system
252:a (completely)
245:
221:
214:
159:
131:thermochemistry
103:ice-calorimeter
38:Thermochemistry
35:
28:
23:
22:
15:
12:
11:
5:
1580:
1578:
1570:
1569:
1564:
1559:
1549:
1548:
1542:
1541:
1539:
1538:
1526:
1514:
1502:
1489:
1486:
1485:
1483:
1482:
1477:
1472:
1467:
1462:
1457:
1452:
1447:
1442:
1437:
1436:
1435:
1425:
1418:
1417:
1416:
1406:
1401:
1395:
1393:
1389:
1388:
1386:
1385:
1384:
1383:
1378:
1373:
1363:
1362:
1361:
1351:
1350:
1349:
1344:
1339:
1334:
1324:
1323:
1322:
1311:
1310:
1309:
1308:
1303:
1293:
1288:
1287:
1286:
1281:
1270:
1269:
1268:
1267:
1265:Soil chemistry
1257:
1256:
1255:
1250:
1243:Food chemistry
1240:
1238:Carbochemistry
1235:
1233:Clay chemistry
1230:
1229:
1228:
1223:
1212:
1211:
1210:
1209:
1204:
1194:
1188:Astrochemistry
1184:Cosmochemistry
1181:
1180:
1179:
1174:
1169:
1167:Radiochemistry
1158:
1156:
1150:
1149:
1147:
1146:
1141:
1136:
1131:
1126:
1124:Neurochemistry
1121:
1116:
1115:
1114:
1104:
1103:
1102:
1092:
1091:
1090:
1085:
1074:
1072:
1066:
1065:
1063:
1062:
1057:
1055:Petrochemistry
1052:
1047:
1042:
1033:
1028:
1023:
1018:
1013:
1008:
1007:
1006:
995:
993:
987:
986:
984:
983:
978:
973:
968:
967:
966:
956:
951:
945:
943:
937:
936:
934:
933:
928:
923:
918:
916:Spin chemistry
913:
911:Photochemistry
908:
903:
898:
896:Femtochemistry
893:
892:
891:
881:
876:
871:
866:
865:
864:
854:
849:
844:
839:
838:
837:
832:
821:
819:
813:
812:
810:
809:
808:
807:
797:
792:
787:
782:
781:
780:
770:
764:
762:
756:
755:
753:
752:
747:
742:
737:
732:
727:
722:
721:
720:
715:
708:Chromatography
705:
700:
699:
698:
693:
688:
678:
677:
676:
671:
666:
661:
651:
646:
640:
638:
632:
631:
629:
628:
626:Periodic table
623:
618:
613:
607:
604:
603:
597:
595:
594:
587:
580:
572:
566:
565:
543:
542:External links
540:
538:
537:
525:
513:
498:, ed. (1911).
496:Chisholm, Hugh
487:
480:
462:
447:
440:
421:
419:
416:
415:
414:
412:Julius Thomsen
409:
404:
402:Thermodynamics
399:
394:
389:
384:
379:
374:
369:
364:
359:
354:
348:
347:
344:Science portal
331:
328:
311:
308:
307:
306:
299:
288:
274:
260:
244:
241:
220:
217:
212:
180:
179:
173:
158:
155:
26:
24:
18:Thermochemical
14:
13:
10:
9:
6:
4:
3:
2:
1579:
1568:
1565:
1563:
1560:
1558:
1555:
1554:
1552:
1537:
1536:
1527:
1525:
1524:
1519:
1515:
1513:
1512:
1503:
1501:
1500:
1491:
1490:
1487:
1481:
1478:
1476:
1473:
1471:
1470:Chemical bond
1468:
1466:
1463:
1461:
1458:
1456:
1453:
1451:
1448:
1446:
1443:
1441:
1438:
1434:
1431:
1430:
1429:
1426:
1423:
1419:
1415:
1412:
1411:
1410:
1407:
1405:
1402:
1400:
1397:
1396:
1394:
1390:
1382:
1379:
1377:
1374:
1372:
1369:
1368:
1367:
1364:
1360:
1359:Stoichiometry
1357:
1356:
1355:
1352:
1348:
1345:
1343:
1340:
1338:
1335:
1333:
1330:
1329:
1328:
1325:
1321:
1318:
1317:
1316:
1315:Nanochemistry
1313:
1312:
1307:
1304:
1302:
1299:
1298:
1297:
1294:
1292:
1289:
1285:
1282:
1280:
1277:
1276:
1275:
1272:
1271:
1266:
1263:
1262:
1261:
1258:
1254:
1251:
1249:
1246:
1245:
1244:
1241:
1239:
1236:
1234:
1231:
1227:
1224:
1222:
1219:
1218:
1217:
1214:
1213:
1208:
1205:
1203:
1200:
1199:
1198:
1195:
1193:
1189:
1185:
1182:
1178:
1175:
1173:
1170:
1168:
1165:
1164:
1163:
1160:
1159:
1157:
1155:
1151:
1145:
1142:
1140:
1137:
1135:
1132:
1130:
1127:
1125:
1122:
1120:
1117:
1113:
1110:
1109:
1108:
1105:
1101:
1098:
1097:
1096:
1093:
1089:
1086:
1084:
1081:
1080:
1079:
1076:
1075:
1073:
1071:
1067:
1061:
1058:
1056:
