429:
688:
572:
701:
668:
264:
449:. Being strongly stabilized by π-donating substituents, NHCs are powerful σ-donors but π-bonding with the metal is weak. For this reason, the bond between the carbon and the metal center is often represented by a single dative bond, whereas Fischer and Schrock carbenes are usually depicted with double bonds to metal. Continuing with this analogy, NHCs are often compared with trialkyl
649:
225:
630:
385:
128:
221:
possible. However this interaction is generally weak since the alpha donor atoms also donate to this orbital. As such, fisher carbenes are characterized as having partial double bond character. The major resonance structures of Fisher carbenes put the negative charge on the metal centre, and the positive on the carbon atom, making it electrophilic.
213:
113:(NHCs) were popularized following Arduengo's isolation of a stable free carbene in 1991. Reflecting the growth of the area, carbene complexes are now known with a broad range of different reactivities and diverse substituents. Often it is not possible to classify a carbene complex solely with regards to its electrophilicity or nucleophilicity.
220:
Fisher carbene complexes are related to the singlet form of carbenes, where both electrons occupy the same sp orbital at the carbon. This lone pair donates to a metal-based empty d orbital, forming a σ bond. π-backbonding from a filled metal d orbital to the empty p orbital of the carbon atom is
397:
is much greater, giving a strong double bond. These bonds are weakly polarized towards carbon and therefore the carbene atom is a nucleophile. Furthermore, the major resonance structures of
Schrock carbene put the negative charge on the carbon atom, making it nucleophilic. Complexes with the
392:
Bonding in such complexes can be viewed as the coupling of a triplet state metal and triplet carbene, forming a true double bond. Both the metal and carbon atom donate 2 electrons, one to each bond. Since there is no donation to the carbene atom from adjacent groups, the extent of
413:
47:, using many different synthetic routes such as nucleophilic addition and alpha-hydrogen abstraction. The term carbene ligand is a formalism since many are not directly derived from carbenes and most are much less reactive than lone carbenes. Described often as
445:(NHCs) are particularly common carbene ligands. They are popular because they are more readily prepared than Schrock and Fischer carbenes. In fact, many NHCs are isolated as the free ligand, since they are
716:, first synthesized as early as 1925, although it was never identified to be a carbene complex. The characterization of (CO)5W(COCH3(Ph)) in the 1960s is often cited as the starting point of the area and
587:
for the synthesis of higher alkenes. A variety of related reactions are used to interconvert light alkenes, e.g. butenes, propylene, and ethylene. Carbene complexes are invoked as intermediates in the
622:
can be used for the olefination of carbonyls, replacing the oxygen atom with a methylidene group. The nucleophilic carbon atom behaves similarly to the carbon atom of the phosphorus ylide in the
1981:
671:
Catalytic cycle for the insertion of carbenes into carbon-hydrogen bonds. The metal carbene is generated by nitrogen elimination from the diazo compound, and then inserts into the C-H bond.
575:
Catalytic cycle of olefin metathesis. The metal complex alternated between a metallocyclobutane ring and carbene complex, catalyzing the formation of new carbon-carbon double bonds.
2548:
2585:
2089:
2435:
1385:
Przyojski JA, Veggeberg KP, Arman HD, Tonzetich ZJ (2015-09-08). "Mechanistic
Studies of Catalytic Carbon–Carbon Cross-Coupling by Well-Defined Iron NHC Complexes".
2615:
240:
Fischer carbenes can be likened to ketones, with the carbene carbon atom being electrophilic, like the carbonyl carbon atom of a ketone. This can be seen from the
2652:
2671:
2742:
2466:
1771:
74:, and are of value in both industrial heterogeneous and in homogeneous catalysis for laboratory- and industrial-scale preparation of fine chemicals.
1814:
1310:
687:
652:
Nucleophilic abstraction of the methyl group of a Fisher carbene. The negatively charge oxygen is a nucleophile which can undergo further reaction.
248:-like reactions. The hydrogen atoms attached to the carbon atom α to the carbene carbon atom are acidic, and can be deprotonated by a base such as
244:, where there is a significant contribution from the structure bearing a positive carbon centre. Like ketones, Fischer carbene species can undergo
2607:
2415:
2286:
1571:
1071:
816:
388:
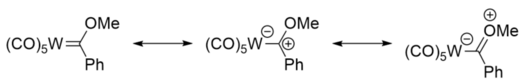
Orbital interaction in the bonding of a
Schrock carbene. Both the metal and carbon provide 2 unpaired electron each, forming the double bond.
2721:
2572:
2493:
2440:
2082:
1829:
2458:
2410:
428:
2733:
2688:
2644:
2628:
2430:
2397:
2387:
2274:
1921:
2805:
2774:
2683:
2540:
2339:
1507:"Guiding principles for site selective and stereoselective intermolecular C–H functionalization by donor/acceptor rhodium carbenes"
952:
2636:
2599:
2594:
2560:
2402:
2351:
2316:
2299:
2227:
2167:
1956:
1916:
779:
2820:
2445:
2218:
2206:
2177:
2172:
2075:
1966:
1961:
1946:
1439:
Herrmann, W. A. (1982). "The methylene bridge: A Challenge to
Synthetic, Mechanistic and Structural Organometallic Chemistry".
1412:
Przyojski JA, Arman HD, Tonzetich ZJ (2012-12-18). "NHC Complexes of Cobalt(II) Relevant to
Catalytic C–C Coupling Reactions".
