825:
312:
237:
39:
813:
48:
981:
76:
882:
67:
634:
477:
1028:
744:, which provides the N-C-C-S backbone of the ring. Thiamine does not fit this pattern however. Several biosynthesis routes lead to the thiazole ring as required for the formation of thiamine. Sulfur of the thiazole is derived from cysteine. In anaerobic bacteria, the CN group is derived from dehydroglycine.
1398:
Alajarín, M.; Cabrera, J.; Pastor, A.; Sánchez-Andrada, P.; Bautista, D. (2006). "On the
Cycloaddition of 2-Aminothiazoles and Dimethyl Acetylenedicarboxylate. Experimental and Computational Evidence of a Thermal Disrotatory Ring Opening of Fused Cyclobutenes".
687:
dyes contain benzothiazole subunits: Algol Yellow 8 (CAS# ), Algol Yellow GC (CAS# ), Indanthren Rubine B (CAS# ), Indanthren Blue CLG (CAS# , and
Indanthren Blue CLB (CAS#). These thiazole dye are used for dyeing
668:, oxazoles are not. It is found in naturally occurring peptides, and utilised in the development of peptidomimetics (i.e. molecules that mimic the function and structure of peptides).
490:
787:
824:
361:
876:-oxide is able to shift the reactivity to reliably favor the 2-position, and allows for these reactions to be carried out under much more mild conditions.
555:
that contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a
648:
Thiazoles are found in a variety of specialized products, often fused with benzene derivatives, the so-called benzothiazoles. In addition to vitamin B
1137:
1099:
625:. The calculated pi-electron density marks C5 as the primary site for electrophilic substitution, and C2-H as susceptible to deprotonation.
679:
are marketed for control of various agricultural pests. Another widely used thiazole derivative is the non-steroidal anti-inflammatory drug
1351:
Campeau, Louis-Charles; Bertrand-Laperle, Mégan; Leclerc, Jean-Philippe; Villemure, Elisia; Gorelsky, Serge; Fagnou, Keith (2008-03-01).
792:
1262:
Dondoni, A.; Merino, P. (1995). "Diastereoselective
Homologation of D-(R)-Glyceraldehyde Acetonide using 2-(Trimethylsilyl)thiazole".
1243:
1074:
326:
1019:
782:
react at this site, replacing the proton. 2-Lithiothiazoles are also generated by metal-halogen exchange from 2-bromothiazole.
1480:
497:
269:
290:
980:
812:
435:
786:
622:
1475:
1316:
721:
454:
1057:
Zoltewicz, J. A.; Deady, L. W. (1978). "Quaternization of
Heteroaromatic Compounds: Quantitative Aspects".
881:
834:
232:
967:
779:
1279:
552:
194:
1435:
Arduengo, A. J.; Goerlich, J. R.; Marshall, W. J. (1997). "A Stable
Thiazol-2-ylidene and Its Dimer".
1007:
939:
928:
94:
307:
120:
1470:
1116:
920:
1124:. Topics in Heterocyclic Chemistry. Vol. 48. Springer Berlin Heidelberg. pp. 235–266.
621:
chemical shift of the ring protons, which absorb between 7.27 and 8.77 ppm, indicating a strong
938:, but in general at high temperatures due to favorable aromatic stabilization of the reactant;
1417:
1380:
1372:
1333:
1239:
1216:
1155:
1133:
1095:
1070:
916:
849:
701:
1446:
1409:
1364:
1325:
1267:
1206:
1164:
1125:
1062:
1003:
796:
725:
641:
599:
515:
384:
770:
with strong bases occurs at C2-H. The negative charge on this position is stabilized as an
278:
38:
1437:
1292:
1182:
Kriek, M.; Martins, F.; Leonardi, R.; Fairhurst, S. A.; Lowe, D. J.; Roach, P. L. (2007).
214:
154:
130:
704:
of thiazoles. Prominent is the
Hantzsch thiazole synthesis, which is a reaction between
311:
236:
174:
606:
468:
1066:
75:
1464:
1401:
1353:"C2, C5, and C4 Azole N -Oxide Direct Arylation Including Room-Temperature Reactions"
935:
908:
767:
729:
728:. Thiazoles can be accessed by acylation of 2-aminothiolates, often available by the
717:
713:
684:
676:
657:
425:
225:
47:
1183:
1015:
857:
1188:: An Investigation of the Substrates and Purified Proteins Required for Activity
258:
1092:
The
Chemistry of Heterocycles: Structure, Reactions, Syntheses, and Applications
804:
775:
665:
610:
66:
17:
1450:
1037:
995:
959:
924:
705:
653:
618:
412:
205:
1376:
1352:
1271:
1168:
974:
in a 6-electron electrocyclic ring, closing before extruding the sulfur atom.