1053:
1051:
1048:
1046:
1043:
1041:
1040:Semisynthesis
1037:
1034:
1032:
1029:
1027:
1024:
1022:
1019:
1017:
1014:
1012:
1009:
1005:
1002:
1001:
1000:
997:
996:
994:
992:
988:
982:
979:
977:
974:
972:
969:
965:
962:
961:
960:
957:
955:
952:
950:
947:
946:
944:
942:
938:
932:
929:
927:
924:
922:
919:
917:
914:
912:
909:
907:
904:
902:
899:
897:
894:
890:
887:
886:
885:
882:
880:
877:
875:
874:Sonochemistry
872:
870:
869:Cryochemistry
867:
863:
862:Micromeritics
860:
859:
858:
855:
853:
850:
848:
845:
843:
840:
836:
833:
831:
828:
827:
826:
823:
822:
820:
818:
814:
806:
803:
802:
801:
798:
796:
793:
791:
788:
786:
783:
779:
776:
775:
774:
771:
769:
766:
765:
763:
761:
757:
751:
748:
746:
743:
741:
740:Wet chemistry
738:
736:
733:
731:
728:
726:
723:
719:
716:
714:
711:
710:
709:
706:
704:
701:
697:
694:
692:
689:
687:
684:
683:
682:
679:
675:
672:
670:
667:
665:
662:
660:
657:
656:
655:
652:
650:
647:
645:
642:
641:
639:
637:
633:
627:
624:
622:
619:
617:
614:
612:
609:
608:
605:
601:
593:
588:
586:
581:
579:
574:
573:
570:
562:
561:
555:
550:
549:Walker, James
546:
545:
541:
534:
529:
526:
522:
517:
514:
509:
508:
502:
497:
491:
488:
483:
481:0-13-014329-4
477:
473:
466:
463:
459:
458:
451:
448:
443:
441:0-19-856552-6
437:
433:
426:
423:
417:
413:
410:
408:
405:
403:
400:
398:
395:
393:
390:
388:
385:
383:
380:
378:
375:
373:
370:
368:
365:
363:
362:Cryochemistry
360:
358:
355:
353:
350:
349:
345:
339:
334:
329:
327:
325:
321:
317:
309:
304:
300:
297:
293:
292:closed system
289:
287:
283:
279:
275:
273:
269:
265:
261:
259:
255:
251:
250:
249:
242:
240:
238:
234:
230:
226:
218:
216:
210:
209:heat capacity
206:
202:
200:
196:
192:
187:
185:
177:
174:
171:
167:
164:
163:
162:
156:
154:
152:
148:
144:
140:
132:
128:
124:
120:
116:
112:
108:
104:
99:
95:
93:
89:
85:
81:
77:
73:
72:heat capacity
69:
65:
61:
59:
55:
51:
47:
43:
39:
33:
19:
1533:
1521:
1509:
1497:
1347:Biosynthesis
1197:Geochemistry
1112:Pharmacology
1088:Cell biology
1078:Biochemistry
906:Spectroscopy
841:
805:VSEPR theory
654:Spectroscopy
598:Branches of
558:
528:
521:Laidler K.J.
516:
505:
490:
471:
465:
455:
450:
431:
425:
313:
295:
281:
246:
233:thermocouple
222:
203:
199:Joseph Black
188:
181:
160:
136:
130:
123:Joseph Black
102:
62:
54:surroundings
37:
36:
1535:WikiProject
760:Theoretical
745:Calorimetry
352:Calorimetry
303:open system
229:thermometer
225:calorimetry
219:Calorimetry
191:latent heat
127:latent heat
92:free energy
1551:Categories
1371:Metallurgy
1070:Biological
636:Analytical
418:References
316:isothermal
284:insulated
1433:Catalysis
941:Inorganic
735:Titration
600:chemistry
533:Atkins P.
324:adiabatic
310:Processes
176:Hess' law
170:Laplace's
166:Lavoisier
1499:Category
1455:Molecule
1392:See also
817:Physical
551:(1911).
330:See also
320:isobaric
84:enthalpy
1511:Commons
1475:Alchemy
991:Organic
272:balloon
243:Systems
157:History
88:entropy
58:entropy
50:boiling
46:melting
1523:Portal
669:UV-Vis
478:
438:
268:piston
90:, and
696:MALDI
664:Raman
1450:Atom
718:HPLC
476:ISBN
436:ISBN
168:and
149:and
143:work
115:heat
109:and
48:and
1460:Ion
691:ICP
674:NMR
301:an
270:or
231:or
193:of
1553::
1190:/
1186:/
1038:/
713:GC
686:EI
659:IR
557:.
504:.
296:un
290:a
282:un
276:a
262:a
239:.
197:.
153:.
94:.
86:,
82:,
78:,
74:,
1424:"
1420:"
591:e
584:t
577:v
484:.
444:.
213:p
133:.
34:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.