804:
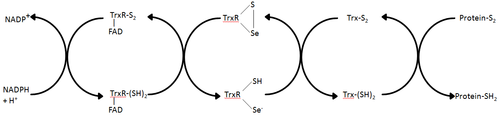
conditions. A second electron transfer from a second molecule of NADPH reduces the active site tihiol bonds with one Cys residue stabilized by an interaction with FAD (Step B). The selenolate anion then attacks the disulfide bonds of Trx and the resulting enzyme-Trx mixed selenenylsulfide (Step C), which is then subsequently attacked by the neighboring Cys residue to regenerate the selenenylsulfide (Step D). This selenenylsulfide is then reduced by the active-site thiolate from the other subunit (Step E). Adapted from Zhong et al. Consistent with findings that (2,2‘:6‘,2‘‘-terpyridine)platinum(II) complexes inhibit human TrxR.
706:
722:
781:
2811:
132:
from NADPH via TrxR and are transferred to the active site of Trx, which goes on to reduce protein disulfides or other substrates. The Trx system exists in all living cells and has an evolutionary history tied to DNA as a genetic material, defense against oxidative damage due to oxygen metabolism, and redox signaling using molecules like hydrogen peroxide and nitric oxide.
136:
905:. Inactivation of mitochondrial TrxR2 in mice results in thinning of the ventricular heart walls and neonatal death. Furthermore two mutations in the TrxR2 gene are found in patients diagnosed with DCM and not in a control population. It is hypothesized that the pathological impact of these mutations is an impaired ability to control oxidative damage in
852:. The disulfide-based TRFS series of fluorescent probes have shown selective detection of TrxR. Mafireyi synthesized the first diselenide probe that was applied in the detection of TrxR. Other detection methods include immunological techniques and the selenocystine-thioredoxin reductase assay (SC-TR assay).
131:
Thioredoxin reductases are enzymes that catalyze the reduction of thioredoxin and hence they are a central component in the thioredoxin system. Together with thioredoxin (Trx) and NADPH this system's most general description is as a system for reducing disulfide bonds in cells. Electrons are taken
803:
Starting from the completely oxidized form, the reaction begins with the reduction of the selenenylsulfide to the selenolate anion (Se(-1)) with electrons received from NADPH via FAD (Step A). Due to the low pKa value of the selenol the selenolate anion is the predominant form under physiological
823:
is rotated 66 degrees with the NADPH domain remaining fixed the two prosthetic groups move into close contact allowing electrons to pass from NADPH to FAD and then to the active site disulfide bond. The conserved active site residues in E. coli are -Cys-Ala-Thr-Cys-.
1987:
Sibbing D, Pfeufer A, Perisic T, Mannes AM, Fritz-Wolf K, Unwin S, Sinner MF, Gieger C, Gloeckner CJ, Wichmann HE, Kremmer E, Schäfer Z, Walch A, Hinterseer M, Näbauer M, Kääb S, Kastrati A, Schömig A, Meitinger T, Bornkamm GW, Conrad M, von
Beckerath N (May 2011).
840:. An additional feature of the mammalian mechanism is the presence of a selenocysteine residue at the C-terminal end of the protein which is required for catalytic activity. The conserved residues in mammalian active site are -Cys-Val-Asn-Val-Gly-Cys-.
797:
690:
but they relative orientation of these domains in ThxR is rotated by 66 degrees. This becomes significant in the enzyme mechanism of action which is described below. ThxR homo-dimerizes with the interface between the two monomers formed by three
107:(Trx). Two classes of thioredoxin reductase have been identified: one class in bacteria and some eukaryotes and one in animals. In bacteria TrxR also catalyzes the reduction of glutaredoxin like proteins known as NrdH. Both classes are
182:
These two classes of TrxR have only ~20% sequence identity in the section of primary sequence where they can be reliably aligned. The net reaction of both classes of TrxR is identical but the mechanism of action of each is distinct.
1552:
Becker K, Herold-Mende C, Park JJ, Lowe G, Schirmer RH (Aug 2001). "Human thioredoxin reductase is efficiently inhibited by (2,2':6',2' '-terpyridine)platinum(II) complexes. Possible implications for a novel antitumor strategy".
1258:"The mechanism of thioredoxin reductase from human placenta is similar to the mechanisms of lipoamide dehydrogenase and glutathione reductase and is distinct from the mechanism of thioredoxin reductase from Escherichia coli"
757:
binding domain between two alpha helices which forms a small pair of beta strands. The active disulfide in the enzyme is located on one of these helices and thus the active disulfide bond is located in the
865:
Since the activity of this enzyme is essential for cell growth and survival, it is a good target for anti-tumor therapy. Furthermore, the enzyme is upregulated in several types of cancer, including
1901:
Nilsonne G, Sun X, Nyström C, Rundlöf AK, Potamitou
Fernandes A, Björnstedt M, Dobra K (Sep 2006). "Selenite induces apoptosis in sarcomatoid malignant mesothelioma cells through oxidative stress".
917:
There has recently been some research to show that low molecular weight thioredoxin reductase could be a target for novel antibiotics (such as auranofin or
Ebselen.) This is especially true for
1717:
Zhao J, Qu Y, Gao H, Zhong M, Li X, Zhang F, et al. (November 2020). "Loss of thioredoxin reductase function in a mouse stroke model disclosed by a two-photon fluorescent probe".
1495:"Structure and mechanism of mammalian thioredoxin reductase: the active site is a redox-active selenolthiol/selenenylsulfide formed from the conserved cysteine-selenocysteine sequence"
1760:
Liu Y, Ma H, Zhang L, Cui Y, Liu X, Fang J (February 2016). "A small molecule probe reveals declined mitochondrial thioredoxin reductase activity in a
Parkinson's disease model".