861:
757:
709:
680:
672:
661:
637:
591:
1421:
1384:
1337:
1220:
1211:
1129:
633:
947:
853:
741:
571:
556:
896:
865:
860:; some of the oxidation takes place at sulfur, leading to non-aromatic
838:
614:
595:
568:
245:
1413:
1368:
1027:
1329:
943:
893:
800:
689:
185:
872:-oxides are useful in Palladium-catalysed C-H arylations, where the
605:
Being planar thiazoles are characterized by significant pi-electron
467:
Except where otherwise noted, data are given for materials in their
1306:
Amir, E.; Rozen, S. (2006). "Easy Access to the Family of
Thiazole
962:
intermediate in a formal cycloaddition to a cyclobutene, then to a
1026:
845:
771:
632:
587:
165:
153:
143:
756:
of 2.5 for the conjugate acid, thiazoles are far less basic than
1115:
Mak, Jeffrey Y. W.; Xu, Weijun; Fairlie, David P. (2015-01-01).
946:
are followed by extrusion of sulfur, and the endproduct is a
724:, thiazoles arise by the condensation of α-aminonitrile with
979:
880:
823:
811:
521:
295:
74:
65:
46:
37:
1238:(3 ed.). Essex, England: Addison Wesley. p. 414.
536:
530:
524:
430:
116 to 118 °C (241 to 244 °F; 389 to 391 K)
1031:
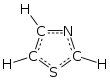
Structure of thiazoles (left) and thiazolium salts (right)
671:
Commercial significant thiazoles include mainly dyes and
712:. For example, 2,4-dimethylthiazole is synthesized from
567:
NS. The thiazole ring is notable as a component of the
485:
533:
958:(DMAD) to a pyridine was found to proceed through a
539:
518:
740:Thiazoles are generally formed via reactions of
257:
1153:George Schwarz (1945). "2,4-Dimethylthiazole".
1002:cation. Thiazolium salts are catalysts in the
129:
8:
1059:Advances in Heterocyclic Chemistry Volume 22
664:. Whereas thiazoles are well represented in
629:Occurrence of thiazoles and thiazolium salts
1257:
1255:
828:Thiazole Nucleophilic Aromatic Substitution
656:. Other important thiazole derivatives are
527:
950:; in one study, a very mild reaction of a
310:
235:
213:
26:
1210:
907:aldehyde takes place with, respectively,
700:Various laboratory methods exist for the
277:
1357:Journal of the American Chemical Society
617:. This aromaticity is evidenced by the
1049:
844:; many oxidizing agents exist, such as
366:
331:
306:
1288:
1277:
559:-like odor and the molecular formula C
226:
338:Key: FZWLAAWBMGSTSO-UHFFFAOYSA-N
193:
173:
7:
660:, for example, the firefly chemical
598:. Thiazole can also be considered a
793:Electrophilic aromatic substitution
675:. Thifluzamide, Tricyclazole, and
348:Key: FZWLAAWBMGSTSO-UHFFFAOYAI
248:
1090:Eicher, T.; Hauptmann, S. (2003).
1020:transition metal carbene complexes
582:Molecular and electronic structure
335:InChI=1S/C3H3NS/c1-2-5-3-4-1/h1-3H
25:
1061:. Vol. 22. pp. 71–121.
1014:-alkyl thiazolium salts give the
998:of thiazoles at nitrogen forms a
613:, moreso than the corresponding
345:InChI=1/C3H3NS/c1-2-5-3-4-1/h1-3H
785:
652:, the thiazole ring is found in
602:when part of a larger molecule.
514:
475:
402:
396:
956:dimethyl acetylenedicarboxylate
471:(at 25 °C , 100 kPa).
405:
390:
1:
1067:10.1016/S0065-2725(08)60103-8
586:Thiazoles are members of the
1234:Thomas L. Gilchrist (1997).
1040:is a thiazolium-based drug.