1849:
563:
Metal carbene complexes have applications in hetereogeneous and homogeneous catalysis, and as reagents for organic reactions.
2521:
2425:
2379:
2262:
2182:
2136:
1971:
457:
that influence catalysis through a combination of electronic and steric effects, but they do not directly bind substrates.
216:
Orbital interaction in a Fisher carbene. The carbene electrons are donated to a sigma bond, and weak pi-backbonding occurs.
2194:
1764:
2713:
2535:
2249:
752:
in 1974 marked the first metal alkylidene complex. In 1991, Anthony J. Arduengo synthesized and crystallized the first
2022:
2027:
660:
can be used for cyclopropanation or to insert into C-H bonds of organic substrates. These reactions are catalyzed by
645:
can be abstracted from the donating group of a
Fischer carbene, making it a strong nucleophile for further reaction.
681:
2815:
728:
and Karl Öfele separately reported metal-bonded N-heterocyclic carbenes. The synthesis and characterization of ((CH
705:
1839:
928:
657:
416:
Major resonance structures of a
Schrock carbene. The negative charge at the carbon atom renders it nucleophilic.
2697:
2257:
2112:
1911:
1875:
1780:
1757:
721:
664:
or related chiral derivatives. Such catalysis is assumed to proceed via the intermediacy of carbene complexes.
638:
691:
General reaction scheme for the Wullf-Dötz reaction, making phenols from Fisher carbene complexes and alkynes.
2485:
24:
2580:
2392:
2056:
1941:
661:
580:
439:
107:
44:
2420:
1976:
292:
241:
102:, are characterized by more nucleophilic carbene carbon centers; these species typically feature higher
579:
The dominant application of metal carbenes involves none of the above classes of compounds, but rather
571:
2371:
2098:
1931:
1794:
1264:
1099:
940:
725:
2761:
1506:
588:
2779:
2051:
1870:
1844:
1799:
626:, attacking the electrophilic carbonyl atom of a ketone, followed by elimination of a metal oxide.
595:
2784:
1251:
Aldeco-Perez E, Rosenthal AJ, Donnadieu B, Parameswaran P, Frenking G, Bertrand G (October 2009).
2766:
2512:
2117:
1865:
1819:
1717:"1,3-Dimethyl-4-imidazolinyliden-(2)-pentacarbonylchrom ein neuer übergangsmetall-carben-komplex"
1059:
753:
749:
717:
446:
99:
87:
619:
598:
and
Schrock molybdenum-imido catalysts have been used for olefin metathesis in laboratory-scale
544:
236:. Structures with a positive charge on carbon are significant and make the carbon electrophilic.
67:
2810:
2046:
2017:
2007:
1936:
1736:
1697:
1658:
1619:
1611:
1567:
1553:
1534:
1526:
1487:
1367:
1290:
1233:
1198:
1159:
1067:
1041:
979:
956:
906:
864:
856:
812:
607:
599:
584:
399:
71:
1677:
1638:
1591:
1253:"Isolation of a C5-deprotonated imidazolium, a crystalline "abnormal" N-heterocyclic carbene"
886:
310:
Schrock carbenes do not have π-accepting ligands on the metal centre. They are often called
2012:
1906:
1809:
1804:
1728:
1689:
1650:
1603:
1559:
1518:
1479:
1448:
1421:
1394:
1357:
1349:
1318:
1280:
1272:
1225:
1190:
1151:
1115:
1107:
1088:"A neutron diffraction study of bis(cyclopentadienyl)(methyl)(methylene)tantalum(V) at 15 K"
1033:
987:
948:
898:
848:
454:
40:
1177:
Hill, Anthony F.; Roper, Warren R.; Waters, Joyce M.; Wright, Anthony H. (September 1983).
1087:
1885:
1880:
1216:
Hahn FE, Jahnke MC (2008). "Heterocyclic carbenes: synthesis and coordination chemistry".
769:
623:
603:
319:
140:
122:
103:
1268:
1103:
944:
306:
distances are 2.37 and 2.04 Å, respectively. Color code: blue = Ta, gray = C, white = H.
2530:
2244:
2002:
1901:
1824:
1362:
1337:
1285:
1252:
394:
249:
245:
1732:
2799:
2239:
2144:
1997:
760:
alkyl groups, accelerating the field of N-heterocarbene ligands to its current use.
720:, for this and other achievements in organometallic chemistry, was awarded the 1973
648:
412:
1467:
1178:
1139:
700:
667:
642:
486:
224:
91:
1483:
1336:
Fillman KL, Przyojski JA, Al-Afyouni MH, Tonzetich ZJ, Neidig ML (February 2015).
629:
1140:"Alkylcarbene complex of tantalum by intramolecular .alpha.-hydrogen abstraction"
2304:
1926:
1716:
1563:
713:
170:
160:
1308:
Arduengo AJ, Goerlich JR, Marshall WJ (2002-05-01). "A stable diaminocarbene".
1021:
384:
263:
127:
1111:
1037:
757:
212:
151:
1740:
1701:
1662:
1615:
1530:
1491:
1398:
1202:
1163:
1045:
910:
860:
836:
2038:
1834:
1452:
1276:
977:
Arduengo III AJ, Harlow RL, Kline M (1991). "A stable crystalline carbene".