1318:
Conrad M, Jakupoglu C, Moreno SG, Lippl S, Banjac A, Schneider M, Beck H, Hatzopoulos AK, Just U, Sinowatz F, Schmahl W, Chien KR, Wurst W, Bornkamm GW, Brielmeier M (Nov 2004).
819:
ThxR the spatial orientation of the FAD and NADPH domains are such that the redox-active rings of FAD and NADPH are not in close proximity to each other. When the FAD domain of
836:. The active-site Cys residues in the FAD domain and bound NADPH domain are in close proximity removing the necessity for a 66 degree rotation for electron transfer found in
721:
2313:
190:(TrxR1, cytosolic), thioredoxin reductase 2 (TrxR2, mitochondrial), thioredoxin reductase 3 (TrxR3, testis specific). Each isozyme is encoded by a separate gene:
522:
371:
220:
2437:
873:(MGd) is a new chemotherapeutic agent that selectively targets tumor cells, leading to cell death and apoptosis via inhibition of thioredoxin reductase and
2343:
780:
705:
2393:
2181:
1436:"Three-dimensional structure of a mammalian thioredoxin reductase: implications for mechanism and evolution of a selenocysteine-dependent enzyme"
1036:
Phulera S, Mande SC (June 2013). "The crystal structure of
Mycobacterium tuberculosis NrdH at 0.87 Å suggests a possible mode of its activity".
2478:
2836:
2831:
2459:
64:
2841:
2306:
2255:
546:
395:
244:
2530:
2339:
1682:
Ma H, Zhang J, Zhang Z, Liu Y, Fang J (October 2016). "A fast response and red emission probe for mammalian thioredoxin reductase".
164:
534:
383:
232:
1964:
1947:
527:
2299:
1109:
Hirt RP, Müller S, Embley TM, Coombs GH (July 2002). "The diversity and evolution of thioredoxin reductase: new perspectives".
376:
225:
2351:
2240:
172:
2686:
1186:
Meyer Y, Buchanan BB, Vignols F, Reichheld JP (2009). "Thioredoxins and glutaredoxins: unifying elements in redox biology".
893:. Thioredoxin reductases are essential proteins for regulating cellular redox balance and mitigating the damage caused by
2801:
2174:
759:
754:
746:
671:
112:
2671:
2787:
2774:
2761:
2748:
2735:
2722:
2709:
2488:
2469:
2447:
2425:
2403:
2388:
2371:
2335:
2220:
2215:
997:"Characterization of Escherichia coli NrdH. A glutaredoxin-like protein with a thioredoxin-like activity profile"
898:
2681:
1320:"Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function"
2635:
2578:
2326:
2153:
890:
874:
587:
436:
285:
97:
753:
binding domain, and an interface between two monomer subunits. In mammalian ThxR there is an insertion in the
2149:
1588:
Lennon BW, Williams CH (Aug 1997). "Reductive half-reaction of thioredoxin reductase from
Escherichia coli".
159:
residue in its active site has been identified in higher eukaryotes including humans. This TxR is related to
2583:
2250:
2235:
2230:
894:
687:
1221:
Lillig CH, Holmgren A (Jan 2007). "Thioredoxin and related molecules--from biology to health and disease".
2210:
2167:
886:
866:
539:
388:
237:
1625:"A fast and specific fluorescent probe for thioredoxin reductase that works via disulphide bond cleavage"
2604:
2523:
2383:
2356:
2205:
1072:
683:
453:
302:
160:
2676:
178:
A low molecular weight (MW = ~ 35,000) type has been identified in archaea, bacteria and other eukarya.
2101:
1808:
1636:
1506:
1447:
1269:
870:
604:
77:
2640:
2291:
2039:
Marshall AC, Kidd SE, Lamont-Friedrich SJ, Arentz G, Hoffmann P, Coad BR, Bruning JB (March 2019).
849:
2573:
2260:
1842:
1742:
1413:
1149:"Thioredoxin and thioredoxin reductase: current research with special reference to human disease"
168:
1948:"Up-regulation of thioredoxin and thioredoxin reductase in human malignant pleural mesothelioma"
1990:"Mutations in the mitochondrial thioredoxin reductase gene TXNRD2 cause dilated cardiomyopathy"
1946:
Kahlos K, Soini Y, Säily M, Koistinen P, Kakko S, Pääkkö P, Holmgren A, Kinnula VL (May 2001).
2846:
2497:
2275:
2270:
2129:
2090:"Auranofin exerts broad-spectrum bactericidal activities by targeting thiol-redox homeostasis"
2070:
2021:
1969:
1928:
1883:
1834:
1777:
1734:
1699:
1664:
1605:
1570:
1534:
1475:
1405:
1349:
1297:
1238:
1203:
1168:
1126:
1084:
1053:
1018:
977:
457:
2619:
2614:
2588:
2516:
2119:
2109:
2060:
2052:
2011:
2001:
1959:
1918:
1910:
1873:
1824:
1816:
1769:
1726:
1691:
1654:
1644:
1597:
1562:
1524:
1514:
1465:
1455:
1395:
1385:
1339:
1331:
1287:
1277:
1230:
1195:
1160:
1118:
1045:
1008:
967:
959:
599:
448:
297:
2666:
2650:
2563:
2415:
2016:
628:
592:
477:
441:
326:
306:
290:
608:
2105:
1914:
1812:
1640:
1510:
1451:
1273:
1199:
848:
Thioredoxin reductase can be quantified by various methods such as the DTNB assay using
2815:
2704:
2645:
2322:
2124:
2089:
2065:
2040:
1829:
1796:
1659:
1624:
972:
951:
906:
156:
120:
1344:
1319:
1122:
2825:
2609:
2568:
2265:
1846:
1795:
Mafireyi TJ, Laws M, Bassett JW, Cassidy PB, Escobedo JO, Strongin RM (August 2020).