837:gives the aromatic thiazole
590:, heterocycles that include
61:
33:
1497:
968:electrocyclic ring opening
1451:10.1002/jlac.199719970213
952:2-(dimethylamino)thiazole
803:group, as illustrated in
640:is a thiazole-containing
465:
448:2.5 (of conjugate acid)
377:
357:
322:
113:
105:
93:
88:
60:
32:
1272:10.15227/orgsyn.072.0021
1184:"Thiazole Synthase from
1169:10.15227/orgsyn.025.0035
972:7-thia-2-azanorcaradiene
623:diamagnetic ring current
609:and have some degree of
1317:Chemical Communications
934:Thiazoles can react in
780:organolithium compounds
722:Cook-Heilbron synthesis
455:Magnetic susceptibility
1287:Cite journal requires
1236:Heterocyclic Chemistry
1212:10.1074/jbc.M700782200
1032:
985:
984:Thiazole cycloaddition
886:
829:
817:
645:
79:
70:
51:
42:
1481:Simple aromatic rings
1130:10.1007/7081_2015_176
1030:
983:
940:Diels-Alder reactions
884:
835:Oxidation at nitrogen
827:
815:
636:
553:heterocyclic compound
78:
69:
50:
41:
1310:-oxides using HOF·CH
1010:. Deprotonation of
1008:Benzoin condensation
929:Mercury(II) chloride
816:Thiazole bromination
95:Preferred IUPAC name
1205:(24): 17413–17423.
420: g·mol
29:
1033:
986:
921:sodium borohydride
887:
885:Thiazole oxidation
830:
818:
795:at C5 but require
646:
551:, is a 5-membered
498:Infobox references
80:
71:
52:
43:
27:
1414:10.1021/jo060664c
1408:(14): 5328–5339.
1369:10.1021/ja7107068
1363:(11): 3276–3277.
1324:(21): 2262–2264.
1156:Organic Syntheses
1139:978-3-319-49117-2
1118:Peptidomimetics I
1101:978-3-527-30720-3
917:organic reduction
850:hypofluorous acid
848:; a novel one is
797:activating groups
702:organic synthesis
506:Chemical compound
504:
503:
461:-50.55·10 cm/mol
291:CompTox Dashboard
155:Interactive image
84:
83:
56:
55:
16:(Redirected from
1488:
1455:
1454:
1432:
1426:
1425:
1395:
1389:
1388:
1348:
1342:
1341:
1330:10.1039/b602594c
1303:
1297:
1296:
1290:
1285:
1283:
1275:
1259:
1250:
1249:
1231:
1225:
1224:
1214:
1196:
1186:Escherichia coli
1179:
1173:
1171:
1150:
1144:
1143:
1123:
1112:
1106:
1105:
1087:
1081:
1080:
1054:
1004:Stetter reaction
991:Thiazolium salts
966:in a 4-electron
899:; conversion of
789:
726:carbon disulfide
683:. The following
642:anti-cancer drug
600:functional group
546:
545:
542:
541:
538:
535:
532:
529:
526:
523:
520:
488:
482:
479:
478:
419:
407:
404:
398:
392:
385:Chemical formula
315:
314:
299:
297:
281:
261:
250:
239:
228:
217:
197:
177:
157:
133:
62:
34:
30:
21:
1496:
1495:
1491:
1490:
1489:
1487:
1486:
1485:
1461:
1460:
1459:
1458:
1438:Liebigs Annalen
1434:
1433:
1429:
1397:
1396:
1392:
1350:
1349:
1345:
1313:
1305:
1304:
1300:
1286:
1276:
1261:
1260:
1253:
1246:
1233:
1232:
1228:
1194:
1181:
1180:
1176:
1152:
1151:
1147:
1140:
1121:
1114:
1113:
1109:
1102:
1089:
1088:
1084:
1077:
1056:
1055:
1051:
1046:
993:
915:-methylation),
763:
755:
750:
738:
698:
651:
631:
584:
577:
566:
562:
517:
513:
507:
500:
495:
494:
493: ?)
484:
480:
476:
472:
458:
444:
417:
401:
395:
387:
373:
370:
365:
364:
353:
350:
349:
346:
340:
339:
336:
330:
329:
318:
300:
293:
284:
264:
251:
220:
200:
180:
160:
147:
136:
123:
109:
101:
100:
23:
22:
18:Thiazolium salt
15:
12:
11:
5:
1494:
1492:
1484:
1483:
1478:
1476:Aromatic bases
1473:
1463:
1462:
1457:
1456:
1445:(2): 365–374.