450:
70:
are used in synthesis. They also feature in catalytic reactions, especially
1749:
1693:
1654:
1623:
1607:
1538:
1371:
1294:
1237:
1229:
960:
902:
868:
953:
10.1002/1521-3773(20000901)39:17<3012::AID-ANIE3012>3.0.CO;2-G
2067:
552:
548:
330:
326:
155:
28:
1322:
1194:
1155:
991:
680:
Fischer carbenes are used with alkynes as the starting reagents for the
1522:
1353:
774:
174:
36:
1425:
1120:
852:
633:
Olefination of an ester using Tebbe's reagent as a methylidene source.
131:
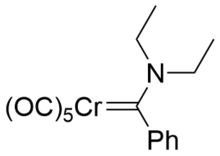
Example Fisher carbene with chromium(0) centre and diethylamine donor.
178:
164:
32:
1020:
de Frémont, Pierre; Marion, Nicolas; Nolan, Steven P. (April 2009).
1179:"A mononuclear, low-valent, electron-rich osmium methylene complex"
699:
686:
666:
647:
628:
411:
383:
262:
223:
211:
182:
126:
1092:
708:, was not recognized as such until decades after its preparation.
82:
Metal carbene complexes are often classified into two types. The
432:
147:
2071:
1753:
1022:"Carbenes: Synthesis, properties, and organometallic chemistry"
314:. Typically this subset of carbene complexes are found with:
1592:"Heterocyclic Carbenes: Synthesis and Coordination Chemistry"
811:(3, compl. rev. and extended ed.). Weinheim: WILEY-VCH.
255:, to give a nucleophile, which can undergo further reaction.
807:
Elschenbroich, Christoph; Elschenbroich, Christoph (2011).
594:
A variety of homogeneous carbene catalysts, especially the
1982:
Arene complexes of univalent gallium, indium, and thallium
712:
The first metal carbene complex to have been reported was
1639:"On the Existence of a Tungsten Carbonyl Carbene Complex"
887:"Organometallic Aspects of the Fischer-Tropsch Synthesis"
465:
An early example of this bonding mode was provided by
1590:
Hahn, F. Ekkehardt; Jahnke, Mareike C. (2008-04-14).
1064:
1678:"Direct Synthesis of a Mercury Salt-Carbene Complex"
338:
hydrogen and alkyl substituents on carbenoid carbon.
39:. Carbene complexes have been synthesized from most
2754:
2664:
2505:
2478:
2364:
2332:
2160:
2129:
2105:
2036:
1990:
1894:
1858:
1787:
1676:Wanzlick, H.-W.; Schönherr, H.-J. (February 1968).
618:Homogeneous Schrock-type carbene complexes such as
1682:Angewandte Chemie International Edition in English
1643:Angewandte Chemie International Edition in English
891:Angewandte Chemie International Edition in English
90:, feature strong π-acceptors at the metal and are
54:, carbene ligands are intermediate between alkyls
1505:Davies, Huw M. L.; Morton, Daniel (2011-03-21).
1066:(4th ed.). New Jersey: Wiley-Interscience.
66:. Many different carbene-based reagents such as
835:Arnold, Polly L.; Casely, Ian J. (2009-08-12).
543:Another example of this family of compounds is
1468:"Industrial applications of olefin metathesis"
2083:
1765:
8:
409:) are the simplest Schrock-type carbenes.
135:The common features of Fisher carbenes are:
1637:Fischer, E. O.; Maasböl, A. (August 1964).
972:
970:
547:. It features a methylene bridge joining
335:σ-donor and sometimes π-donor metal ligands
2090:
2076:
2068:
1866:Oxidative addition / reductive elimination
1772:
1758:
1750:
1472:Journal of Molecular Catalysis A: Chemical
837:"F-Block N-Heterocyclic Carbene Complexes"
1361:
1284:
1119:
1815:Polyhedral skeletal electron pair theory
1183:Journal of the American Chemical Society
1144:Journal of the American Chemical Society
570:
535:
531:
527:
523:
519:
515:
511:
507:
503:
499:
495:
480:
476:
472:
468:
453:ligands. Like phosphines, NHCs serve as
427:
405:
376:
372:
368:
361:
357:
353:
349:
345:
302:
298:
286:
282:
278:
274:
270:
231:
204:
200:
192:
57:
50:
1596:Angewandte Chemie International Edition
885:Herrmann, Wolfgang A. (February 1982).
790:
1585:
1583:
1552:Denmark, Scott E., ed. (2004-04-30).
7:
1922:Transition metal fullerene complexes
1138:Schrock, Richard R. (October 1974).
1133:
1131:
1015:
1013:
1011:
1009:
1007:
1005:
1003:
1001:
922:
920:
880:
878:
830:
828:
802:
800:
798:
796:
794:
1721:Journal of Organometallic Chemistry
1957:Transition metal carbyne complexes
1952:Transition metal carbene complexes
1917:Transition metal indenyl complexes
1338:"N-heterocyclic carbene complexes"
146:middle and late transition metals
14:
1967:Transition metal alkyne complexes
1962:Transition metal alkene complexes
704:The first metal carbene complex,
1972:Transition-metal allyl complexes
780:Transition metal carbyne complex
21:transition metal carbene complex
16:Class of organometalic compounds
1947:Transition metal acyl complexes
1026:Coordination Chemistry Reviews
929:"Olefin Metathesis and Beyond"
927:Fürstner, Alois (2000-09-01).
228:Major resonance structures of
1:
1733:10.1016/s0022-328x(00)88691-x
1484:10.1016/j.molcata.2003.10.049
559:Application of Metal Carbenes
461:Bimetallic carbene complexes
94:at the carbene carbon atom.