1746:
1529:
1494:
1470:
1435:
1335:
1292:
1257:
832:
Mammalian TrxRs have a much higher sequence homology with glutathione reductase than
692:
108:
69:
1417:
2558:
2225:
2194:
2088:
Harbut MB, Vilchèze C, Luo X, Hensler ME, Guo H, Yang B, et al. (April 2015).
1797:"A Diselenide Turn-On Fluorescent Probe for the Detection of Thioredoxin Reductase"
902:
1878:
1861:
563:
412:
261:
45:
2782:
2717:
2553:
1390:
1373:
963:
771:
696:
638:
487:
336:
104:
2810:
2094:
Proceedings of the
National Academy of Sciences of the United States of America
2006:
1989:
1649:
1499:
Proceedings of the
National Academy of Sciences of the United States of America
1440:
Proceedings of the
National Academy of Sciences of the United States of America
1262:
Proceedings of the
National Academy of Sciences of the United States of America
1164:
796:
2375:
2245:
679:
570:
419:
268:
1887:
1088:
1013:
996:
2756:
2730:
2429:
2114:
2133:
2074:
2025:
1973:
1932:
1838:
1820:
1781:
1738:
1703:
1668:
1574:
1538:
1519:
1479:
1460:
1353:
1282:
1242:
1207:
1172:
1130:
1057:
981:
678:. The connection between these two domains is a two-stranded anti-parallel
1609:
1409:
1400:
1301:
1234:
1022:
135:
73:
2159:
2056:
575:
424:
273:
40:
1374:"Mechanism and structure of thioredoxin reductase from Escherichia coli"
682:. Each domain individually is very similar to the analogous domains in
2451:
2190:
1965:
10.1002/1097-0215(20010520)95:3<198::AID-IJC1034>3.0.CO;2-F
1923:
1773:
1730:
1695:
1434:
Sandalova T, Zhong L, Lindqvist Y, Holmgren A, Schneider G (Aug 2001).
100:
52:
1601:
1566:
1049:
2769:
2539:
2407:
1256:
Arscott LD, Gromer S, Schirmer RH, Becker K, Williams CH (Apr 1997).
558:
407:
256:
213:
187:
57:
1623:
Li X, Zhang B, Yan C, Li J, Wang S, Wei X, et al. (June 2019).
1148:
2743:
995:
Jordan A, Aslund F, Pontis E, Reichard P, Holmgren A (July 1997).
763:
750:
675:
134:
116:
151:
Two classes of thioredoxin reductase have evolved independently:
551:
400:
249:
2512:
2295:
2163:
695:
and two loops. Each monomer can separately bind a molecule of
119:
binding domain, and an active site containing a redox-active
2508:
155:
A high molecular weight (MW = ~55,000) type containing a
1862:"Fluorogenic probes for thioredoxin reductase activity"
1071:
Phulera S, Akif M, Sardesai AA, Mande SC (2014-01-01).
921:, and could be used for antibiotic resistant bacteria.
786:
Structure of human ThxR FAD and NADPH prosthetic groups
111:
which function as homodimers. Each monomer contains a
2799:
801:
Proposed mechanism in mammals and presumably humans:
186:
Humans express three thioredoxin reductase isozymes:
1860:
Mafireyi TJ, Escobedo JO, Strongin RM (2021-03-29).
2695:
2659:
2628:
2597:
2546:
2487:
2468:
2446:
2424:
2402:
2370:
2334:
1153:
Biochemical and Biophysical Research Communications
634:
624:
619:
598:
586:
581:
569:
557:
545:
533:
521:
513:
508:
503:
483:
473:
468:
447:
435:
430:
418:
406:
394:
382:
370:
362:
357:
352:
332:
322:
317:
296:
284:
279:
267:
255:
243:
231:
219:
209:
204:
199:
63:
51:
39:
31:
26:
21:
731:ThxR with FAD and NADPH prosthetic groups labeled
1429:
1427:
1313:
1311:
1367:
1365:
1363:
1142:
1140:
1073:"Redox Proteins of Mycobacterium tuberculosis"
945:
943:
941:
939:
937:
935:
933:
2524:
2307:
2175:
2041:"Aspergillus fumigatus Thioredoxin Reductase"
8:
670:ThxR there are two binding domains, one for
1104:
1102:
1100:
1098:
2531:
2517:
2509:
2314:
2300:
2292:
2182:
2168:
2160:
1493:Zhong L, Arnér ES, Holmgren A (May 2000).
1077:Journal of the Indian Institute of Science
616:
465:
314:
2152:at the U.S. National Library of Medicine
2123:
2113:
2064:
2015:
2005:
1963:
1922:
1877:
1828:
1658:
1648:
1528:
1518:
1469:
1459:
1399:
1389:
1343:
1291:
1281:
1012:
971:
140:Schematic diagram of TrxR's cellular role
2438:Glutathione—homocystine transhydrogenase
795:
2806:
2394:Flavocytochrome c sulfide dehydrogenase
929:
776:
741:Mammalian TrxR structure is similar to
701:
950:Mustacich D, Powis G (February 2000).
