155:
29:
328:. Pseudorotation is similar in concept to the movement of a conformational diastereomer, though no full revolutions are completed. In the process of pseudorotation, two equatorial ligands (both of which have a shorter bond length than the third) "shift" toward the molecule's axis, while the axial ligands simultaneously "shift" toward the equator, creating a constant cyclical movement. Pseudorotation is particularly notable in simple molecules such as
736:
236:
The VSEPR theory also predicts that substitution of a ligand at a central atom by a lone pair of valence electrons leaves the general form of the electron arrangement unchanged with the lone pair now occupying one position. For molecules with five pairs of valence electrons including both bonding
195:
of molecular geometry, an axial position is more crowded because an axial atom has three neighboring equatorial atoms (on the same central atom) at a 90° bond angle, whereas an equatorial atom has only two neighboring axial atoms at a 90° bond angle. For molecules with five identical ligands, the
227:
and also with pi-electron withdrawing ability, as in the sequence Cl < F < CN. Both factors decrease electron density in the bonding region near the central atom so that crowding in the axial position is less important.
237:
pairs and lone pairs, the electron pairs are still arranged in a trigonal bipyramid but one or more equatorial positions is not attached to a ligand atom so that the molecular geometry (for the nuclei only) is different.
252:) with a central sulfur atom surrounded by four fluorine atoms occupying two axial and two equatorial positions, as well as one equatorial lone pair, corresponding to an AX
423:
716:
721:
700:
498:
674:
664:
121:
690:
648:
643:
565:
408:
65:
669:
622:
466:
124:), because there is no geometrical arrangement with five terminal atoms in equivalent positions. Examples of this molecular geometry are
617:
560:
491:
300:
with terminal iodine atoms in the two axial positions only and the three equatorial positions occupied by lone pairs of electrons (AX
596:
695:
586:
196:
axial bond lengths tend to be longer because the ligand atom cannot approach the central atom as closely. As examples, in PF
638:
484:
280:
molecule with fluorine atoms in two axial and one equatorial position, as well as two equatorial lone pairs. Finally, the
172:
The five atoms bonded to the central atom are not all equivalent, and two different types of position are defined. For
765:
739:
570:
261:
591:
539:
456:
297:
241:
760:
329:
173:
136:
125:
544:
176:
as an example, the phosphorus atom shares a plane with three chlorine atoms at 120° angles to each other in
120:. This is one geometry for which the bond angles surrounding the central atom are not identical (see also
154:
265:
245:
117:
523:
75:
461:
507:
350:
324:
Isomers with a trigonal bipyramidal geometry are able to interconvert through a process known as
113:
60:
49:
28:
404:
224:
93:
219:
the chlorines occupy two of the equatorial positions, indicating that fluorine has a greater
437:
309:
471:
325:
370:
223:
or tendency to occupy an axial position. In general ligand apicophilicity increases with
220:
754:
433:
518:
345:
257:
192:
16:
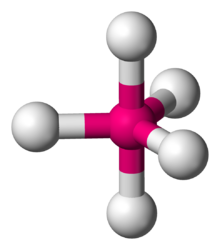
Molecular structure with atoms at the center and vertices of a triangular bipyramid
428:
296:) is also based upon a trigonal bipyramid, but the actual molecular geometry is
441:
432:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
281:
201:
105:
476:
180:
positions, and two more chlorine atoms above and below the plane (
153:
116:
with one atom at the center and 5 more atoms at the corners of a
480:
208:
the axial and equatorial are 214 and 202 pm respectively.
40:
168:= equatorial ligand (in plane perpendicular to unique axis)
709:
683:
657:
631:
605:
579:
553:
532:
308:); another example of this geometry is provided by
92:
84:
74:
59:
35:
21:
462:Interactive molecular examples for point groups
492:
457:Indiana University Molecular Structure Center
204:and the equatorial is 152 pm, and in PCl
8:
403:(2nd ed.). Prentice Hall. p. 407.
394:
392:
390:
499:
485:
477:
150:Axial (or apical) and equatorial positions
27:
399:Housecroft, C. E.; Sharpe, A. G. (2004).
142:
131:
51:
42:
362:
22:Trigonal bipyramidal molecular geometry
200:the axial P−F bond length is 158
18:
7:
158:Trigonal bipyramidal molecular shape
429:Compendium of Chemical Terminology
232:Related geometries with lone pairs
14:
735:
734:
371:"Trigonal bipyramidal molecules"
472:Animated Trigonal Planar Visual
163:= axial ligand (on unique axis)
1:
717:Tricapped trigonal prismatic
722:Capped square antiprismatic
701:Bicapped trigonal prismatic
262:T-shaped molecular geometry
782:
730:
675:Capped trigonal prismatic
514:
242:seesaw molecular geometry
26:
442:10.1351/goldbook.AT06990
330:phosphorus pentafluoride
174:phosphorus pentachloride
137:phosphorus pentachloride
126:phosphorus pentafluoride
665:Pentagonal bipyramidal
211:In the mixed halide PF
169:
710:Coordination number 9
684:Coordination number 8
658:Coordination number 7
632:Coordination number 6
606:Coordination number 5
580:Coordination number 4
554:Coordination number 3
533:Coordination number 2
157:
691:Square antiprismatic
649:Pentagonal pyramidal
613:Trigonal bipyramidal
326:Berry pseudorotation
320:Berry pseudorotation
266:chlorine trifluoride
246:sulfur tetrafluoride
146:) in the gas phase.