2023:Shell higher olefin process
1830:Dewar–Chatt–Duncanson model
1564:10.1002/0471264180.or070.02
2837:
1912:Cyclopentadienyl complexes
1876:β-hydride elimination
1850:Metal–ligand multiple bond
1441:Pure and Applied Chemistry
1098:(3): 439–443. 1988-03-15.
120:
1977:Transition metal carbides
1112:10.1107/S0108270187010527
1038:10.1016/j.ccr.2008.05.018
658:methyl phenyldiazoacetate
2806:Organometallic chemistry
1781:Organometallic chemistry
1511:Chemical Society Reviews
1399:10.1021/acscatal.5b01445
722:Nobel Prize in Chemistry
639:nucleophilic abstraction
614:Stoichiometric reactions
325:early transition metals
1942:Half sandwich compounds
1715:Öfele, K. (June 1968).
1453:10.1351/pac198254010065
1277:10.1126/science.1178206
591:route to hydrocarbons.
581:heterogeneous catalysts
435:is a common NHC ligand.
35:, itself also called a
25:organometallic compound
2821:Coordination chemistry
2099:Coordination complexes
2057:Bioinorganic chemistry
1694:10.1002/anie.196801412
1655:10.1002/anie.196405801
1608:10.1002/anie.200703883
1230:10.1002/anie.200703883
903:10.1002/anie.198201171
709:
692:
672:
662:dirhodium tetraacetate
653:
634:
576:
443:-Heterocyclic carbenes
436:
424:-Heterocyclic carbenes
417:
389:
307:
237:
217:
132:
111:-Heterocyclic carbenes
2028:Ziegler–Natta process
1932:Metal tetranorbornyls
1558:(1 ed.). Wiley.
1466:Mol, J (2004-04-13).
703:
690:
670:
656:Diazo compounds like
651:
632:
574:
431:
415:
387:
293:X-ray crystallography
266:
227:
215:
130:
2037:Related branches of
1795:Crystal field theory
756:, an NHC with large
726:Hans-Werner Wanzlick
312:alkylidene complexes
242:resonance structures
2052:Inorganic chemistry
1871:Migratory insertion
1845:Agostic interaction
1800:Ligand field theory
1323:10.1021/ja00149a034
1317:(44): 11027–11028.
1269:2009Sci...326..556A
1195:10.1021/ja00356a050
1156:10.1021/ja00828a061
1104:1988AcCrC..44..439.
992:10.1021/ja00001a054
945:2000AngCh..39.3012F
684:, forming phenols.
682:Wulff–Dötz reaction
676:Wulff-Dötz Reaction
447:persistent carbenes
291:, as determined by
1937:Sandwich compounds
1895:Types of compounds
1820:Isolobal principle
1523:10.1039/C0CS00217H
1354:10.1039/c4sc02791d
754:persistent carbene
750:Richard R. Schrock
718:Ernst Otto Fischer
714:Chugaev's red salt
710:
706:Chugaev's red salt
693:
673:
654:
635:
577:
437:
418:
390:
308:
238:
218:
133:
106:(valency) metals.
100:Richard R. Schrock
88:Ernst Otto Fischer
2816:Transition metals
2793:
2792:
2065:
2064:
2047:Organic chemistry
2018:Olefin metathesis
2008:Grignard reaction
1907:Grignard reagents
1602:(17): 3122–3172.
1573:978-0-471-26418-7
1555:Organic Reactions
1426:10.1021/om3010756
1393:(10): 5938–5946.
1311:J. Am. Chem. Soc.
1218:Angewandte Chemie
1189:(18): 5939–5940.
1150:(21): 6796–6797.
1073:978-0-471-66256-3
980:J. Am. Chem. Soc.
939:(17): 3012–3043.
933:Angewandte Chemie
853:10.1021/cr8005203
818:978-3-527-29390-2
608:materials science
596:Grubbs' ruthenium
585:alkene metathesis
455:spectator ligands
342:Examples include
189:Examples include
84:Fischer carbenes,
72:alkene metathesis
41:transition metals
2828:
2092:
2085:
2078:
2069:
2013:Monsanto process
1810:d electron count
1805:18-electron rule
1774:
1767:
1760:
1751:
1745:
1744:
1712:
1706:
1705:
1673:
1667:
1666:
1634:
1628:
1627:
1587:
1578:
1577:
1549:
1543:
1542:
1517:(4): 1857–1869.
1502:
1496:
1495:
1463:
1457:
1456:
1436:
1430:
1429:
1409:
1403:
1402:
1382:
1376:
1375:
1365:
1348:(2): 1178–1188.
1342:Chemical Science
1333:
1327:
1326:
1305:
1299:
1298:
1288:
1248:
1242:
1241:
1213:
1207:
1206:
1174:
1168:
1167:
1135:
1126:
1125:
1123:
1084:
1078:
1077:
1056:
1050:
1049:
1032:(7–8): 862–892.
1017:
996:
995:
974:
965:
964:
924:
915:
914:
882:
873:
872:
847:(8): 3599–3611.