500:
349:
196:
18:
2460:Glutathione dehydrogenase (ascorbate)
2045:Antimicrobial Agents and Chemotherapy
7:
889:) is a common diagnosis in cases of
2256:Methylenetetrahydrofolate reductase
1915:10.1016/j.freeradbiomed.2006.04.031
1903:Free Radical Biology & Medicine
1200:10.1146/annurev-genet-102108-134201
1001:The Journal of Biological Chemistry
1223:Antioxidants & Redox Signaling
14:
2479:CoB—CoM heterodisulfide reductase
2809:
1336:10.1128/MCB.24.21.9414-9423.2004
779:
720:
704:
1952:International Journal of Cancer
2352:Dihydrolipoamide dehydrogenase
2241:Dihydrolipoamide dehydrogenase
2017:11858/00-001M-0000-0024-1F10-3
1555:Journal of Medicinal Chemistry
1324:Molecular and Cellular Biology
1:
2150:Thioredoxin+Reductase+(NADPH)
1147:Holmgren A, Lu J (May 2010).
1123:10.1016/S1471-4922(02)02293-6
2837:Genes on human chromosome 22
2832:Genes on human chromosome 12
1879:10.1016/j.rechem.2021.100127
715:ThxR dimer bound thioredoxin
142:Adapted from Holmgren et al.
2842:Genes on human chromosome 3
1391:10.1096/fasebj.9.13.7557016
2863:
2325:: sulfur oxidoreductases (
1650:10.1038/s41467-019-10807-8
1165:10.1016/j.bbrc.2010.03.083
103:) are enzymes that reduce
2687:Michaelis–Menten kinetics
2389:Thiosulfate dehydrogenase
2221:Butyryl CoA dehydrogenase
2216:Apoptosis-inducing factor
2201:
1188:Annual Review of Genetics
964:10.1042/0264-6021:3460001
919:Mycobacterium Haemophilum
899:oxidative phosphorylation
615:
464:
313:
2579:Diffusion-limited enzyme
2154:Medical Subject Headings
2007:10.1093/eurheartj/ehq507
1372:Williams CH (Oct 1995).
1014:10.1074/jbc.272.29.18044
891:congestive heart failure
885:Dilated cardiomyopathy (
875:ribonucleotide reductase
2251:Methemoglobin reductase
2236:Cytokinin dehydrogenase
2231:Cytochrome b5 reductase
2115:10.1073/pnas.1504022112
1762:Chemical Communications
1719:Chemical Communications
1684:Chemical Communications
956:The Biochemical Journal
952:"Thioredoxin reductase"
895:reactive oxygen species
688:lipoamide dehydrogenase
504:thioredoxin reductase 3
353:thioredoxin reductase 2
200:thioredoxin reductase 1
188:thioredoxin reductase 1
173:lipoamide dehydrogenase
165:trypanothione reductase
2211:Acyl CoA dehydrogenase
1994:European Heart Journal
1821:10.1002/ange.202004094
1520:10.1073/pnas.100114897
1461:10.1073/pnas.171178698
1283:10.1073/pnas.94.8.3621
1111:Trends in Parasitology
867:malignant mesothelioma
805:
143:
86:Thioredoxin reductases
2672:Eadie–Hofstee diagram
2605:Allosteric regulation
2384:Sulfite dehydrogenase
2362:Thioredoxin reductase
2357:Glutathione reductase
2281:Thioredoxin reductase
2206:Acetolactate synthase
1629:Nature Communications
1235:10.1089/ars.2007.9.25
958:. 346 Pt 1 (1): 1–8.
856:Clinical significance
799:
684:glutathione reductase
161:glutathione reductase
138:
22:Thioredoxin reductase
2682:Lineweaver–Burk plot
2057:10.1128/AAC.02281-18
1866:Results in Chemistry
871:motexafin gadolinium
115:prosthetic group, a
2106:2015PNAS..112.4453H
1813:2020AngCh.13215259M
1807:(35): 15147–15151.
1725:(90): 14075–14078.
1690:(81): 12060–12063.
1641:2019NatCo..10.2745L
1511:2000PNAS...97.5854Z
1452:2001PNAS...98.9533S
1274:1997PNAS...94.3621A
762:domain and not the
2641:Enzyme superfamily
2574:Enzyme promiscuity
2261:NADH dehydrogenase
1774:10.1039/c5cc09998f
1731:10.1039/D0CC05900E
1696:10.1039/C6CC04984B
806:
169:mercuric reductase
144:
2797:
2796:
2506:
2505:
2498:Sulfite reductase
2289:
2288:
2276:Sarcosine oxidase
2271:Nitrate reductase
1801:Angewandte Chemie
1602:10.1021/bi970307j
1567:10.1021/jm001014i
1050:10.1021/bi400191z
844:Detection methods
652:
651:
648:
647:
644:
643:
497:
496:
493:
492:
346:
345:
342:
341:
83:
82:
2854:
2814:
2813:
2805:
2677:Hanes–Woolf plot
2620:Enzyme activator
2615:Enzyme inhibitor
2589:Enzyme catalysis
2533:
2526:
2519:
2510:
2316:
2309:
2302:
2293:
2184:
2177:
2170:
2161:
2138:
2137:
2127:
2117:
2085:
2079:
2078:
2068:
2036:
2030:
2029:
2019:
2009:
1984:
1978:
1977:
1967:
1943:
1937:
1936:
1926:
1898:
1892:
1891:
1881:
1857:
1851:
1850:
1832:
1792:
1786:
1785:
1757:
1751:
1750:
1714:
1708:
1707:
1679:
1673:
1672:
1662:
1652:
1620:
1614:
1613:
1585:
1579:
1578:
1549:
1543:
1542:
1532:
1522:
1490:
1484:
1483:
1473:
1463:
1431:
1422:
1421:
1403:
1393:
1369:
1358:
1357:
1347:
1315:
1306:
1305:
1295:
1285:
1253:
1247:
1246:
1218:
1212:
1211:
1183:
1177:
1176:
1144:
1135:
1134:
1106:
1093:
1092:
1068:
1062:
1061:
1033:
1027:
1026:
1016:
1007:(29): 18044–50.