1427:
1390:
1343:
1311:
1298:
1289:|journal=
1251:
1244:
1226:
1174:
1145:
1138:
1107:
1100:
1082:
1075:
1048:
1047:
1045:
1042:
1035:
1034:
992:
989:
988:
987:
976:
975:
970:and then to a
964:1,3-thiazepine
936:cycloadditions
932:
892:Thiazoles are
889:
888:
852:prepared from
832:
831:
820:
819:
761:
753:
749:
746:
737:
734:
697:
694:
658:benzothiazoles
649:
630:
627:
607:delocalization
583:
580:
575:
564:
560:
505:
502:
501:
496:
474:
473:
469:standard state
466:
463:
462:
459:
453:
450:
449:
446:
442:
432:
431:
428:
422:
421:
415:
409:
408:
399:
393:
388:
383:
380:
379:
375:
374:
372:
371:
368:
360:
359:
358:
355:
354:
352:
351:
347:
344:
343:
341:
337:
334:
333:
325:
324:
323:
320:
319:
317:
316:
303:
301:
289:
286:
285:
283:
282:
274:
272:
266:
265:
263:
262:
254:
252:
244:
241:
240:
230:
222:
221:
219:
218:
210:
208:
202:
201:
199:
198:
190:
188:
182:
181:
179:
178:
170:
168:
162:
161:
159:
158:
150:
148:
141:
138:
137:
135:
134:
126:
124:
119:
116:
115:
111:
110:
107:
103:
102:
98:
97:
91:
90:
86:
85:
82:
81:
72:
58:
57:
54:
53:
44:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1493:
1482:
1479:
1477:
1474:
1472:
1469:
1468:
1466:
1452:
1448:
1444:
1440:
1439:
1431:
1428:
1423:
1419:
1415:
1411:
1407:
1404:
1403:
1402:J. Org. Chem.
1394:
1391:
1386:
1382:
1378:
1374:
1370:
1366:
1362:
1358:
1354:
1347:
1344:
1339:
1335:
1331:
1327:
1323:
1319:
1318:
1309:
1302:
1299:
1294:
1281:
1273:
1269:
1265:
1258:
1256:
1252:
1247:
1245:0-582-27843-0
1241:
1237:
1230:
1227:
1222:
1218:
1213:
1208:
1204:
1200:
1199:J. Biol. Chem
1193:
1191:
1187:
1178:
1175:
1170:
1166:
1162:
1158:
1157:
1149:
1146:
1141:
1135:
1131:
1127:
1120:
1119:
1111:
1108:
1103:
1097:
1093:
1086:
1083:
1078:
1076:9780120206223
1072:
1068:
1064:
1060:
1053:
1050:
1043:
1041:
1039:
1029:
1025:
1024:
1023:
1021:
1017:
1016:free carbenes
1013:
1009:
1005:
1001:
997:
990:
982:
978:
977:
973:
969:
965:
961:
957:
953:
949:
945:
941:
937:
933:
930:
926:
922:
918:
914:
910:
909:methyl iodide
906:
902:
898:
895:
891:
890:
883:
879:
878:
877:
875:
871:
867:
863:
859:
856:and water in
855:
851:
847:
843:
841:
836:
826:
822:
821:
814:
810:
809:
808:
806:
802:
798:
794:
790:
788:
783:
781:
777:
773:
769:
768:Deprotonation
765:
759:
747:
745:
743:
735:
733:
731:
730:Herz reaction
727:
723:
719:
718:chloroacetone
715:
714:thioacetamide
711:
707:
703:
695:
693:
691:
686:
685:anthroquinone
682:
678:
677:Thiabendazole
674:
669:
667:
663:
659:
655:
643:
639:
635:
628:
626:
624:
620:
616:
612:
608:
603:
601:
597:
593:
589:
581:
579:
573:
570:
558:
554:
550:
544:
511:
499:
492:
487:
470:
464:
460:
456:
452:
451:
447:
441:
437:
434:
433:
429:
427:
426:Boiling point
424:
423:
416:
414:
411:
410:
389:
386:
382:
381:
376:
367:
363:
356:
342:
332:
328:
321:
313:
309:
308:DTXSID2059776
305:
304:
302:
292:
288:
287:
280:
276:
275:
273:
271:
268:
267:
260:
256:
255:
253:
247:
243:
242:
238:
234:
231:
229:
227:ECHA InfoCard
224:
223:
216:
212:
211:
209:
207:
204:
203:
196:
192:
191:
189:
187:
184:
183:
176:
172:
171:
169:
167:
164:
163:
156:
152:
151:
149:
145:
140:
139:
132:
128:
127:
125:
122:
118:
117:
112:
104:
96:
92:
87:
77:
73:
68:
64:
63:
59:
49:
45:
40:
36:
35:
31:
19:
1442:
1436:
1430:
1405:
1400:
1393:
1360:
1356:
1346:
1321:
1315:
1307:
1301:
1280:cite journal
1263:
1235:
1229:
1202:
1198:
1189:
1185:
1177:
1160:
1154:
1148:
1117:
1110:
1091:
1085:
1058:
1052:
1036:
1011:
999:
994:
971:
963:
960:zwitterionic
955:
951:
912:
904:
900:
873:
869:
858:acetonitrile
839:
833:
791:
784:
776:Hauser bases
766:
751:
739:
736:Biosynthesis
699:
670:
666:biomolecules
647:
604:
585:
549:1,3-thiazole
548:
509:
508:
439:
114:Identifiers
106:Other names
99:1,3-Thiazole
868:: Thiazole
805:bromination
706:haloketones
611:aromaticity
378:Properties
233:100.005.475
195:ChEMBL15605
175:CHEBI:43732
1465:Categories
1044:References
1038:Alagebrium
1000:thiazolium
996:Alkylation
925:hydrolysis
799:such as a
710:thioamides
673:fungicides
654:epothilone
592:imidazoles
413:Molar mass
279:320RCW8PEF
206:ChemSpider
142:3D model (
121:CAS Number
1471:Thiazoles
1377:0002-7863
1094:. Wiley.
931:in water.
862:sulfoxide
758:imidazole
752:With a pK
748:Reactions
720:. In the
696:Synthesis
681:Meloxicam
662:luciferin
638:Bleomycin
28:Thiazole
1422:16808523
1385:18302383
1338:16718323
1221:17403671
1190:in vitro
1006:and the
948:pyridine
897:synthons
854:fluorine
742:cysteine
615:oxazoles
596:oxazoles
572:thiamine
557:pyridine
510:Thiazole
457:(χ)
131:288-47-1
108:Thiazole
944:alkynes
903:to the
866:sulfone
569:vitamin
491:what is
489: (
436:Acidity
369:n1ccsc1
246:PubChem
1420:
1383:
1375:
1336:
1266:: 21.
1242:
1219:
1163:: 35.
1136:
1098:
1073:
923:, and
901:R-thia
894:formyl
842:-oxide
801:methyl
764:=7).
690:cotton
588:azoles
547:), or
486:verify
483:
362:SMILES
186:ChEMBL
89:Names
1314:CN".
1195:(PDF)
1122:(PDF)
954:with
942:with
927:with
919:with
905:R-CHO
846:mCPBA
772:ylide
619:H NMR
418:85.12
327:InChI
166:ChEBI
144:JSmol
1443:1997
1418:PMID
1381:PMID
1373:ISSN
1334:PMID
1322:2006
1293:help
1240:ISBN
1217:PMID
1134:ISBN
1096:ISBN
1071:ISBN
1018:and
778:and
716:and
708:and
594:and
270:UNII
259:9256
215:8899
1447:doi
1410:doi
1365:doi
1361:130
1326:doi
1268:doi
1207:doi
1203:282
1165:doi
1126:doi
1063:doi
760:(pK
578:).
296:EPA
249:CID
1467::
1441:.
1416:.
1406:71
1379:.
1371:.
1359:.
1355:.
1332:.
1320:.
1284::
1282:}}
1278:{{
1264:72
1254:^
1215:.
1201:.
1197:.
1161:25
1159:.
1132:.
1069:.
1022:.
807::
774:.
732:.
692:.
574:(B
537:oʊ
525:aɪ
445:)
438:(p
1453:.
1449::
1424:.
1412::
1387:.
1367::
1340:.
1328::
1312:3
1308:N
1295:)
1291:(
1274:.
1270::
1248:.
1223:.
1209::
1192:"
1172:.
1167::
1142:.
1128::
1104:.
1079:.
1065::
1012:N
913:N
911:(
874:N
870:N
864:/
840:N
762:a
754:a
650:1
644:.
576:1
565:3
563:H
561:3
543:/
540:l
534:z
531:ə
528:.
522:θ
519:ˈ
516:/
512:(
481:Y
443:a
440:K
406:S
403:N
400:3
397:H
394:3
391:C
298:)
294:(
146:)
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.