841:Chemical Reviews
832:
823:
822:
804:
604:natural products
539:
484:
408:
380:
364:
305:
290:
259:Schrock carbenes
235:
208:
196:
163:-acceptor metal
117:Fischer carbenes
96:Schrock carbenes
65:
61:
53:
2836:
2835:
2831:
2830:
2829:
2827:
2826:
2825:
2796:
2795:
2794:
2789:
2770:
2750:
2737:
2729:
2725:
2717:
2709:
2705:
2701:
2692:
2679:
2675:
2660:
2656:
2648:
2640:
2632:
2623:
2619:
2611:
2603:
2589:
2576:
2568:
2564:
2556:
2552:
2544:
2525:
2516:
2501:
2497:
2489:
2474:
2462:
2453:
2449:
2406:
2383:
2375:
2360:
2355:
2347:
2343:
2328:
2324:
2320:
2312:
2308:
2295:
2282:
2278:
2270:
2266:
2253:
2235:
2231:
2222:
2214:
2210:
2202:
2198:
2190:
2186:
2156:
2152:
2148:
2140:
2125:
2121:
2101:
2096:
2066:
2061:
2032:
1986:
1902:Gilman reagents
1890:
1886:Carbometalation
1881:Transmetalation
1854:
1783:
1778:
1748:
1714:
1713:
1709:
1675:
1674:
1670:
1636:
1635:
1631:
1589:
1588:
1581:
1574:
1551:
1550:
1546:
1504:
1503:
1499:
1465:
1464:
1460:
1438:
1437:
1433:
1414:Organometallics
1411:
1410:
1406:
1384:
1383:
1379:
1335:
1334:
1330:
1307:
1306:
1302:
1263:(5952): 556–9.
1250:
1249:
1245:
1224:(17): 3122–72.
1215:
1214:
1210:
1176:
1175:
1171:
1137:
1136:
1129:
1086:
1085:
1081:
1074:
1058:
1057:
1053:
1019:
1018:
999:
976:
975:
968:
926:
925:
918:
884:
883:
876:
834:
833:
826:
819:
809:Organometallics
806:
805:
792:
788:
770:Carbene radical
766:
747:
743:
739:
735:
731:
698:
678:
624:Wittig reaction
620:Tebbe's reagent
616:
589:Fischer–Tropsch
569:
561:
545:Tebbe's reagent
537:
533:
529:
525:
521:
517:
513:
509:
505:
501:
497:
493:
482:
478:
474:
470:
466:
463:
426:
407:
403:
378:
374:
370:
366:
363:
359:
355:
351:
347:
343:
320:oxidation state
304:
300:
296:
288:
284:
280:
276:
272:
268:
261:
233:
229:
206:
202:
198:
194:
190:
141:oxidation state
125:
123:Fischer carbene
119:
104:oxidation state
80:
68:Tebbe's reagent
63:
59:
55:
52:
48:
17:
12:
11:
5:
2834:
2832:
2824:
2823:
2818:
2813:
2808:
2798:
2797:
2791:
2790:
2788:
2787:
2782:
2777:
2772:
2768:
2764:
2758:
2756:
2755:Halide donors:
2752:
2751:
2749:
2748:
2740:
2735:
2731:
2727:
2723:
2719:
2715:
2711:
2707:
2703:
2699:
2695:
2690:
2686:
2681:
2677:
2673:
2668:
2666:
2662:
2661:
2659:
2658:
2654:
2650:
2646:
2642:
2638:
2634:
2630:
2626:
2621:
2617:
2613:
2609:
2605:
2601:
2597:
2592:
2587:
2583:
2578:
2574:
2570:
2566:
2562:
2558:
2554:
2550:
2546:
2542:
2538:
2533:
2528:
2523:
2519:
2514:
2509:
2507:
2503:
2502:
2500:
2499:
2495:
2491:
2487:
2482:
2480:
2476:
2475:
2473:
2472:
2464:
2460:
2456:
2451:
2447:
2443:
2438:
2433:
2428:
2423:
2418:
2413:
2408:
2404:
2400:
2395:
2390:
2385:
2381:
2377:
2373:
2368:
2366:
2362:
2361:
2359:
2358:
2353:
2349:
2345:
2341:
2336:
2334:
2330:
2329:
2327:
2326:
2322:
2318:
2314:
2310:
2306:
2302:
2297:
2293:
2289:
2284:
2280:
2276:
2272:
2268:
2264:
2260:
2255:
2251:
2247:
2242:
2237:
2233:
2229:
2225:
2220:
2216:
2212:
2208:
2204:
2200:
2196:
2192:
2188:
2184:
2180:
2175:
2170:
2164:
2162:
2158:
2157:
2155:
2154:
2150:
2146:
2142:
2138:
2133:
2131:
2127:
2126:
2124:
2123:
2119:
2115:
2109:
2107:
2103:
2102:
2097:
2095:
2094:
2087:
2080:
2072:
2063:
2062:
2060:
2059:
2054:
2049:
2043:
2041:
2034:
2033:
2031:
2030:
2025:
2020:
2015:
2010:
2005:
2003:Cativa process
2000:
1994:
1992:
1988:
1987:
1985:
1984:
1979:
1974:
1969:
1964:
1959:
1954:
1949:
1944:
1939:
1934:
1929:
1924:
1919:
1914:
1909:
1904:
1898:
1896:
1892:
1891:
1889:
1888:
1883:
1878:
1873:
1868:
1862:
1860:
1856:
1855:
1853:
1852:
1847:
1842:
1837:
1832:
1827:
1822:
1817:
1812:
1807:
1802:
1797:
1791:
1789:
1785:
1784:
1779:
1777:
1776:
1769:
1762:
1754:
1747:
1746:
1727:(3): P42–P43.
1707:
1688:(2): 141–142.