992:
986:
985:
975:
947:
907:cardiac myocytes
861:Cancer treatment
850:Ellman's reagent
783:
745:. It contains a
724:
708:
674:and another for
617:
501:
466:
350:
315:
197:
193:
192:
19:
16:Class of enzymes
2862:
2861:
2857:
2856:
2855:
2853:
2852:
2851:
2822:
2821:
2820:
2808:
2800:
2798:
2793:
2705:Oxidoreductases
2691:
2667:Enzyme kinetics
2655:
2651:List of enzymes
2624:
2593:
2564:Catalytic triad
2542:
2537:
2507:
2502:
2483:
2464:
2442:
2420:
2416:Sulfite oxidase
2398:
2366:
2330:
2323:Oxidoreductases
2320:
2290:
2285:
2197:
2188:
2146:
2141:
2087:
2086:
2082:
2038:
2037:
2033:
1986:
1985:
1981:
1945:
1944:
1940:
1900:
1899:
1895:
1859:
1858:
1854:
1794:
1793:
1789:
1759:
1758:
1754:
1716:
1715:
1711:
1681:
1680:
1676:
1622:
1621:
1617:
1596:(31): 9464–77.
1587:
1586:
1582:
1561:(17): 2784–92.
1551:
1550:
1546:
1492:
1491:
1487:
1433:
1432:
1425:
1384:(13): 1267–76.
1371:
1370:
1361:
1330:(21): 9414–23.
1317:
1316:
1309:
1255:
1254:
1250:
1220:
1219:
1215:
1185:
1184:
1180:
1146:
1145:
1138:
1108:
1107:
1096:
1070:
1069:
1065:
1044:(23): 4056–65.
1035:
1034:
1030:
994:
993:
989:
949:
948:
931:
927:
915:
883:
869:. For example,
863:
858:
846:
830:
813:
794:
787:
784:
739:
732:
725:
716:
709:
664:
657:
149:
129:
17:
12:
11:
5:
2860:
2858:
2850:
2849:
2844:
2839:
2834:
2824:
2823:
2819:
2818:
2795:
2794:
2792:
2791:
2778:
2765:
2752:
2739:
2726:
2713:
2699:
2697:
2693:
2692:
2690:
2689:
2684:
2679:
2674:
2669:
2663:
2661:
2657:
2656:
2654:
2653:
2648:
2643:
2638:
2632:
2630:
2629:Classification
2626:
2625:
2623:
2622:
2617:
2612:
2607:
2601:
2599:
2595:
2594:
2592:
2591:
2586:
2581:
2576:
2571:
2566:
2561:
2556:
2550:
2548:
2544:
2543:
2538:
2536:
2535:
2528:
2521:
2513:
2504:
2503:
2501:
2500:
2494:
2492:
2485:
2484:
2482:
2481:
2475:
2473:
2472:: Other, known
2466:
2465:
2463:
2462:
2456:
2454:
2444:
2443:
2441:
2440:
2434:
2432:
2422:
2421:
2419:
2418:
2412:
2410:
2400:
2399:
2397:
2396:
2391:
2386:
2380:
2378:
2368:
2367:
2365:
2364:
2359:
2354:
2348:
2346:
2332:
2331:
2321:
2319:
2318:
2311:
2304:
2296:
2287:
2286:
2284:
2283:
2278:
2273:
2268:
2263:
2258:
2253:
2248:
2243:
2238:
2233:
2228:
2223:
2218:
2213:
2208:
2202:
2199:
2198:
2189:
2187:
2186:
2179:
2172:
2164:
2158:
2157:
2145:
2144:External links
2142:
2140:
2139:
2100:(14): 4453–8.
2080:
2031:
2000:(9): 1121–33.
1979:
1958:(3): 198–204.
1938:
1893:
1852:
1787:
1768:(11): 2296–9.
1752:
1709:
1674:
1615:
1580:
1544:
1505:(11): 5854–9.
1485:
1446:(17): 9533–8.
1423:
1401:2027.42/154540
1359:
1307:
1248:
1213:
1178:
1136:
1094:
1083:(1): 127–138.