122:pentagonal bipyramid
118:triangular bipyramid
524:Coordination number
401:Inorganic Chemistry
76:Coordination number
766:Molecular geometry
644:Trigonal prismatic
566:Trigonal pyramidal
508:Molecular geometry
467:Molecular Modeling
375:Creative Chemistry
351:Molecular geometry
256:E molecule in the
170:
114:molecular geometry
110:trigonal bipyramid
748:
747:
670:Capped octahedral
623:Pentagonal planar
410:978-0-13-039913-7
225:electronegativity
191:According to the
102:
101:
773:
738:
737:
618:Square pyramidal
501:
494:
487:
478:
444:
421:
415:
414:
396:
385:
384:
382:
381:
367:
310:xenon difluoride
295:
294:
293:
145:
134:
55:
46:
31:
19:
781:
780:
776:
775:
774:
772:
771:
770:
761:Stereochemistry
751:
750:
749:
744:
726:
705:
679:
653:
627:
601:
575:
561:Trigonal planar
549:
528:
510:
505:
453:
448:
447:
422:
418:
411:
398:
397:
388:
379:
377:
369:
368:
364:
359:
342:
335:
322:
315:
307:
303:
292:
289:
288:
287:
285:
279:
275:
271:
255:
251:
234:
218:
214:
207:
199:
164:
159:
152:
144:
140:
133:
129:
112:formation is a
69:
53:
48:
44:
39:
17:
12:
11:
5:
779:
777:
769:
768:
763:
753:
752:
746:
745:
743:
742:
731:
728:
727:
725:
724:
719:
713:
711:
707:
706:
704:
703:
698:
693:
687:
685:
681:
680:
678:
677:
672:
667:
661:
659:
655:
654:
652:
651:
646:
641:
635:
633:
629:
628:
626:
625:
620:
615:
609:
607:
603:
602:
600:
599:
594:
589:
583:
581:
577:
576:
574:
573:
568:
563:
557:
555:
551:
550:
548:
547:
542:
536:
534:
530:
529:
527:
526:
521:
515:
512:
511:
506:
504:
503:
496:
489:
481:
475:
474:
469:
464:
459:
452:
451:External links
449:
446:
445:
434:Apicophilicity
416:
409:
386:
361:
360:
358:
355:
354:
353:
348:
341:
338:
333:
321:
318:
313:
305:
301:
290:
277:
273:
269:
253:
249:
233:
230:
221:apicophilicity
216:
212:
205:
197:
151:
148:
100:
99:
96:
90:
89:
86:
82:
81:
78:
72:
71:
67:
63:
57:
56:
37:
33:
32:
24:
23:
15:
13:
10:
9:
6:
4:
3:
2:
778:
767:
764:
762:
759:
758:
756:
741:
733:
732:
729:
723:
720:
718:
715:
714:
712:
708:
702:
699:
697:
694:
692:
689:
688:
686:
682:
676:
673:
671:
668:
666:
663:
662:
660:
656:
650:
647:
645:
642:
640:
637:
636:
634:
630:
624:
621:
619:
616:
614:
611:
610:
608:
604:
598:
597:Square planar
595:
593:
590:
588:
585:
584:
582:
578:
572:
569:
567:
564:
562:
559:
558:
556:
552:
546:
543:
541:
538:
537:
535:
531:
525:
522:
520:
517:
516:
513:
509:
502:
497:
495:
490:
488:
483:
482:
479:
473:
470:
468:
465:
463:
460:
458:
455:
454:
450:
443:
439:
435:
431:
430:
425:
420:
417:
412:
406:
402:
395:
393:
391:
387:
376:
372:
366:
363:
356:
352:
349:
347:
344:
343:
339:
337:
331:
327:
319:
317:
311:
299:
283:
267:
263:
259:
247:
243:
238:
231:
229:
226:
222:
209:
203:
194:
189:
187:
183:
179:
175:
167:
162:
156:
149:
147:
138:
127:
123:
119:
115:
111:
107:
97:
95:
91:
87:
85:Bond angle(s)
83:
79:
77:
73:
70:
64:
62:
58:
54:
45:
38:
34:
30:
25:
20:
696:Dodecahedral
612:
519:VSEPR theory
427:
419:
400:
378:. Retrieved
374:
365:
323:
264:is found in
258:AXE notation
244:is found in
239:
235:
210:
193:VSEPR theory
190:
188:positions).
185:
181:
177:
171:
165:
160:
109:
103:
94:μ (Polarity)
587:Tetrahedral
61:Point group
755:Categories
639:Octahedral
380:2023-02-07
357:References
346:AXE method
178:equatorial
282:triiodide
106:chemistry
88:90°, 120°
740:Category
571:T-shaped
340:See also
272:), an AX
36:Examples
135:), and
592:Seesaw
540:Linear
407:
298:linear
186:apical
50:Fe(CO)
424:IUPAC
312:, XeF
284:ion (
182:axial
545:Bent
405:ISBN
268:(ClF
260:. A
240:The
108:, a
438:doi
436:".
336:).
332:(PF
248:(SF
184:or
141:PCl
104:In
757::
426:,
389:^
373:.
316:.
215:Cl
202:pm
166:eq
161:ax
130:PF
68:3h
47:,
41:PF
500:e
493:t
486:v
440::
413:.
383:.
334:5
314:2
306:3
304:E
302:2
291:3
286:I
278:2
276:E
274:3
270:3
254:4
250:4
217:2
213:3
206:5
198:5
143:5
139:(
132:5
128:(
98:0
80:5
66:D
52:5
43:5
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.