1668:
1649:(8): 580–581.
1629:
1579:
1572:
1544:
1497:
1458:
1431:
1420:(3): 723–732.
1404:
1377:
1328:
1300:
1243:
1208:
1169:
1127:
1079:
1072:
1051:
997:
986:(1): 361–363.
966:
916:
897:(2): 117–130.
874:
824:
817:
789:
787:
784:
783:
782:
777:
772:
765:
762:
745:
741:
737:
733:
729:
697:
694:
677:
674:
615:
612:
568:
565:
560:
557:
541:
540:
485:prepared from
462:
459:
425:
419:
395:pi backbonding
340:
339:
336:
333:
323:
260:
257:
187:
186:
181:and alkylated
167:
158:
144:
121:Main article:
118:
115:
98:, named after
79:
78:Classification
76:
45:f-block metals
15:
13:
10:
9:
6:
4:
3:
2:
2833:
2822:
2819:
2817:
2814:
2812:
2809:
2807:
2804:
2803:
2801:
2786:
2783:
2781:
2778:
2776:
2773:
2771:
2765:
2763:
2760:
2759:
2757:
2753:
2747:
2746:
2741:
2739:
2732:
2730:
2720:
2718:
2712:
2710:
2696:
2694:
2687:
2685:
2682:
2680:
2670:
2669:
2667:
2663:
2657:
2651:
2649:
2643:
2641:
2635:
2633:
2627:
2625:
2614:
2612:
2606:
2604:
2598:
2596:
2593:
2591:
2584:
2582:
2579:
2577:
2571:
2569:
2559:
2557:
2547:
2545:
2539:
2537:
2534:
2532:
2529:
2527:
2520:
2518:
2511:
2510:
2508:
2504:
2498:
2492:
2490:
2484:
2483:
2481:
2477:
2471:
2469:
2465:
2463:
2457:
2455:
2444:
2442:
2439:
2437:
2434:
2432:
2429:
2427:
2424:
2422:
2419:
2417:
2414:
2412:
2409:
2407:
2401:
2399:
2396:
2394:
2391:
2389:
2386:
2384:
2378:
2376:
2370:
2369:
2367:
2363:
2357:
2350:
2348:
2338:
2337:
2335:
2331:
2325:
2315:
2313:
2303:
2301:
2298:
2296:
2290:
2288:
2285:
2283:
2273:
2271:
2261:
2259:
2256:
2254:
2248:
2246:
2243:
2241:
2238:
2236:
2226:
2224:
2217:
2215:
2205:
2203:
2193:
2191:
2181:
2179:
2176:
2174:
2171:
2169:
2166:
2165:
2163:
2159:
2153:
2143:
2141:
2135:
2134:
2132:
2128:
2122:
2116:
2114:
2111:
2110:
2108:
2104:
2100:
2093:
2088:
2086:
2081:
2079:
2074:
2073:
2070:
2058:
2055:
2053:
2050:
2048:
2045:
2044:
2042:
2040:
2035:
2029:
2026:
2024:
2021:
2019:
2016:
2014:
2011:
2009:
2006:
2004:
2001:
1999:
1998:Carbonylation
1996:
1995:
1993:
1989:
1983:
1980:
1978:
1975:
1973:
1970:
1968:
1965:
1963:
1960:
1958:
1955:
1953:
1950:
1948:
1945:
1943:
1940:
1938:
1935:
1933:
1930:
1928:
1925:
1923:
1920:
1918:
1915:
1913:
1910:
1908:
1905:
1903:
1900:
1899:
1897:
1893:
1887:
1884:
1882:
1879:
1877:
1874:
1872:
1869:
1867:
1864:
1863:
1861:
1857:
1851:
1848:
1846:
1843:
1841:
1838:
1836:
1833:
1831:
1828:
1826:
1825:π backbonding
1823:
1821:
1818:
1816:
1813:
1811:
1808:
1806:
1803:
1801:
1798:
1796:
1793:
1792:
1790:
1786:
1782:
1775:
1770:
1768:
1763:
1761:
1756:
1755:
1752:
1742:
1738:
1734:
1730:
1726:
1722:
1718:
1711:
1708:
1703:
1699:
1695:
1691:
1687:
1683:
1679:
1672:
1669:
1664:
1660:
1656:
1652:
1648:
1644:
1640:
1633:
1630:
1625:
1621:
1617:
1613:
1609:
1605:
1601:
1597:
1593:
1586:
1584:
1580:
1575:
1569:
1565:
1561:
1557:
1556:
1548:
1545:
1540:
1536:
1532:
1528:
1524:
1520:
1516:
1512:
1508:
1501:
1498:
1493:
1489:
1485:
1481:
1477:
1473:
1469:
1462:
1459:
1454:
1450:
1446:
1442:
1435:
1432:
1427:
1423:
1419:
1415:
1408:
1405:
1400:
1396:
1392:
1388:
1387:ACS Catalysis
1381:
1378:
1373:
1369:
1364:
1359:
1355:
1351:
1347:
1343:
1339:
1332:
1329:
1324:
1320:
1316:
1313:
1312:
1304:
1301:
1296:
1292:
1287:
1282:
1278:
1274:
1270:
1266:
1262:
1258:
1254:
1247:
1244:
1239:
1235:
1231:
1227:
1223:
1219:
1212:
1209:
1204:
1200:
1196:
1192:
1188:
1184:
1180:
1173:
1170:
1165:
1161:
1157:
1153:
1149:
1145:
1141:
1134:
1132:
1128:
1122:
1117:
1113:
1109:
1105:
1101:
1097:
1093:
1089:
1083:
1080:
1075:
1069:
1065:
1061:
1055:
1052:
1047:
1043:
1039:
1035:
1031:
1027:
1023:
1016:
1014:
1012:
1010:
1008:
1006:
1004:
1002:
998:
993:
989:
985:
982:
981:
973:
971:
967:
962:
958:
954:
950:
946:
942:
938:
934:
930:
923:
921:
917:
912:
908:
904:
900:
896:
892:
888:
881:
879:
875:
870:
866:
862:
858:
854:
850:
846:
842:
838:
831:
829:
825:
820:
814:
810:
803:
801:
799:
797:
795:
791:
785:
781:
778:
776:
773:
771:
768:
767:
763:
761:
759:
755:
751:
727:
723:
719:
715:
707:
702:
695:
689:
685:
683:
675:
669:
665:
663:
659:
650:
646:
644:
640:
631:
627:
625:
621:
613:
611:
609:
605:
601:
597:
592:
590:
586:
582:
573:
566:
564:
558:
556:
554:
550:
546:
492:
491:
490:
488:
460:
458:
456:
452:
448:
444:
442:
434:
430:
423:
420:
414:
410:
401:
396:
386:
382:
337:
334:
332:
328:
324:
321:
317:
316:
315:
313:
294:
267:Structure of
265:
258:
256:
254:
253:-butyllithium
252:
247:
243:
226:
222:
214:
210:
184:
180:
177:atom such as
176:
172:
168:
166:
162:
159:
157:
153:
149:
145:
142:
138:
137:
136:
129:
124:
116:
114:
112:
110:
105:
101:
97:
93:
92:electrophilic
89:
85:
77:
75:
73:
69:
62:and carbynes
46:
42:
38:
34:
30:
26:
22:
2744:
2467:
2291:
1991:Applications
1951:
1927:Metallocenes
1724:
1720:
1710:
1685:
1681:
1671:
1646:
1642:
1632:
1599:
1595:
1554:
1547:
1514:
1510:
1500:
1478:(1): 39–45.
1475:
1471:
1461:
1444:
1440:
1434:
1417:
1413:
1407:
1390:
1386:
1380:
1345:
1341:
1331:
1314:
1309:
1303:
1260:
1256:
1246:
1221:
1217:
1211:
1186:
1182:
1172:
1147:
1143:
1095:
1091:
1082:
1063:
1054:
1029:
1025:
983:
978:
936:
932:
894:
890:
844:
840:
808:
711:
679:
655:
643:methyl group
641:reaction, a
636:
617:
593:
578:
562:
542:
506:(thf) + CH
487:diazomethane
464:
440:
438:
421:
391:
341:
322:metal center
311:
309:
250:
239:
219:
188:
171:substituents
143:metal center
134:
108:
95:
86:named after
83:
81:
27:featuring a
20:
18:
1840:spin states
1060:Crabtree RH
724:. In 1968,
400:methylidene
2800:Categories
2416:amino acid
2333:Si donors:
1788:Principles
1121:1808/17672
786:References
758:adamantane
740:)Ta=CHC(CH
514:→ [C
375:(NO)Cl(=CH
356:)Ta=CHC(CH
2665:S donors:
2506:O donors:
2479:P donors:
2441:porphyrin
2388:imidazole
2365:N donors:
2161:C donors:
2130:B donors:
2106:H donors:
2039:chemistry
1859:Reactions
1835:Hapticity
1741:0022-328X
1702:0570-0833
1663:0570-0833
1616:1433-7851
1531:1460-4744
1492:1381-1169
1447:: 65–82.
1203:0002-7863
1164:0002-7863
1046:0010-8545
911:0570-0833
861:0009-2665
600:synthesis
583:used for
567:Catalysis
451:phosphine
301:and Ta=CH
2811:Carbenes
1624:18398856
1539:21359404
1372:25621143
1295:19900893
1238:18398856
1062:(2005).
961:11028025
869:19358527
764:See also
553:aluminum
549:titanium
538:+ 2 thf
402:ligand (
234:W=COMePh
195:W=COMePh
169:π-donor
29:divalent
2279:& C
1363:4302958
1286:2871154
1265:Bibcode
1257:Science
1100:Bibcode
941:Bibcode
775:Carbyne
696:History
637:In the
534:] + N
203:Cr=C(NR
185:groups.
175:carbene
173:on the
165:ligands
37:carbene
31:carbon
2187:=CH-CH
1739:
1700:
1661:
1622:
1614:
1570:
1537:
1529:
1490:
1370:
1360:
1293:
1283:
1236:
1201:
1162:
1070:
1044:
959:
909:
867:
859:
815:
522:Mn(CO)
502:Mn(CO)
483:(μ−CO)
475:Mn(CO)
467:[C
367:Os(PPh
327:Ti(IV)
295:. The
179:alkoxy
33:ligand
23:is an
2178:HC(O)
2173:RC(O)
530:(μ−CH
331:Ta(V)
318:high
297:Ta−CH
246:aldol
183:amino
156:Cr(0)
152:Mo(0)
148:Fe(0)
64:(≡CR)
2581:acac
2553:/HCO
2436:bipy
2195:C(CH
1737:ISSN
1698:ISSN
1659:ISSN
1620:PMID
1612:ISSN
1568:ISBN
1535:PMID
1527:ISSN
1488:ISSN
1368:PMID
1291:PMID
1234:PMID
1199:ISSN
1160:ISSN
1068:ISBN
1042:ISSN
957:PMID
907:ISSN
865:PMID
857:ISSN
813:ISBN
606:and
551:and
433:IMes
381:.