1063:
1028:
987:
928:
926:
923:
914:
911:
897:generated via
882:
881:Cardiomyopathy
879:
862:
859:
857:
854:
845:
842:
829:
826:
812:
807:
793:
790:
789:
788:
785:
778:
738:
735:
734:
733:
726:
719:
717:
710:
703:
663:
658:
656:
653:
650:
649:
646:
645:
642:
641:
636:
632:
631:
626:
622:
621:
613:
612:
602:
596:
595:
590:
584:
583:
579:
578:
573:
567:
566:
561:
555:
554:
549:
543:
542:
537:
531:
530:
525:
519:
518:
515:
511:
510:
506:
505:
498:
495:
494:
491:
490:
485:
481:
480:
475:
471:
470:
462:
461:
451:
445:
444:
439:
433:
432:
428:
427:
422:
416:
415:
410:
404:
403:
398:
392:
391:
386:
380:
379:
374:
368:
367:
364:
360:
359:
355:
354:
347:
344:
343:
340:
339:
334:
330:
329:
324:
320:
319:
311:
310:
300:
294:
293:
288:
282:
281:
277:
276:
271:
265:
264:
259:
253:
252:
247:
241:
240:
235:
229:
228:
223:
217:
216:
211:
207:
206:
202:
201:
180:
179:
176:
157:selenocysteine
148:
145:
128:
125:
121:disulfide bond
81:
80:
67:
61:
60:
55:
49:
48:
43:
37:
36:
33:
29:
28:
24:
23:
15:
13:
10:
9:
6:
4:
3:
2:
2859:
2848:
2845:
2843:
2840:
2838:
2835:
2833:
2830:
2829:
2827:
2817:
2812:
2807:
2803:
2789:
2785:
2784:
2779:
2776:
2772:
2771:
2766:
2763:
2759:
2758:
2753:
2750:
2746:
2745:
2740:
2737:
2733:
2732:
2727:
2724:
2720:
2719:
2714:
2711:
2707:
2706:
2701:
2700:
2698:
2694:
2688:
2685:
2683:
2680:
2678:
2675:
2673:
2670:
2668:
2665:
2664:
2662:
2658:
2652:
2649:
2647:
2646:Enzyme family
2644:
2642:
2639:
2637:
2634:
2633:
2631:
2627:
2621:
2618:
2616:
2613:
2611:
2610:Cooperativity
2608:
2606:
2603:
2602:
2600:
2596:
2590:
2587:
2585:
2582:
2580:
2577:
2575:
2572:
2570:
2569:Oxyanion hole
2567:
2565:
2562:
2560:
2557:
2555:
2552:
2551:
2549:
2545:
2541:
2534:
2529:
2527:
2522:
2520:
2515:
2514:
2511:
2499:
2496:
2495:
2493:
2490:
2486:
2480:
2477:
2476:
2474:
2471:
2467:
2461:
2458:
2457:
2455:
2453:
2449:
2445:
2439:
2436:
2435:
2433:
2431:
2427:
2423:
2417:
2414:
2413:
2411:
2409:
2405:
2401:
2395:
2392:
2390:
2387:
2385:
2382:
2381:
2379:
2377:
2373:
2369:
2363:
2360:
2358:
2355:
2353:
2350:
2349:
2347:
2345:
2341:
2337:
2333:
2328:
2324:
2317:
2312:
2310:
2305:
2303:
2298:
2297:
2294:
2282:
2279:
2277:
2274:
2272:
2269:
2267:
2266:NADPH oxidase
2264:
2262:
2259:
2257:
2254:
2252:
2249:
2247:
2244:
2242:
2239:
2237:
2234:
2232:
2229:
2227:
2224:
2222:
2219:
2217:
2214:
2212:
2209:
2207:
2204:
2203:
2200:
2196:
2195:flavoproteins
2192:
2185:
2180:
2178:
2173:
2171:
2166:
2165:
2162:
2155:
2151:
2148:
2147:
2143:
2135:
2131:
2126:
2121:
2116:
2111:
2107:
2103:
2099:
2095:
2091:
2084:
2081:
2076:
2072:
2067:
2062:
2058:
2054:
2050:
2046:
2042:
2035:
2032:
2027:
2023:
2018:
2013:
2008:
2003:
1999:
1995:
1991:
1983:
1980:
1975:
1971:
1966:
1961:
1957:
1953:
1949:
1942:
1939:
1934:
1930:
1925:
1920:
1916:
1912:
1909:(6): 874–85.
1908:
1904:
1897:
1894:
1889:
1885:
1880:
1875:
1871:
1867:
1863:
1856:
1853:
1848:
1844:
1840:
1836:
1831:
1826:
1822:
1818:
1814:
1810:
1806:
1802:
1798:
1791:
1788:
1783:
1779:
1775:
1771:
1767:
1763:
1756:
1753:
1748:
1744:
1740:
1736:
1732:
1728:
1724:
1720:
1713:
1710:
1705:
1701:
1697:
1693:
1689:
1685:
1678:
1675:
1670:
1666:
1661:
1656:
1651:
1646:
1642:
1638:
1634:
1630:
1626:
1619:
1616:
1611:
1607:
1603:
1599:
1595:
1591:
1584:
1581:
1576:
1572:
1568:
1564:
1560:
1556:
1548:
1545:
1540:
1536:
1531:
1526:
1521:
1516:
1512:
1508:
1504:
1500:
1496:
1489:
1486:
1481:
1477:
1472:
1467:
1462:
1457:
1453:
1449:
1445:
1441:
1437:
1430:
1428:
1424:
1419:
1415:
1411:
1407:
1402:
1397:
1392:
1387:
1383:
1379:
1378:FASEB Journal
1375:
1368:
1366:
1364:
1360:
1355:
1351:
1346:
1341:
1337:
1333:
1329:
1325:
1321:
1314:
1312:
1308:
1303:
1299:
1294:
1289:
1284:
1279:
1275:
1271:
1268:(8): 3621–6.
1267:
1263:
1259:
1252:
1249:
1244:
1240:
1236:
1232:
1228:
1224:
1217:
1214:
1209:
1205:
1201:
1197:
1193:
1189:
1182:
1179:
1174:
1170:
1166:
1162:
1158:
1154:
1150:
1143:
1141:
1137:
1132:
1128:
1124:
1120:
1116:
1112:
1105:
1103:
1101:
1099:
1095:
1090:
1086:
1082:
1078:
1074:
1067:
1064:
1059:
1055:
1051:
1047:
1043:
1039:
1032:
1029:
1024:
1020:
1015:
1010:
1006:
1002:
998:
991:
988:
983:
979:
974:
969:
965:
961:
957:
953:
946:
944:
942:
940:
938:
936:
934:
930:
924:
922:
920:
912:
910:
908:
904:
900:
896:
892:
888:
880:
878:
876:
872:
868:
860:
855:
853:
851:
843:
841:
839:
835:
827:
825:
822:
818:
811:
808:
802:
798:
791:
782:
777:
775:
773:
769:
766:domain as in
765:
761:
756:
752:
748:
744:
736:
730:
727:Structure of
723:
718:
714:
711:Structure of
707:
702:
700:
698:
694:
693:alpha-helices
689:
685:
681:
677:
673:
669:
662:
659:
654:
640:
637:
633:
630:
627:
623:
618:
614:
611:
610:
606:
603:
601:
597:
594:
591:
589:
585:
580:
577:
574:
572:
568:
565:
562:
560:
556:
553:
550:
548:
544:
541:
538:
536:
532:
529:
526:
524:
520:
516:
512:
507:
502:
499:
489:
486:
482:
479:
476:
472:
467:
463:
460:
459:
455:
452:
450:
446:
443:
440:
438:
434:
429:
426:
423:
421:
417:
414:
411:
409:
405:
402:
399:
397:
393:
390:
387:
385:
381:
378:
375:
373:
369:
365:
361:
356:
351:
348:
338:
335:
331:
328:
325:
321:
316:
312:
309:
308:
304:
301:
299:
295:
292:
289:
287:
283:
278:
275:
272:
270:
266:
263:
260:
258:
254:
251:
248:
246:
242:
239:
236:
234:
230:
227:
224:
222:
218:
215:
212:
208:
203:
198:
195:
194:
191:
189:
184:
177:
174:
170:
166:
162:
158:
154:
153:
152:
146:
141:
137:
133:
127:Cellular role
126:
124:
122:
118:
114:
110:
109:flavoproteins
106:
102:
99:
95:
91:
87:
79:
75:
71:
68:
66:
62:
59:
56:
54:
50:
47:
44:
42:
38:
34:
30:
25:
20:
2783:Translocases
2780:
2767:
2754:
2741:
2728:
2718:Transferases
2715:
2702:
2559:Binding site
2361:
2280:
2226:Cryptochrome
2097:
2093:
2083:
2048:
2044:
2034:
1997:
1993:
1982:
1955:
1951:
1941:
1906:
1902:
1896:
1869:
1865:
1855:
1804:
1800:
1790:
1765:
1761:
1755:
1722:
1718:
1712:
1687:
1683:
1677:
1632:
1628:
1618:
1593:
1590:Biochemistry
1589:
1583:
1558:
1554:
1547:
1502:
1498:
1488:
1443:
1439:
1381:
1377:
1327:
1323:
1265:
1261:
1251:
1229:(1): 25–47.
1226:
1222:
1216:
1191:
1187:
1181:
1159:(1): 120–4.
1156:
1152:
1117:(7): 302–8.
1114:
1110:
1080:
1076:
1066:
1041:
1038:Biochemistry
1037:
1031:
1004:
1000:
990:
955:
918:
916:
903:mitochondria
884:
864:
847:
837:
833:
831:
820:
816:
814:
809:
800:
767:
742:
740:
728:
712:
667:
665:
660:
607:
456:
305:
185:
181:
150:
139:
130:
93:
89:
85:
84:
2554:Active site
1924:10616/47514
1635:(1): 2745.
772:prokaryotes
697:thioredoxin
629:Swiss-model
509:Identifiers
478:Swiss-model
358:Identifiers
327:Swiss-model
205:Identifiers
105:thioredoxin
27:Identifiers
2826:Categories
2757:Isomerases
2731:Hydrolases
2598:Regulation
2376:cytochrome
2246:Flavodoxin
1872:: 100127.
1194:: 335–67.
925:References
913:Antibiotic
770:and other
625:Structures
620:Search for
609:p13-q13.33
582:Other data
474:Structures
469:Search for
431:Other data
323:Structures
318:Search for
280:Other data
2636:EC number
2430:disulfide
1888:2211-7156
1847:229142596
1747:225082279
1089:0970-4140
828:Mammalian
792:Mechanism
737:Mammalian
655:Structure
588:EC number
564:XM_051264
523:NCBI gene
437:EC number
413:NM_006440
372:NCBI gene
307:q23-q24.1
286:EC number
262:NM_003330
221:NCBI gene
147:Diversity
46:IPR005982
2847:EC 1.8.1
2660:Kinetics
2584:Cofactor
2547:Activity
2134:25831516
2075:30642940
2026:21247928
1974:11307155
1933:16934670
1839:32449244
1782:26725656
1739:33107534
1704:27709154
1669:31227705
1575:11495589
1539:10801974
1480:11481439
1418:26055087
1354:15485910
1243:17115886
1208:19691428
1173:20494123
1131:12379950
1058:23675692
982:10657232
639:InterPro
488:InterPro
337:InterPro
41:InterPro
2816:Biology
2770:Ligases
2540:Enzymes
2491:: Other
2452:quinone
2191:Protein
2125:4394260
2102:Bibcode
2066:6395915
1830:9438933
1809:Bibcode
1660:6588570
1637:Bibcode
1610:9235991
1507:Bibcode
1448:Bibcode
1410:7557016
1302:9108027
1270:Bibcode
1023:9218434
973:1220815
901:in the
838:E. coli
834:E. coli
821:E. coli
817:E. coli
810:E. coli
768:E. coli
743:E. coli
729:E. coli
713:E. coli
680:β-sheet
668:E. coli
661:E. coli
635:Domains
593:1.8.1.9
571:UniProt
484:Domains
454:Chr. 22
442:1.8.1.9
420:UniProt
333:Domains
303:Chr. 12
291:1.8.1.9
269:UniProt
101:1.8.1.9
58:PS00573
53:PROSITE
2802:Portal
2744:Lyases
2489:1.8.99
2470:1.8.98
2408:oxygen
2156:(MeSH)
2132:
2122:
2073:
2063:
2024:
1972:
1931:
1886:
1845:
1837:
1827:
1780:
1745:
1737:
1702:
1667:
1657:
1608:
1573:
1537:
1527:
1478:
1468:
1416:
1408:
1352:
1345:522221
1342:
1300:
1290:
1241:
1206:
1171:
1129:
1087:
1056:
1021:
980:
970:
686:, and
605:Chr. 3
576:Q86VQ6
559:RefSeq
552:606235
528:114112
517:TXNRD3
514:Symbol
458:q11.21
425:Q9NNW7
408:RefSeq
401:606448
366:TXNRD2
363:Symbol
274:Q16881
257:RefSeq
250:601112
214:TXNRD1
210:Symbol
78:SUPFAM
32:Symbol
2696:Types
2448:1.8.5
2426:1.8.4
2404:1.8.3
2372:1.8.2
2336:1.8.1
2051:(3).