365:and
344:((CH
281:TaCH
230:(CO)
199:(OC)
197:and
191:(CO)
139:low
56:(−CR
43:and
2676:NCS
2653:OPR
2629:OSR
2608:ClO
2595:ONO
2573:RCO
2450:Si)
2446:(Me
2431:RCN
2398:RNO
2346:4−n
2344:SiR
2300:≡CR
2292:=CR
2287:RNC
2211:=CH
1729:doi
1690:doi
1651:doi
1604:doi
1560:doi
1519:doi
1480:doi
1476:213
1449:doi
1422:doi
1395:doi
1358:PMC
1350:doi
1319:doi
1315:117
1281:PMC
1273:doi
1261:326
1226:doi
1191:doi
1187:105
1152:doi
1116:hdl
1108:doi
1034:doi
1030:253
988:doi
984:113
949:doi
899:doi
849:doi
845:109
748:by
736:CCH
602:of
494:2 C
404:=CH
352:CCH
285:(CH
207:)Ph
49:=CR
2802::
2780:Br
2775:Cl
2743:NC
2734:SR
2714:SO
2684:RS
2645:PO
2637:SO
2624:NO
2600:NO
2590:CO
2549:CO
2531:RO
2494:PR
2486:PR
2470:CS
2426:RN
2411:py
2403:NO
2393:NO
2372:NH
2356:Si
2281:70
2277:60
2250:CO
2245:CO
2240:CN
2219:RC
2207:CH
2183:CH
2137:BR
1735:.
1725:12
1723:.
1719:.
1696:.
1684:.
1680:.
1657:.
1645:.
1641:.
1618:.
1610:.
1600:47
1598:.
1594:.
1582:^
1566:.
1533:.
1525:.
1515:40
1513:.
1509:.
1486:.
1474:.
1470:.
1445:54
1443:.
1418:32
1416:.
1389:.
1366:.
1356:.
1344:.
1340:.
1289:.
1279:.
1271:.
1259:.
1255:.
1232:.
1222:47
1220:.
1197:.
1185:.
1181:.
1158:.
1148:96
1146:.
1142:.
1130:^
1114:.
1106:.
1096:44
1094:.
1090:.
1040:.
1028:.
1024:.
1000:^
969:^
955:.
947:.
937:39
935:.
931:.
919:^
905:.
895:21
893:.
889:.
877:^
863:.
855:.
843:.
839:.
827:^
793:^
610:.
555:.
518:Me
498:Me
489::
471:Me
329:,
269:(C
209:.
154:,
150:,
19:A
2785:I
2769:2
2767:F
2762:F
2745:S
2738:O
2736:2
2728:3
2726:O
2724:2
2722:S
2716:2
2708:2
2706:S
2704:2
2702:C
2700:2
2698:R
2693:S
2691:2
2689:R
2678:2
2674:2
2672:R
2655:3
2647:4
2639:4
2631:2
2622:5
2620:H
2618:5
2616:C
2610:4
2602:3
2588:2
2586:R
2575:2
2567:4
2565:O
2563:2
2561:C
2555:3
2551:3
2543:2
2541:O
2536:O
2526:O
2524:2
2522:R
2517:O
2515:2
2513:H
2496:2
2488:3
2468:N
2461:2
2459:N
2454:N
2452:2
2448:3
2421:N
2405:2
2382:3
2380:N
2374:3
2354:3
2352:R
2342:n
2340:H
2323:7
2321:H
2319:9
2317:C
2311:5
2309:H
2307:5
2305:C
2294:2
2275:C
2269:6
2267:R
2265:6
2263:C
2258:C
2252:2
2234:4
2232:H
2230:6
2228:C
2223:R
2221:2
2213:2
2209:2
2201:3
2199:)
2197:2
2189:2
2185:2
2168:R
2151:n
2149:H
2147:m
2145:B
2139:2
2120:2
2118:H
2113:H
2091:e
2084:t
2077:v
1773:e
1766:t
1759:v
1743:.
1731::
1704:.
1692::
1686:7
1665:.
1653::
1647:3
1626:.
1606::
1576:.
1562::
1541:.
1521::
1494:.
1482::
1455:.
1451::
1428:.
1424::
1401:.
1397::
1391:5
1374:.
1352::
1346:6
1325:.
1321::
1297:.
1275::
1267::
1240:.
1228::
1205:.
1193::
1166:.
1154::
1124:.
1118::
1110::
1102::
1076:.
1048:.
1036::
994:.
990::
963:.
951::
943::
913:.
901::
871:.
851::
821:.
746:3
744:)
742:3
738:2
734:3
732:)
730:3
536:2
532:2
528:2
526:]
524:2
520:5
516:5
512:2
510:N
508:2
504:2
500:5
496:5
481:2
479:]
477:2
473:5
469:5
441:N
422:N
406:2
379:)
377:2
373:2
371:)
369:3
362:3
360:)
358:3
354:2
350:3
348:)
346:3
303:2
299:3
289:)
287:2
283:3
279:2
277:)
275:5
273:H
271:5
251:n
232:5
205:2
201:5
193:5
161:π
109:N
60:)
58:3
51:2
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.