1843:S2CID
1743:S2CID
1530:18523
1471:55487
1414:S2CID
1293:20490
764:NADPH
751:NADPH
676:NADPH
600:Locus
540:20667
449:Locus
389:18155
377:10587
298:Locus
238:12437
117:NADPH
74:SCOPe
65:SCOP2
2788:list
2781:EC7
2775:list
2768:EC6
2762:list
2755:EC5
2749:list
2742:EC4
2736:list
2729:EC3
2723:list
2716:EC2
2710:list
2703:EC1
2344:NADP
2329:1.8)
2130:PMID
2071:PMID
2022:PMID
1970:PMID
1929:PMID
1884:ISSN
1835:PMID
1778:PMID
1735:PMID
1700:PMID
1665:PMID
1606:PMID
1571:PMID
1535:PMID
1476:PMID
1406:PMID
1350:PMID
1298:PMID
1239:PMID
1204:PMID
1169:PMID
1127:PMID
1085:ISSN
1054:PMID
1019:PMID
978:PMID
749:and
547:OMIM
535:HGNC
396:OMIM
384:HGNC
245:OMIM
233:HGNC
226:7296
171:and
94:TrxR
70:1zof
2342:or
2340:NAD
2120:PMC
2110:doi
2098:112
2061:PMC
2053:doi
2012:hdl
2002:doi
1960:doi
1919:hdl
1911:doi
1874:doi
1825:PMC
1817:doi
1770:doi
1727:doi
1692:doi
1655:PMC
1645:doi
1598:doi
1563:doi
1525:PMC
1515:doi
1466:PMC
1456:doi
1396:hdl
1386:doi
1340:PMC
1332:doi
1288:PMC
1278:doi
1231:doi
1196:doi
1161:doi
1157:396
1119:doi
1046:doi
1009:doi
1005:272
968:PMC
960:doi
887:DCM
815:In
760:FAD
755:FAD
747:FAD
672:FAD
666:In
113:FAD
96:) (
2828::
2450::
2428::
2406::
2374::
2338::
2327:EC
2193::
2128:.
2118:.
2108:.
2096:.
2092:.
2069:.
2059:.
2049:63
2047:.
2043:.
2020:.
2010:.
1998:32
1996:.
1992:.
1968:.
1956:95
1954:.
1950:.
1927:.
1917:.
1907:41
1905:.
1882:.
1868:.
1864:.
1841:.
1833:.
1823:.
1815:.
1805:59
1803:.
1799:.
1776:.
1766:52
1764:.
1741:.
1733:.
1723:56
1721:.
1698:.
1688:52
1686:.
1663:.
1653:.
1643:.
1633:10
1631:.
1627:.
1604:.
1594:36
1592:.
1569:.
1559:44
1557:.
1533:.
1523:.
1513:.
1503:97
1501:.
1497:.
1474:.
1464:.
1454:.
1444:98
1442:.
1438:.
1426:^
1412:.
1404:.
1394:.
1380:.
1376:.
1362:^
1348:.
1338:.
1328:24
1326:.
1322:.
1310:^
1296:.
1286:.
1276:.
1266:94
1264:.
1260:.
1237:.
1225:.
1202:.
1192:43
1190:.
1167:.
1155:.
1151:.
1139:^
1125:.
1115:18
1113:.
1097:^
1081:94
1079:.
1075:.
1052:.
1042:52
1040:.
1017:.
1003:.
999:.
976:.
966:.
954:.
932:^
909:.
877:.
774:.
699:.
167:,
163:,
123:.
98:EC
92:,
90:TR
76:/
72:/
2804::
2790:)
2786:(
2777:)
2773:(
2764:)
2760:(
2751:)
2747:(
2738:)
2734:(
2725:)
2721:(
2712:)
2708:(
2532:e
2525:t
2518:v
2315:e
2308:t
2301:v
2183:e
2176:t
2169:v
2136:.
2112::
2104::
2077:.
2055::
2028:.
2014::
2004::
1976:.
1962::
1935:.
1921::
1913::
1890:.
1876::
1870:3
1849:.
1819::
1811::
1784:.
1772::
1749:.
1729::
1706:.
1694::
1671:.
1647::
1639::
1612:.
1600::
1577:.
1565::
1541:.
1517::
1509::
1482:.
1458::
1450::
1420:.
1398::
1388::
1382:9
1356:.
1334::
1304:.
1280::
1272::
1245:.
1233::
1227:9
1210:.
1198::
1175:.
1163::
1133:.
1121::
1091:.
1060:.
1048::
1025:.
1011::
984:.
962::
175:.
88:(
35:?
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.